Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. CA125 in Relation to Extra-Uterine Disease and LNM
3.3. Imaging in Relation to Extra-Uterine Disease and LNM
3.4. CA125 and CT Results in Relation to LNM
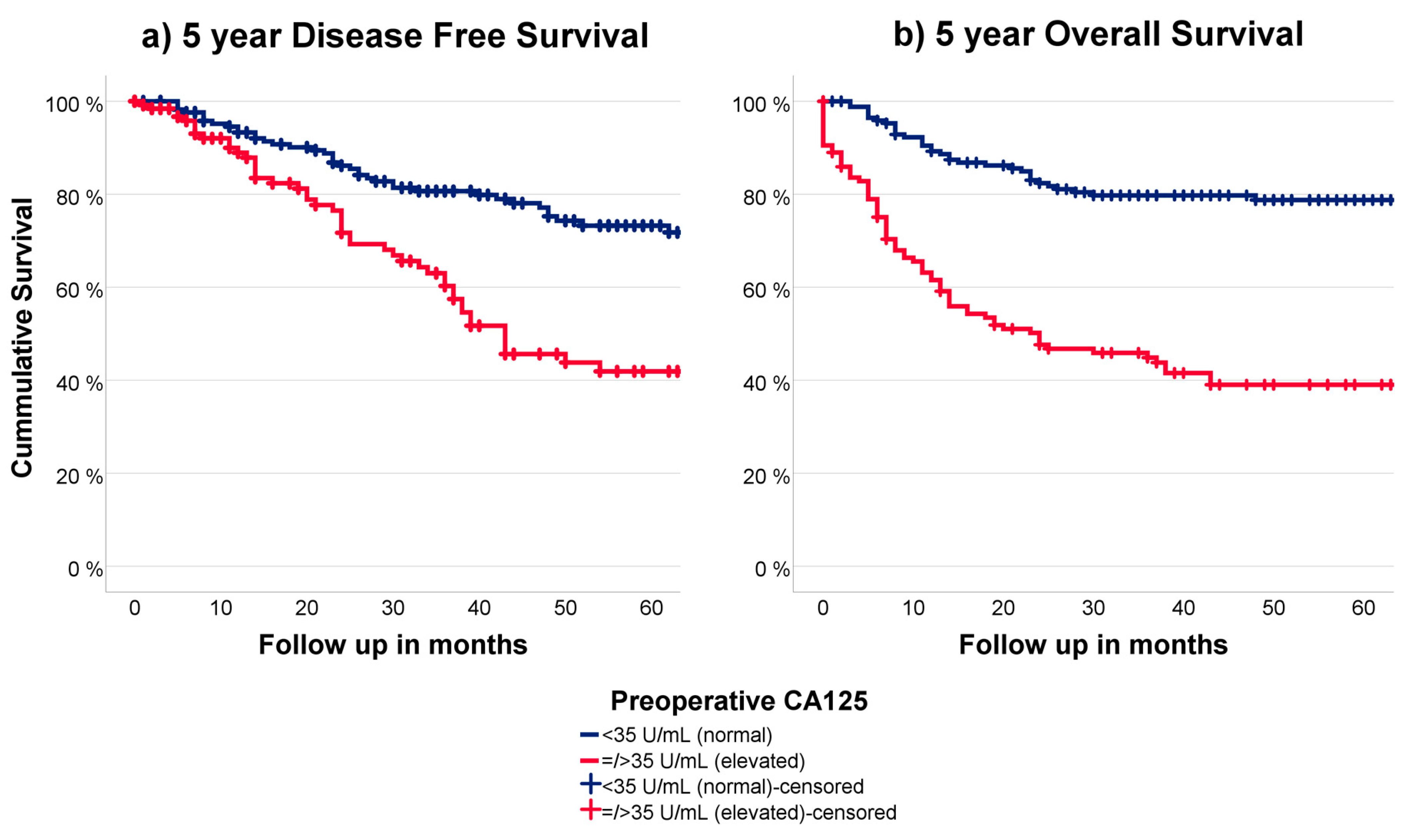
3.5. CA125 in Relation to Outcome
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stålberg, K.; Kjølhede, P.; Bjurberg, M.; Borgfeldt, C.; Dahm-Kähler, P.; Falconer, H. Risk factors for lymph node metastases in women with endometrial cancer: A population-based, nation-wide register study-On behalf of the Swedish Gynecological Cancer Group. Int. J. Cancer 2017, 140, 2693–2700. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014, 134, 85–92. [Google Scholar] [CrossRef]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Lowery, W.J.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Ali, S.; Mutch, D.G.; Zaino, R.J.; Powell, M.A.; Mannel, R.S.; Backes, F.J.; DiSilvestro, P.A.; Argenta, P.A.; Pearl, M.L.; et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol. Oncol. 2017, 145, 519–525. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Salvesen, H.B. What Is the Best Preoperative Imaging for Endometrial Cancer? Curr. Oncol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Fasmer, K.E.; Gulati, A.; Dybvik, J.A.; Wagner-Larsen, K.S.; Lura, N.; Salvesen, Ø. Preoperative pelvic MRI and 2-[(18)F]FDG PET/CT for lymph node staging and prognostication in endometrial cancer-time to revisit current imaging guidelines? Eur. Radiol. 2022, 33, 221–232. [Google Scholar] [CrossRef]
- Reijnen, C.; IntHout, J.; Massuger, L.F.; Strobbe, F.; Küsters-Vandevelde, H.V.; Haldorsen, I.S.; Snijders, M.P.; Pijnenborg, J.M. Diagnostic Accuracy of Clinical Biomarkers for Preoperative Prediction of Lymph Node Metastasis in Endometrial Carcinoma: A Systematic Review and Meta-Analysis. Oncologist 2019, 24, e880–e890. [Google Scholar] [CrossRef]
- Tuomi, T.; Pasanen, A.; Luomaranta, A.; Leminen, A.; Bützow, R.; Loukovaara, M. Risk-stratification of endometrial carcinomas revisited: A combined preoperative and intraoperative scoring system for a reliable prediction of an advanced disease. Gynecol. Oncol. 2015, 137, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, W.D.; Chung, H.H.; Jeong, D.H.; Seo, S.-S.; Lee, J.-M.; Lee, J.-K.; Kim, J.-W.; Kim, S.-M.; Park, S.-Y.; et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: A Korean gynecologic oncology group study. J. Clin. Oncol. 2012, 30, 1329–1334. [Google Scholar] [CrossRef]
