Biomolecules Involved in Both Metastasis and Placenta Accreta Spectrum—Does the Common Pathophysiological Pathway Exist?
Abstract
:Simple Summary
Abstract
1. Introduction
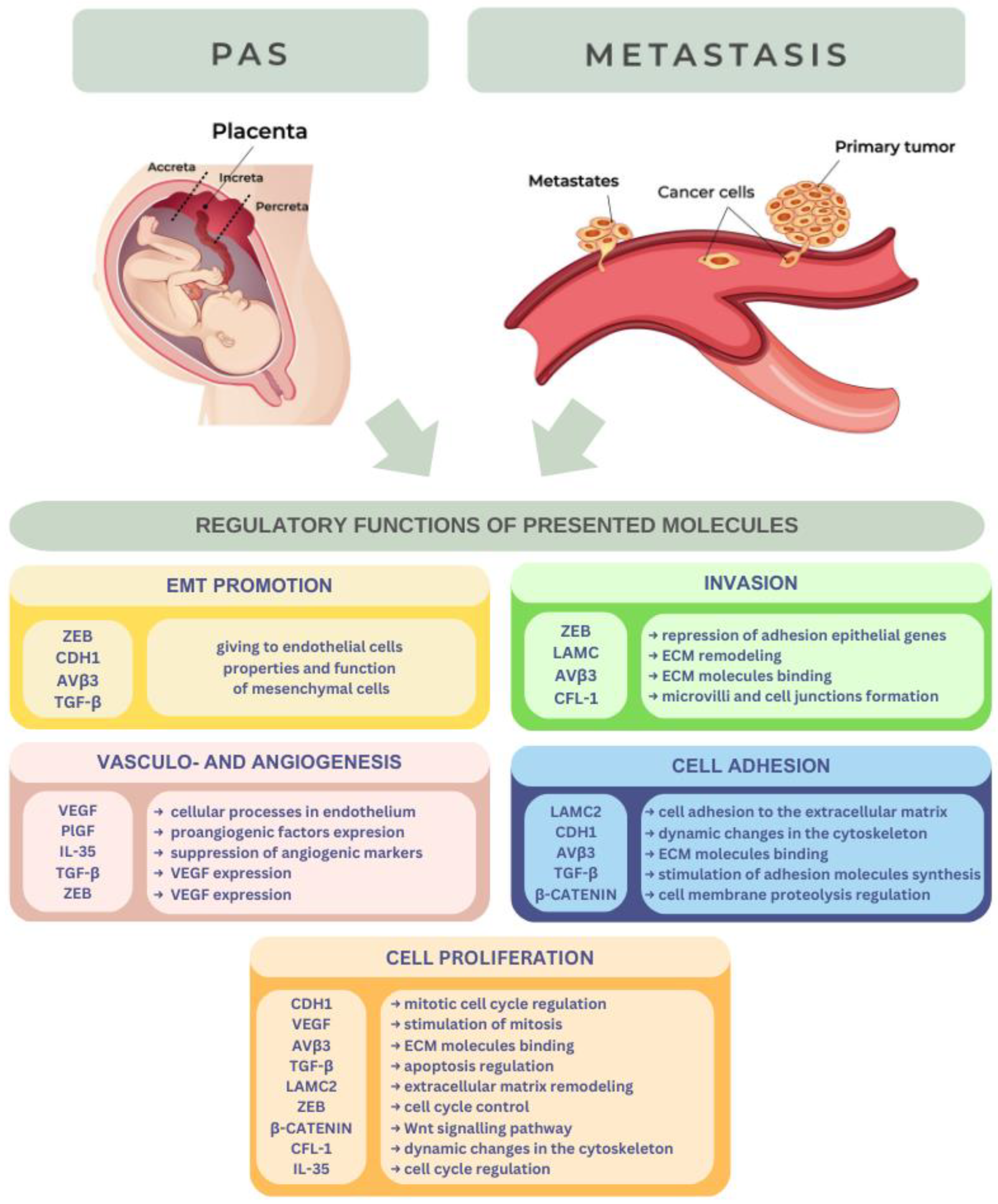
2. Biomolecules
2.1. PIGF
2.2. VEGF
- VEGF-A (also called VEGF) is the best-known and most important factor, mainly involved in vasculogenesis and angiogenesis. The main targets of VEGF-A are endothelial cells, in which it stimulates migration and mitosis, inhibits apoptosis, and dilates vessels with NO. There are many specific isoforms of VEGF-A, with various features, formed during alternative splicing. VEGF-A is secreted by kidney mesangial cells, macrophages, cells of the retina, osteoblasts, keratinocytes, platelets, and many others. VEGF-A is bound to extracellular matrix elements, and proteolytic enzymes (metalloproteinases and plasmin) can release free, diffusible forms of it, which are active in the extracellular environment.