- Kang, S.; Nam, J.-H.; Bae, D.-S.; Kim, J.-W.; Kim, M.-H.; Chen, X.; No, J.-H.; Lee, J.-M.; Watari, H.; Kim, S.M.; et al. Preoperative assessment of lymph node metastasis in endometrial cancer: A Korean Gynecologic Oncology Group study. Cancer 2017, 123, 263–272. [Google Scholar] [CrossRef]
- Tsikouras, P.; Koukouli, Z.; Bothou, A.; Manav, B.; Iatrakis, G.; Zervoudis, S. Preoperative assessment in endometrial cancer. Is triage for lymphadenectomy possible? J. Buon. 2017, 22, 34–43. [Google Scholar] [PubMed]
- Reijnen, C.; Gogou, E.; Visser, N.C.M.; Engerud, H.; Ramjith, J.; Van Der Putten, L.J.M.; Van De Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; et al. Preoperative risk stratification in endometrial cancer (ENDORISK) by a Bayesian network model: A development and validation study. PLoS Med. 2020, 17, e1003111. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Leung, S.; Lum, A.; Morin, C.; Ennour-Idrissi, K.; Sebastianelli, A.; Renaud, M.-C.; Gregoire, J.; et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol. Oncol. 2022, 165, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Anton, C.; e Silva, A.S.; Baracat, E.C.; Dogan, N.U.; Köhler, C.; Carvalho, J.P.; di Favero, G.M. A novel model to estimate lymph node metastasis in endometrial cancer patients. Clinics 2017, 72, 30–35. [Google Scholar] [CrossRef]
- Bogani, G.; Gostout, B.S.; Dowdy, S.C.; Multinu, F.; Casarin, J.; Cliby, W.A.; Frigerio, L.; Kim, B.; Weaver, A.L.; Glaser, G.E.; et al. Clinical Utility of Preoperative Computed Tomography in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 1685–1693. [Google Scholar] [CrossRef]
- Steenbeek, M.P.; Bulten, J.; Snijders, M.P.; Lombaers, M.; Hendriks, J.; Brand, M.V.D.; Kraayenbrink, A.A.; Massuger, L.F.; Sweegers, S.; de Hullu, J.A.; et al. Fallopian tube abnormalities in uterine serous carcinoma. Gynecol. Oncol. 2020, 158, 339–346. [Google Scholar] [CrossRef]
- Visser, N.C.M.; Bulten, J.; Van Der Wurff, A.A.M.; Boss, E.A.; Bronkhorst, C.M.; Feijen, H.W.H.; Haartsen, J.E.; Van Herk, H.A.D.M.; De Kievit, I.M.; Klinkhamer, P.J.J.M.; et al. PIpelle Prospective ENDOmetrial carcinoma (PIPENDO) study, pre-operative recognition of high risk endometrial carcinoma: A multicentre prospective cohort study. BMC Cancer 2015, 15, 487. [Google Scholar] [CrossRef]