- VEGF-B is primarily involved in embryonic tissues in the development of the cardiovascular system. In adult individuals, it takes part in myocardial remodeling.
- VEGF-C initiates the development of lymphatic tissue during lymphangiogenesis but is not a strong angiogenic factor.
- VEGF-D, similar to VEGF-C, controls lymphangiogenesis, but in the lungs.
- VEGF-E (viral) is not found in humans.
- VEGFR-1 (Flt-1) is responsible for binding VEGF-A, VEGF-B, and PlGF. VEGFR-1 has also been produced in the ECM as a soluble isoform (sVEGFR-1/sFlt-1) and, similarly to the transmembrane type, can bind the same factors. Surprisingly, sFlt-1 can cooperate with VEGFR-2 to decrease its activity. Consequently, sVEGFR-1 employs anti-angiogenic, anti-edema, and anti-inflammatory activities, and its dysregulation has been connected with other pathological processes. The pathogenesis of preeclampsia, which usually occurs in the last trimester of pregnancy and is linked to sVEGFR-1 production because of the placenta, and subsequent neutralization of VEGF-A and PIGF signaling. A poor quantity of sVEGFR-1 to VEGF-A has been tied to excessive tumor malignancy/invasiveness and inferior patient survival. Additionally, sVEGFR-1 may play a proangiogenic and protumoral role as well, through the activation of β1 integrin, which stimulates endothelial cell adhesion and chemotaxis [31].
- VEGFR-2 (KDR/Flk-1) binds VEGF-A and, on special occasions, VEGF-E, C, and D. Its main function is to initiate vasculogenesis and can be found not only on epithelial cells but also on hemangioblasts.
- VEGFR-3 (Flt-4) is a receptor for VEGF-C and VEGF-D and is a mediator in the lymphangiogenesis process.
2.3. CDH1
2.4. LAMC2
2.5. ZEB Proteins
2.6. αVβ3 Integrin
2.7. TGF-β
2.8. β-Catenin
2.9. CFL-1
2.10. IL-35
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef] [PubMed]
- Krstic, J.; Deutsch, A.; Fuchs, J.; Gauster, M.; Gorsek Sparovec, T.; Hiden, U.; Krappinger, J.C.; Moser, G.; Pansy, K.; Szmyra, M.; et al. (Dis)Similarities between the Decidual and Tumor Microenvironment. Biomedicines 2022, 10, 1065. [Google Scholar] [CrossRef]
- Gheldof, A.; Hulpiau, P.; van Roy, F.; De Craene, B.; Berx, G. Evolutionary Functional Analysis and Molecular Regulation of the ZEB Transcription Factors. Cell. Mol. Life Sci. 2012, 69, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M.; Barbour, K.D. Placenta Accreta Spectrum: Accreta, Increta, and Percreta. Obstet. Gynecol. Clin. N. Am. 2015, 42, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of Implantation: Strategies for Successful Pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Weitzner, O.; Seraya-Bareket, C.; Biron-Shental, T.; Fishamn, A.; Yagur, Y.; Tzadikevitch-Geffen, K.; Farladansky-Gershnabel, S.; Kidron, D.; Ellis, M.; Ashur-Fabian, O. Enhanced Expression of AVβ3 Integrin in Villus and Extravillous Trophoblasts of Placenta Accreta. Arch. Gynecol. Obstet. 2021, 303, 1175–1183. [Google Scholar] [CrossRef]
- Matsukawa, S.; Sumigama, S.; Kotani, T.; Wang, J.; Miki, R.; Moriyama, Y.; Nakano, T.; Mano, Y.; Tsuda, H.; Tamakoshi, K.; et al. Possible Association between Cathepsin V and the Development of Placenta Accreta Spectrum Disorders. Gynecol. Obstet. Investig. 2019, 84, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta Accreta Spectrum: Pathophysiology and Evidence-Based Anatomy for Prenatal Ultrasound Imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Gireesan, K.V.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Kshitiz Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Bo, A.B.; Mihu, C.; Istrate, M.; Moldovan, I.-M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (VEGF)—Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Dewerchin, M.; Carmeliet, P. Placental Growth Factor in Cancer. Expert Opin. Ther. Targets 2014, 18, 1339–1354. [Google Scholar] [CrossRef]
- Dewerchin, M.; Carmeliet, P. PlGF: A Multitasking Cytokine with Disease-Restricted Activity. Cold Spring Harb. Perspect. Med. 2012, 2, a011056. [Google Scholar] [CrossRef]
- Tseng, J.J.; Chou, M.M.; Hsieh, Y.T.; Wen, M.C.; Ho, E.S.C.; Hsu, S.L. Differential Expression of Vascular Endothelial Growth Factor, Placenta Growth Factor and Their Receptors in Placentae from Pregnancies Complicated by Placenta Accreta. Placenta 2006, 27, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E.; Murray, A.J. Oxygen and Placental Development; Parallels and Differences with Tumour Biology. Placenta 2017, 56, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Kalra, V.K. Placenta Growth Factor-Induced Early Growth Response 1 (Egr-1) Regulates Hypoxia-Inducible Factor-1α (HIF-1α) in Endothelial Cells*. J. Biol. Chem. 2010, 285, 20570–20579. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Atzori, M.G.; Ceci, C.; Ruffini, F.; Scimeca, M.; Cicconi, R.; Mattei, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor 1 (VEGFR-1) D16F7 Monoclonal Antibody Inhibits Melanoma Adhesion to Soluble VEGFR-1 and Tissue Invasion in Response to Placenta Growth Factor. Cancers 2022, 14, 5578. [Google Scholar] [CrossRef] [PubMed]
- Pagani, E.; Ruffini, F.; Antonini Cappellini, G.C.; Scoppola, A.; Fortes, C.; Marchetti, P.; Graziani, G.; D’Atri, S.; Lacal, P.M. Placenta Growth Factor and Neuropilin-1 Collaborate in Promoting Melanoma Aggressiveness. Int. J. Oncol. 2016, 48, 1581–1589. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.; Zhang, Y.; Zhao, X.Y.; Huang, B.; Wu, P.F.; Li, Q.; Li, H.; Liu, Y.S.; Cao, L.Y.; et al. Elevated PLGF Contributes to Small-Cell Lung Cancer Brain Metastasis. Oncogene 2013, 32, 2952–2962. [Google Scholar] [CrossRef]
- He, J.; Shen, N.; Huang, X. Thyroid Carcinoma Cells Produce PLGF to Enhance Metastasis. Tumor Biol. 2015, 36, 8601–8607. [Google Scholar] [CrossRef]
- Meng, Q.; Duan, P.; Li, L.; Miao, Y. Expression of Placenta Growth Factor Is Associated with Unfavorable Prognosis of Advanced-Stage Serous Ovarian Cancer. Tohoku J. Exp. Med. 2018, 244, 291–296. [Google Scholar] [CrossRef]
- Bu, J.; Bu, X.; Liu, B.; Chen, F.; Chen, P. Inhibition of Metastasis of Oral Squamous Cell Carcinoma by Anti-PLGF Treatment. Tumor Biol. 2015, 36, 2695–2701. [Google Scholar] [CrossRef]
- Faraji, A.; Akbarzadeh-Jahromi, M.; Bahrami, S.; Gharamani, S.; Raeisi Shahraki, H.; Kasraeian, M.; Vafaei, H.; Zare, M.; Asadi, N. Predictive Value of Vascular Endothelial Growth Factor and Placenta Growth Factor for Placenta Accreta Spectrum. J. Obstet. Gynaecol. 2022, 42, 900–905. [Google Scholar] [CrossRef]