- Visser, N.C.; van der Wurff, A.A.; IntHout, J.; Reijnen, C.; Dabir, P.D.; Soltani, G.G.; Alcala, L.S.; Boll, D.; Bronkhorst, C.M.; Bult, P.; et al. Improving preoperative diagnosis in endometrial cancer using systematic morphological assessment and a small immunohistochemical panel. Hum. Pathol. 2021, 117, 68–78. [Google Scholar] [CrossRef]
- Faria, S.C.; Sagebiel, T.; Balachandran, A.; Devine, C.; Lal, C.; Bhosale, P.R. Imaging in endometrial carcinoma. Indian J. Radiol. Imaging 2015, 25, 137–147. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Jung, D.-C.; Park, S.-H.; Lim, M.-C.; Seo, S.-S.; Park, S.-Y.; Kang, S. Preoperative prediction model of lymph node metastasis in endometrial cancer. Int. J. Gynecol. Cancer 2010, 20, 1350–1355. [Google Scholar]
- Reijnen, C.; Visser, N.C.; Kasius, J.C.; Boll, D.; Geomini, P.M.; Ngo, H.; van Hamont, D.; Pijlman, B.M.; Vos, M.C.; Bulten, J.; et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J. Gynecol. Oncol. 2019, 30, e70. [Google Scholar] [CrossRef]
- Solmaz, U.; Mat, E.; Dereli, M.; Turan, V.; Güngördük, K.; Hasdemir, P.S.; Tosun, G.; Dogan, A.; Ozdemir, A.; Adiyeke, M.; et al. Lymphovascular space invasion and cervical stromal invasion are independent risk factors for nodal metastasis in endometrioid endometrial cancer. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Pothuri, B.; Arend, R.C.; Backes, F.J.; Gehrig, P.A.; Soliman, P.T.; Thompson, J.S.; Urban, R.R.; Burke, W.M. Endometrial cancer: A society of gynecologic oncology evidence-based review and recommendations. Gynecol. Oncol. 2021, 160, 817–826. [Google Scholar] [CrossRef]
- Sebastianelli, A.; Renaud, M.-C.; Grégoire, J.; Roy, M.; Plante, M. Preoperative CA 125 tumour marker in endometrial cancer: Correlation with advanced stage disease. J. Obstet. Gynaecol. Can. 2010, 32, 856–860. [Google Scholar] [CrossRef]
- Schmidt, M.; Segev, Y.; Sadeh, R.; Suzan, E.; Feferkorn, I.; Kaldawy, A.; Kligun, G.; Lavie, O. Cancer Antigen 125 Levels are Significantly Associated With Prognostic Parameters in Uterine Papillary Serous Carcinoma. Int. J. Gynecol. Cancer 2018, 28, 1311–1317. [Google Scholar] [CrossRef]