- Umapathy, A.; Chamley, L.W.; James, J.L. Reconciling the Distinct Roles of Angiogenic/Anti-Angiogenic Factors in the Placenta and Maternal Circulation of Normal and Pathological Pregnancies. Angiogenesis 2020, 23, 105–117. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, M.; Chen, P.; Wan, S.; Zhou, Q.; Lu, Y.; Li, L. Distinguishing Placenta Accreta from Placenta Previa via Maternal Plasma Levels of SFlt-1 and PLGF and the SFlt-1/PLGF Ratio. Placenta 2022, 124, 48–54. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and Angiogenesis. Cancer Treat. Res. 2004, 117, 3–32. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, X.; Zhou, M.; Deng, Y.; Li, Y.; Peng, C. SFlt-1: A Double Regulator in Angiogenesis-Related Diseases. Curr. Pharm. Des. 2021, 27, 4160–4170. [Google Scholar] [CrossRef]
- Macklin, P.S.; McAuliffe, J.; Pugh, C.W.; Yamamoto, A. Hypoxia and HIF Pathway in Cancer and the Placenta. Placenta 2017, 56, 8–13. [Google Scholar] [CrossRef]
- Devery, A.M.; Wadekar, R.; Bokobza, S.M.; Weber, A.M.; Jiang, Y.; Ryan, A.J. Vascular Endothelial Growth Factor Directly Stimulates Tumour Cell Proliferation in Non-Small Cell Lung Cancer. Int. J. Oncol. 2015, 47, 849–856. [Google Scholar] [CrossRef]
- Adamcic, U.; Skowronski, K.; Peters, C.; Morrison, J.; Coomber, B.L. The Effect of Bevacizumab on Human Malignant Melanoma Cells with Functional VEGF/VEGFR2 Autocrine and Intracrine Signaling Loops. Neoplasia 2012, 14, 612-IN16. [Google Scholar] [CrossRef]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular Endothelial Growth Factor Induces Proliferation of Breast Cancer Cells and Inhibits the Anti-Proliferative Activity of Anti-Hormones. Endocr. Relat. Cancer 2006, 13, 905–919. [Google Scholar] [CrossRef]
- Gasparini, G. Prognostic Value of Vascular Endothelial Growth Factor in Breast Cancer. Oncologist 2000, 5, 37–44. [Google Scholar] [CrossRef]
- Guibourdenche, J.; Fournier, T.; MalassinĂŠ, A.; Evain-Brion, D. Development and Hormonal Functions of the Human Placenta. Folia Histochem. Cytobiol. 2009, 47, 35–40. [Google Scholar] [CrossRef]
- Wang, N.; Shi, D.; Li, N.; Qi, H. Clinical Value of Serum VEGF and SFlt-1 in Pernicious Placenta Previa. Ann. Med. 2021, 53, 2041–2049. [Google Scholar] [CrossRef]
- Manna, C.; Lacconi, V.; Rizzo, G.; De Lorenzo, A.; Massimiani, M. Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. Int. J. Mol. Sci. 2022, 23, 659. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia—Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Shibuya, M. Involvement of Flt-1 (VEGF Receptor-1) in Cancer and Preeclampsia. Proc. Jpn. Acad. Ser. B 2011, 87, 167–178. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F.; Berx, G. The Cell-Cell Adhesion Molecule E-Cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Beavon, I.R.G. The E-Cadherin–Catenin Complex in Tumour Metastasis: Structure, Function and Regulation. Eur. J. Cancer 2000, 36, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Coopman, P.; Djiane, A. Adherens Junction and E-Cadherin Complex Regulation by Epithelial Polarity. Cell. Mol. Life Sci. 2016, 73, 3535–3553. [Google Scholar] [CrossRef]
- Na, T.-Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The Functional Activity of E-Cadherin Controls Tumor Cell Metastasis at Multiple Steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Duzyj, C.M.; Buhimschi, I.A.; Motawea, H.; Laky, C.A.; Cozzini, G.; Zhao, G.; Funai, E.F.; Buhimschi, C.S. The Invasive Phenotype of Placenta Accreta Extravillous Trophoblasts Associates with Loss of E-Cadherin. Placenta 2015, 36, 645–651. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Sipos, F.; Galamb, O. Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transitions in the Colon. World J. Gastroenterol. 2012, 18, 601–608. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Fedorov, I.S.; Pirogova, M.M.; Vasilchenko, O.N.; Chagovets, V.V.; Ezhova, L.S.; Zabelina, T.M.; Shmakov, R.G.; Sukhikh, G.T. Clusterin and Its Potential Regulatory MicroRNAs as a Part of Secretome for the Diagnosis of Abnormally Invasive Placenta: Accreta, Increta, and Percreta Cases. Life 2021, 11, 270. [Google Scholar] [CrossRef]
- Trillsch, F.; Kuerti, S.; Eulenburg, C.; Burandt, E.; Woelber, L.; Prieske, K.; Eylmann, K.; Oliveira-Ferrer, L.; Milde-Langosch, K.; Mahner, S. E-Cadherin Fragments as Potential Mediators for Peritoneal Metastasis in Advanced Epithelial Ovarian Cancer. Br. J. Cancer 2016, 114, 213–220. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; e Silva, F.F.-V.; Pérez-Jardón, A.; Chamorro-Petronacci, C.M.; Oliveira-Alves, M.G.; Álvarez-Calderón-Iglesias, Ó.; Caponio, V.C.A.; Pinti, M.; Perrotti, V.; Pérez-Sayáns, M. Overexpression of E-Cadherin Is a Favorable Prognostic Biomarker in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Biology 2023, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, A.; Peeters, J.K.; Smid, M.; Timmermans, M.; Verhoog, L.C.; Westenend, P.J.; Heine, A.A.J.; Chan, A.; Sieuwerts, A.M.; Wiemer, E.A.C.; et al. Loss of E-Cadherin Is Not a Necessity for Epithelial to Mesenchymal Transition in Human Breast Cancer. Breast Cancer Res. Treat. 2013, 138, 47–57. [Google Scholar] [CrossRef]
- Pęksa, M.; Kamieniecki, A.; Gabrych, A.; Lew-Tusk, A.; Preis, K.; Świątkowska-Freund, M. Loss of E-Cadherin Staining Continuity in the Trophoblastic Basal Membrane Correlates with Increased Resistance in Uterine Arteries and Proteinuria in Patients with Pregnancy-Induced Hypertension. J. Clin. Med. 2022, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Wang, F.; Oliver, M.T.; Tipton, T.; Gao, Y.; Jiang, S.-W. Syncytin-1 Modulates Placental Trophoblast Cell Proliferation by Promoting G1/S Transition. Cell. Signal. 2013, 25, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Jie, Q.; Sun, F.; Li, Q.; Zhu, J.; Wei, Y.; Yang, H.; Long, P.; Wang, Z.; Yang, X.; Li, D.; et al. Downregulated Ribosomal Protein L39 Inhibits Trophoblast Cell Migration and Invasion by Targeting E-Cadherin in the Placenta of Patients with Preeclampsia. FASEB J. 2021, 35, e21322. [Google Scholar] [CrossRef]
- Ortega, M.A.; Chaowen, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cruza, I.; Pereda-Cerquella, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Fatych, Y.; et al. Chronic Venous Disease in Pregnant Women Causes an Increase in ILK in the Placental Villi Associated with a Decrease in E-Cadherin. J. Pers. Med. 2022, 12, 277. [Google Scholar] [CrossRef]
- Incebiyik, A.; Kocarslan, S.; Camuzcuoglu, A.; Hilali, N.G.; Incebiyik, H.; Camuzcuoglu, H. Trophoblastic E-Cadherin and TGF-Beta Expression in Placenta Percreta and Normal Pregnancies. J. Matern. Fetal Neonatal Med. 2016, 29, 126–129. [Google Scholar] [CrossRef]
- El-Hussieny, M.; Mohammed, E.M.; Zenhom, N.M.; Refaie, M.M.; Okasha, A.M.; Tawab, M.A.E. Possible Role of TGF-Β1, MMP-2, E-CAD, β-Catenin and Antioxidants in Pathogenesis of Placenta Accreta. Fetal Pediatr. Pathol. 2021, 40, 222–232. [Google Scholar] [CrossRef]