- Cetinkaya, N.; Selcuk, I.; Ozdal, B.; Meydanli, M.M.; Gungor, T. Diagnostic Impacts of Serum CA-125 Levels, Pap Smear Evaluation, and Endometrial Sampling in Women with Endometrial Clear Cell Carcinoma. Oncol. Res. Treat 2016, 39, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of <sup>18</sup>F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar]
- D’Ambrosio, V.; Brunelli, R.; Musacchio, L.; Del Negro, V.; Vena, F.; Boccuzzi, G.; Boccherini, C.; Di Donato, V.; Piccioni, M.G.; Panici, P.B.; et al. Adnexal masses in pregnancy: An updated review on diagnosis and treatment. Tumori 2021, 107, 12–16. [Google Scholar] [CrossRef]
- Jacobs, I.; Bast, R.C., Jr. The CA 125 tumour-associated antigen: A review of the literature. Hum. Reprod 1989, 4, 1–12. [Google Scholar] [CrossRef]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur. J. Contracept. Reprod. Health Care 2022, 27, 335–340. [Google Scholar] [CrossRef]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. A systematic review of tests for lymph node status in primary endometrial cancer. BMC Womens Health 2008, 8, 8. [Google Scholar]
- Haldorsen, I.S.; Berg, A.; Werner, H.M.; Magnussen, I.J.; Helland, H.; Salvesen, O.; Trovik, J.; Salvesen, H.B. Magnetic resonance imaging performs better than endocervical curettage for preoperative prediction of cervical stromal invasion in endometrial carcinomas. Gynecol. Oncol. 2012, 126, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Gulìa, C.; Signore, F.; Gaffi, M.; Gigli, S.; Votino, R.; Nucciotti, R.; Bertacca, L.; Zaami, S.; Baffa, A.; Santini, E.; et al. Y RNA: An Overview of Their Role as Potential Biomarkers and Molecular Targets in Human Cancers. Cancers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Piergentili, R.; Zaami, S.; Cavaliere, A.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef] [PubMed]
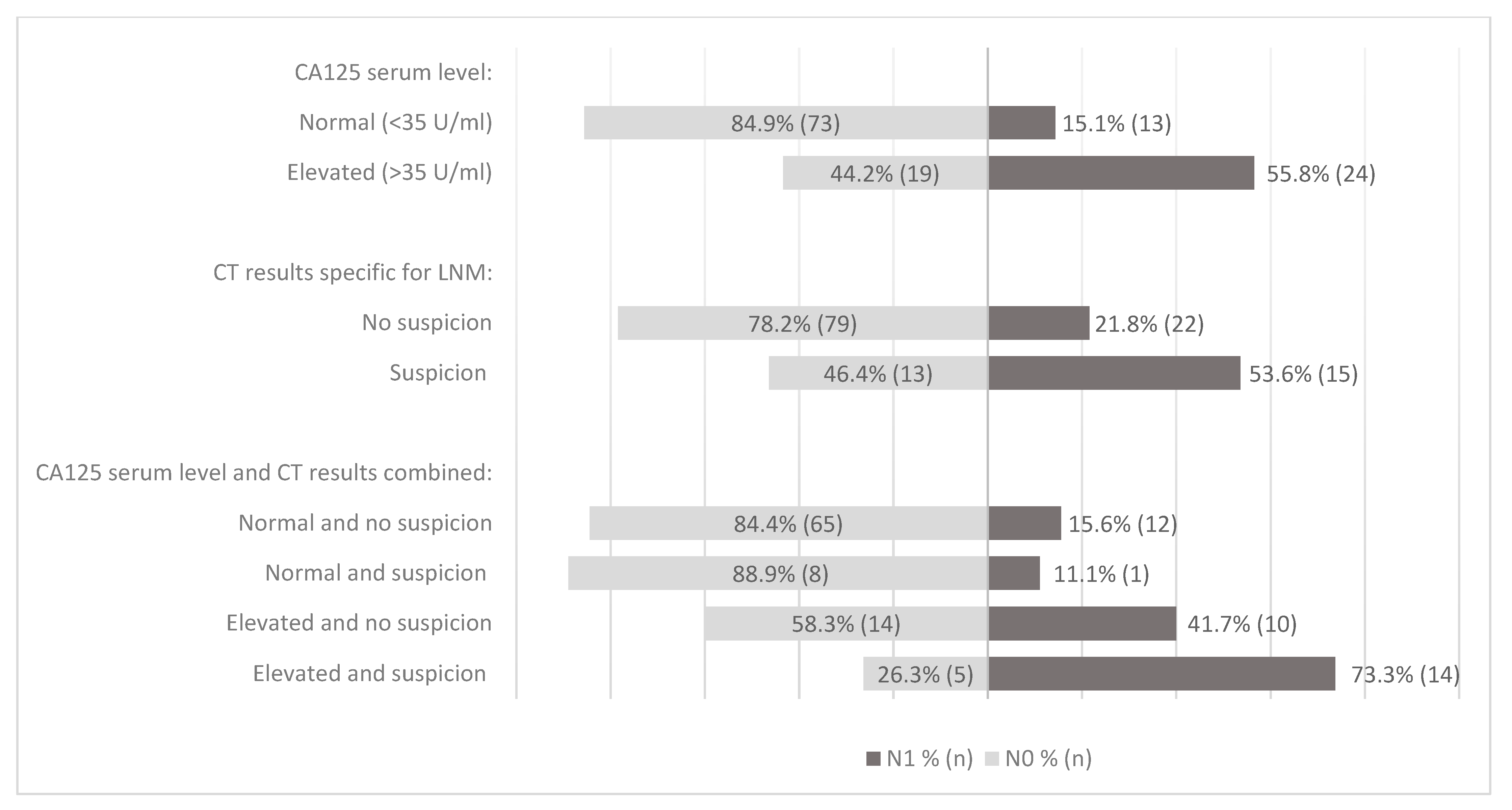
| Surgical LN Staging (n = 193) | No Surgical LN Staging (n = 140) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 193) | CA125 <35 U/mL (n = 125) | CA125 >35 U/mL (n = 68) | p Value | Total (n = 140) | CA125 <35 U/mL (n = 61) | CA125 >35 U/mL (n = 79) | p Value | ||
| Age (years) | 66 (35–83) | 66 (35–83) | 66 (44–82) | 0.477 | 72 (48–93) | 74 (48–91) | 71 (51–93) | 0.234 | |
| BMI (kg/m2) | 26.8 (17.6–56.0) | 26.8 (17.6–56.0) | 28.2 (18.0–42.6) | 0.750 | 28.2 (16.4–49.5) | 27.9 (19.5–47.8) | 29.1 (16.4–49.5) | 0.486 | |