- Fu, T.; Liu, J.-X.; Xie, J.; Gao, Z.; Yang, Z. LAMC2 as a Prognostic Biomarker in Human Cancer: A Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e063682. [Google Scholar] [CrossRef]
- Salo, S.; Haakana, H.; Kontusaari, S.; Hujanen, E.; Kallunki, T.; Tryggvason, K. Laminin-5 Promotes Adhesion and Migration of Epithelial Cells: Identification of a Migration-Related Element in the γ2 Chain Gene ž LAMC2/with Activity in Transgenic Mice. Matrix Biol. 1999, 18, 197–210. [Google Scholar] [CrossRef]
- Pei, Y.-F.; Liu, J.; Cheng, J.; Wu, W.-D.; Liu, X.-Q. Silencing of LAMC2 Reverses Epithelial-Mesenchymal Transition and Inhibits Angiogenesis in Cholangiocarcinoma via Inactivation of the Epidermal Growth Factor Receptor Signaling Pathway. Am. J. Pathol. 2019, 189, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Kanojia, D.; Okamoto, R.; Jain, S.; Madan, V.; Chien, W.; Sampath, A.; Ding, L.-W.; Xuan, M.; Said, J.W.; et al. Laminin-5γ-2 (LAMC2) Is Highly Expressed in Anaplastic Thyroid Carcinoma and Is Associated with Tumor Progression, Migration, and Invasion by Modulating Signaling of EGFR. J. Clin. Endocrinol. Metab. 2014, 99, E62–E72. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, J.; Du, S.; Wei, W.; Shen, X. LAMC2 Modulates the Acidity of Microenvironments to Promote Invasion and Migration of Pancreatic Cancer Cells via Regulating AKT-Dependent NHE1 Activity. Exp. Cell Res. 2020, 391, 111984. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nishiwada, S.; Yamamura, K.; Sho, M.; Baba, H.; Takayama, T.; Goel, A. Identification of LAMC2 as a Prognostic and Predictive Biomarker for Determining Response to Gemcitabine-Based Therapy in Pancreatic Ductal Adenocarcinoma. Eur. J. Cancer 2021, 146, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Pandey, A.K.; Ramachandran, B.; Mishra, D.P.; Dawson, D.W.; Sethi, G.; Ganesan, T.S.; Koeffler, H.P.; Garg, M. Overexpression of Laminin-5 Gamma-2 Promotes Tumorigenesis of Pancreatic Ductal Adenocarcinoma through EGFR/ERK1/2/AKT/MTOR Cascade. Cell. Mol. Life Sci. 2022, 79, 362. [Google Scholar] [CrossRef]
- Cave, D.D.; Buonaiuto, S.; Sainz, B.; Fantuz, M.; Mangini, M.; Carrer, A.; Di Domenico, A.; Iavazzo, T.T.; Andolfi, G.; Cortina, C.; et al. LAMC2 Marks a Tumor-Initiating Cell Population with an Aggressive Signature in Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 315. [Google Scholar] [CrossRef]
- Zhou, Q.-H.; Deng, C.-Z.; Chen, J.-P.; Huang, K.-B.; Liu, T.-Y.; Yao, K.; Liu, Z.-W.; Qin, Z.-K.; Li, Y.-H.; Guo, S.-J.; et al. Elevated Serum LAMC2 Is Associated with Lymph Node Metastasis and Predicts Poor Prognosis in Penile Squamous Cell Carcinoma. Cancer Manag. Res. 2018, 10, 2983–2995. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Zhao, J.; Liu, L.; Li, S.; Duan, Y.; Huo, Y. Overexpressed LAMC2 Promotes Trophoblast Over-Invasion through the PI3K/Akt/MMP2/9 Pathway in Placenta Accreta Spectrum. J. Obstet. Gynaecol. Res. 2023, 49, 548–559. [Google Scholar] [CrossRef]
- Rao, G.; Pierobon, M.; Kim, I.-K.; Hsu, W.-H.; Deng, J.; Moon, Y.-W.; Petricoin, E.F.; Zhang, Y.-W.; Wang, Y.; Giaccone, G. Inhibition of AKT1 Signaling Promotes Invasion and Metastasis of Non-Small Cell Lung Cancer Cells with K-RAS or EGFR Mutations. Sci. Rep. 2017, 7, 7066. [Google Scholar] [CrossRef]
- Kent, L.N.; Ohboshi, S.; Soares, M.J. Akt1 and Insulin-like Growth Factor 2 (Igf2) Regulate Placentation and Fetal/Postnatal Development. Int. J. Dev. Biol. 2012, 56, 255–261. [Google Scholar] [CrossRef]
- Fardi, M.; Alivand, M.; Baradaran, B.; Farshdousti Hagh, M.; Solali, S. The Crucial Role of ZEB2: From Development to Epithelial-to-mesenchymal Transition and Cancer Complexity. J. Cell. Physiol. 2019, 234, 14783–14799. [Google Scholar] [CrossRef] [PubMed]
- Yalim-Camci, I.; Balcik-Ercin, P.; Cetin, M.; Odabas, G.; Tokay, N.; Sayan, A.E.; Yagci, T. ETS1 Is Coexpressed with ZEB2 and Mediates ZEB2-induced Epithelial-mesenchymal Transition in Human Tumors. Mol. Carcinog. 2019, 58, 1068–1081. [Google Scholar] [CrossRef]
- Soyama, H.; Miyamoto, M.; Ishibashi, H.; Iwahashi, H.; Matsuura, H.; Kakimoto, S.; Suzuki, R.; Sakamoto, T.; Hada, T.; Takano, M. Placenta Previa May Acquire Invasive Nature by Factors Associated with Epithelial-mesenchymal Transition and Matrix Metalloproteinases. J. Obstet. Gynaecol. Res. 2020, 46, 2526–2533. [Google Scholar] [CrossRef]
- DaSilva-Arnold, S.; James, J.L.; Al-Khan, A.; Zamudio, S.; Illsley, N.P. Differentiation of First Trimester Cytotrophoblast to Extravillous Trophoblast Involves an Epithelial–Mesenchymal Transition. Placenta 2015, 36, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- DaSilva-Arnold, S.C.; Kuo, C.-Y.; Davra, V.; Remache, Y.; Kim, P.C.W.; Fisher, J.P.; Zamudio, S.; Al-Khan, A.; Birge, R.B.; Illsley, N.P. ZEB2, a Master Regulator of the Epithelial–Mesenchymal Transition, Mediates Trophoblast Differentiation. MHR Basic Sci. Reprod. Med. 2019, 25, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ang, H.L.; Moghadam, E.R.; Mohammadi, S.; Zarrin, V.; Hushmandi, K.; Samarghandian, S.; Zarrabi, A.; Najafi, M.; Mohammadinejad, R.; et al. MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules 2020, 10, 1040. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, S.; Mao, L.; Zhang, H.; Sun, D.; Zhang, J.; Li, J.; Tang, J. Crosstalk between TGF-β Signaling and MiRNAs in Breast Cancer Metastasis. Tumor Biol. 2016, 37, 10011–10019. [Google Scholar] [CrossRef]
- Takamaru, N.; Fukuda, N.; Akita, K.; Kudoh, K.; Miyamoto, Y. Association of PD-L1 and ZEB-1 Expression Patterns with Clinicopathological Characteristics and Prognosis in Oral Squamous Cell Carcinoma. Oncol. Lett. 2022, 23, 75. [Google Scholar] [CrossRef]
- Perez-Oquendo, M.; Gibbons, D.L. Regulation of ZEB1 Function and Molecular Associations in Tumor Progression and Metastasis. Cancers 2022, 14, 1864. [Google Scholar] [CrossRef]
- Li, M.-Z.; Wang, J.-J.; Yang, S.-B.; Li, W.-F.; Xiao, L.-B.; He, Y.-L.; Song, X.-M. ZEB2 Promotes Tumor Metastasis and Correlates with Poor Prognosis of Human Colorectal Cancer. Am. J. Transl. Res. 2017, 9, 2838–2851. [Google Scholar]
- Park, M.K.; Lee, H.; Lee, C.H. Post-Translational Modification of ZEB Family Members in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 15127. [Google Scholar] [CrossRef] [PubMed]
- Illsley, N.P.; DaSilva-Arnold, S.C.; Zamudio, S.; Alvarez, M.; Al-Khan, A. Trophoblast Invasion: Lessons from Abnormally Invasive Placenta (Placenta Accreta). Placenta 2020, 102, 61–66. [Google Scholar] [CrossRef]
- Gong, H.; Lu, F.; Zeng, X.; Bai, Q. E2F Transcription Factor 1 (E2F1) Enhances the Proliferation, Invasion and EMT of Trophoblast Cells by Binding to Zinc Finger E-Box Binding Homeobox 1 (ZEB1). Bioengineered 2022, 13, 2360–2370. [Google Scholar] [CrossRef]