| Imaging modality | CT | 145 (86.3) | 94 (84.7) | 51 (89.5) | 0.393 | 69 (69.7) | 30 (55.6) | 39 (86.7) | <0.001 |
| MRI | 10 (12.0) | 8 (15.4) | 2 (8.0) | 0.485 | 4 (16.0) | 3 (18.8) | 1 (11.1) | 1 | |
| CT results | No extra-uterine disease | 101 (69.7) | 77 (81.9) | 24 (47.1) | <0.001 | 43 (62.3) | 22 (73.3) | 21 (53.8) | 0.052 |
| Suspected LNM with or without suspected distant metastasis | 28 (19.3) | 9 (9.6) | 19 (37.3) | 11 (15.9) | 6 (20.0) | 5 (12.8) | |||
| Suspected distant metastasis without suspected LNM | 5 (3.4) | 2 (2.1) | 3 (5.9) | 14 (20.3) | 2 (6.7) | 12 (30.8) | |||
| Inconclusive | 11 (7.6) | 6 (6.4) | 5 (9.8) | 1 (1.4) | 0 (0.0) | 1 (2.6) | |||
| FIGO stage a | IA | 69 (35.8) | 59 (47.2) | 10 (14.7) | <0.001 | 32 (22.5) | 25 (41.0) | 7 (8.9) | <0.001 |
| IB | 40 (20.7) | 30 (24.0) | 10 (14.7) | 36 (25.7) | 22 (36.1) | 14 (17.7) | |||
| II | 17 (8.8) | 10 (8.0) | 7 (10.3) | 10 (7.1) | 5 (8.2) | 5 (6.3) | |||
| IIIA | 7 (3.6) | 5 (4.0) | 2 (2.9) | 12 (8.6) | 3 (4.9) | 9 (11.4) | |||
| IIIB | 1 (0.5) | 1 (0.8) | 0 (0.0) | 5 (3.6) | 1 (1.6) | 4 (5.1) | |||
| IIIC1-2 | 40 (20.7) | 17 (13.6) | 23 (33.8) | 3 (2.1) | 0 (0.0) | 3 (3.8) | |||
| IVA | 1 (0.5) | 1(0.8) | 0 (0.0) | 7 (5.0) | 1 (1.6) | 6 (7.6) | |||
| IVB | 18 (9.3) | 2 (1.6) | 16 (23.5) | 37 (25.0) | 4 (6.6) | 31 (39.2) | |||
| Tumor grade a | 1 | 3 (1.6) | 0 (0.0) | 3 (4.4) | 0.020 | 6 (4.3) | 5 (8.3) | 1 (1.3) | 0.040 |
| 2 | 12 (6.2) | 10 (8.0) | 2 (2.9) | 4 (2.9) | 3 (5.0) | 1 (1.3) | |||
| 3 | 178 (92.2) | 115 (92.0) | 63 (92.6) | 129 (92.8) | 52 (86.7) | 77 (97.5) | |||
| Total (n = 193) | CA125 <35 U/mL (n = 125) | CA125 >35 U/mL (n = 68) | p value | Total (n = 140) | CA125 <35 U/mL (n = 61) | CA125 >35 U/mL (n = 79) | p value | ||
| Histology a | Endometrioid | 80 (41.5) | 54 (43.2) | 26 (38.2) | 0.480 | 56 (40.0) | 38 (62.3) | 18 (22.8) | <0.001 |
| Serous | 89 (46.1) | 53 (42.4) | 36 (52.9) | 74 (52.9) | 19 (31.1) | 55 (69.6) | |||
| Clear cell | 9 (4.7) | 7 (5.6) | 2 (2.9) | 1 (0.7) | 1 (1.6) | 0 (0.0) | |||
| Other | 15 (7.8) | 11 (8.8) | 4 (5.9) | 9 (6.4) | 3 (4.9) | 6 (7.6) | |||
| MI a | <50% | 94 (48.7) | 70 (56.0) | 24 (35.3) | 0.006 | 55 (41.7) | 30 (50.0) | 25 (34.7) | 0.076 |
| =/>50% | 99 (51.3) | 55 (44.0) | 44 (64.7) | 77 (58.3) | 30 (50.0) | 47 (65.3) | |||
| CI a | No | 143 (74.1) | 102 (83.6) | 41 (62.1) | <0.001 | 94 (71.8) | 47 (78.3) | 47 (66.2) | 0.124 |
| Yes | 45 (23.3) | 20 (16.4) | 25 (37.9) | 37 (28.2) | 13 (21.7) | 24 (33.8) | |||
| LVSI a | No | 110 (57.0) | 91 (72.8) | 19 (27.9) | <0.001 | 70 (52.6) | 39 (65.0) | 31 (42.5) | 0.010 |
| Yes | 83 (43.0) | 34 (27.2) | 49 (72.1) | 63 (47.4) | 21 (35.0) | 42 (57.5) | |||
| LNM b | No (N0) | 136 (70.5) | 107 (85.6) | 29 (42.6) | <0.001 | - | - | - | - |
| Yes (N1) | 57 (29.5) | 18 (14.4) | 39 (57.4) | - | - | - | |||
| Adjuvant therapy | Radiotherapy | 82 (42.5) | 60 (48.0) | 22 (32.4) | 0.006 | 59 (42.1) | 36 (59.0) | 23 (29.1) | <0.001 |
| Chemotherapy | 60 (31.1) | 28 (22.4) | 32 (47.1) | 32 (22.9) | 4 (6.6) | 28 (35.4) | |||
| Radio- and chemo-therapy | 10 (5.2) | 7 (5.6) | 3 (4.4) | 5 (3.6) | 2 (3.3) | 3 (3.8) | |||
| No adjuvant therapy | 41 (21.2) | 30 (24.0) | 11 (16.2) | 44 (31.4) | 19 (31.1) | 25 (31.6) | |||
| Radio-therapy | VBT | 36 (39.1) | 24 (35.8) | 12 (48.0) | 0.287 | 23 (35.9) | 15 (39.5) | 8 (30.8) | 0.476 |
| EBRT ± VBT | 56 (60.9) | 43 (64.2) | 13 (52.0) | 41 (64.1) | 23 (60.5) | 18 (69.2) | |||
| Follow up (months) | 39.0 (0–193) | 47.5 (0–193) | 33.8 (0–123) | 0.003 | 21.0 (0–132) | 36.0 (0–132) | 9.0 (0–83) | <0.001 | |
| Recurrence | No | 120 (62.5) | 92 (74.2) | 28 (41.2) | <0.001 | 64 (46.0) | 41 (68.3) | 23 (29.1) | <0.001 |
| Yes | 53 (27.6) | 27 (21.8) | 26 (38.2) | 40 (28.8) | 17 (28.3) | 23 (29.1) | |||
| Progression | 19 (9.9) | 5 (4.0) | 14 (20.6) | 35 (25.2) | 2 (3.3) | 33 (41.8) | |||
| Death | No | 125 (65.4) | 94 (75.8) | 31 (46.3) | <0.001 | 68 (49.6) | 43 (70.5) | 25 (32.9) | <0.001 |
| Yes, caused by EC | 54 (28.3) | 23 (18.5) | 31 (46.3) | 56 (40.9) | 12 (19.7) | 44 (57.9) | |||
| Yes, not caused by EC | 6 (3.1) | 5 (4.0) | 1 (1.5) | 8 (5.8) | 4 (6.6) | 4 (5.3) | |||
| Yes, unknown cause | 6 (3.1) | 2 (1.6) | 4 (6.0) | 5 (3.6) | 2 (3.3) | 3 (3.9) | |||
| Total (n) | Sensitivity % [95% CI] (n) | Specificity % [95% CI] (n) | PPV % [95% CI] (n) | NPV % [95% CI] (n) | ROC AUC (95% CI) | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| LNM | CA125 a | 193 | 68.4 [54.8–80.1] (39/57) | 78.7 [70.8–85.2] (107/136) | 57.4 [44.8–69.2] (39/68) | 85.6 [78.2–91.2] (107/125) | 0.721 (0.619–0.824) | <0.001 | |
| CT b | 129 | 40.5 [24.8–7.9] (15/37) | 85.9 [77.1–92.3] (79/92) | 53.6 [33.9–72.5] (15/28) | 78.2 [68.9–85.8] (79/101) | 0.623 (0.520–0.744) | <0.001 | ||
| CA125 <35 U/mL | CT b | 86 | 7.7 [0.0–36.0] (1/13) | 89.0 [79.5–95.2] (65/73) | 11.1 [0.0–48.3] (1/9) | 84.4 [74.4–91.7] (65/77) | 0.484 (0.316–0.652) | 1.000 | |
| CA125 >35 U/mL | CT b | 43 | 58.3 [36.6–77.9] (14/24) | 73.7 [48.8–90.9] (14/19) | 73.7 [48.8–90.9] (14/19) | 58.3 [36.6–77.9] (14/24) | 0.660 (0.495–0.826) | 0.036 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| p Value | Adjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | |
| Age > 65 years * | 0.964 | 0.99 | 0.53–1.83 | - | - | - |
| BMI >30 kg/m2 * | 0.790 | 0.91 | 0.46–1.81 | - | - | - |
| NEEC histology | <0.001 | 3.75 | 1.82–7.70 | 0.028 | 3.72 | 1.16–11.95 |
| CA125 > 35 U/mL | <0.001 | 7.99 | 4.00–15.99 | 0.003 | 6.25 | 1.85–21.09 |
| CT with suspected LNM | 0.002 | 4.14 | 1.72–9.99 | 0.427 | 1.680 | 0.48–6.05 |
| Deep myometrial involvement | <0.001 | 3.45 | 1.76 6.74 | 0.007 | 4.88 | 1.54–15.46 |
| Cervical stromal involvement | 0.009 | 4.21 | 1.42–12.50 | 0.018 | 7.87 | 1.42–43.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombaers, M.S.; Cornel, K.M.C.; Visser, N.C.M.; Bulten, J.; Küsters-Vandevelde, H.V.N.; Amant, F.; Boll, D.; Bronsert, P.; Colas, E.; Geomini, P.M.A.J.; et al. Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer. Cancers 2023, 15, 2605. https://doi.org/10.3390/cancers15092605
Lombaers MS, Cornel KMC, Visser NCM, Bulten J, Küsters-Vandevelde HVN, Amant F, Boll D, Bronsert P, Colas E, Geomini PMAJ, et al. Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer. Cancers. 2023; 15(9):2605. https://doi.org/10.3390/cancers15092605
Chicago/Turabian StyleLombaers, Marike S., Karlijn M. C. Cornel, Nicole C. M. Visser, Johan Bulten, Heidi V. N. Küsters-Vandevelde, Frédéric Amant, Dorry Boll, Peter Bronsert, Eva Colas, Peggy M. A. J. Geomini, and et al. 2023. "Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer" Cancers 15, no. 9: 2605. https://doi.org/10.3390/cancers15092605
APA StyleLombaers, M. S., Cornel, K. M. C., Visser, N. C. M., Bulten, J., Küsters-Vandevelde, H. V. N., Amant, F., Boll, D., Bronsert, P., Colas, E., Geomini, P. M. A. J., Gil-Moreno, A., van Hamont, D., Huvila, J., Krakstad, C., Kraayenbrink, A. A., Koskas, M., Mancebo, G., Matías-Guiu, X., Ngo, H., ... Pijnenborg, J. M. A. (2023). Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer. Cancers, 15(9), 2605. https://doi.org/10.3390/cancers15092605