- Li, N.; Yang, T.; Yu, W.; Liu, H.; Qiao, C.; Liu, C. The Role of Zeb1 in the Pathogenesis of Morbidly Adherent Placenta. Mol. Med. Rep. 2019, 20, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Zheng, Y.; Zhu, Y.; Zhou, Z.; Liu, Z.; Lin, C.; Wan, Y.; Wen, Y.; Liu, C.; et al. Autocrine Pro-Legumain Promotes Breast Cancer Metastasis via Binding to Integrin Avβ3. Oncogene 2022, 41, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin Avβ3-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chang, W.-J.; Chu, H.-Y.; De Luca, R.; Pedersen, J.Z.; Incerpi, S.; Li, Z.-L.; Shih, Y.-J.; Lin, H.-Y.; Wang, K.; et al. Nano-Strategies Targeting the Integrin Avβ3 Network for Cancer Therapy. Cells 2021, 10, 1684. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Lai, Y.-Y.; Hsu, H.-C.; Fong, Y.-C.; Lien, M.-Y.; Tang, C.-H. CCL4 Stimulates Cell Migration in Human Osteosarcoma via the Mir-3927-3p/Integrin Avβ3 Axis. Int. J. Mol. Sci. 2021, 22, 12737. [Google Scholar] [CrossRef]
- Kariya, Y.; Oyama, M.; Suzuki, T.; Kariya, Y. Avβ3 Integrin Induces Partial EMT Independent of TGF-β Signaling. Commun. Biol. 2021, 4, 490. [Google Scholar] [CrossRef]
- Wang, L.; Song, L.; Li, J.; Wang, Y.; Yang, C.; Kou, X.; Xiao, B.; Zhang, W.; Li, L.; Liu, S.; et al. Bone Sialoprotein-αvβ3 Integrin Axis Promotes Breast Cancer Metastasis to the Bone. Cancer Sci. 2019, 110, 3157–3172. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Yang, D.; Xu, R.; Radisky, E.S.; Bissell, M.J.; Bishop, J.M. MYC Suppresses Cancer Metastasis by Direct Transcriptional Silencing of Av and Β3 Integrin Subunits. Nat. Cell Biol. 2012, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J. Pathobiology of Transforming Growth Factor b in Cancer, Fibrosis and Immunologic Disease, and Therapeutic Considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef]
- Chuva de Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules 2020, 10, 453. [Google Scholar] [CrossRef]
- Wang, L.; Tong, X.; Zhou, Z.; Wang, S.; Lei, Z.; Zhang, T.; Liu, Z.; Zeng, Y.; Li, C.; Zhao, J.; et al. Circular RNA Hsa_circ_0008305 (CircPTK2) Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition and Metastasis by Controlling TIF1γ in Non-Small Cell Lung Cancer. Mol. Cancer 2018, 17, 140. [Google Scholar] [CrossRef]
- Huynh, L.K.; Hipolito, C.J.; ten Dijke, P. A Perspective on the Development of TGF-β Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming Growth Factor-β: A Therapeutic Target for Cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H. TGF-β Signaling in Cancer: Control by Negative Regulators and Crosstalk with Proinflammatory and Fibrogenic Pathways. Cancers 2019, 11, 384. [Google Scholar] [CrossRef]
- Dong, C.; Wu, K.; Gu, S.; Wang, W.; Xie, S.; Zhou, Y. PTBP3 Mediates TGF-β-Induced EMT and Metastasis of Lung Adenocarcinoma. Cell Cycle 2022, 21, 1406–1421. [Google Scholar] [CrossRef]
- Huang, M.; Fu, M.; Wang, J.; Xia, C.; Zhang, H.; Xiong, Y.; He, J.; Liu, J.; Liu, B.; Pan, S.; et al. TGF-Β1-Activated Cancer-Associated Fibroblasts Promote Breast Cancer Invasion, Metastasis and Epithelial-Mesenchymal Transition by Autophagy or Overexpression of FAP-α. Biochem. Pharmacol. 2021, 188, 114527. [Google Scholar] [CrossRef]
- Fosslien, E. Review: Molecular Pathology of Cyclooxygenase-2 in Cancer-Induced Angiogenesis. Ann. Clin. Lab. Sci. 2001, 31, 325–348. [Google Scholar]
- Zhang, L.; Qu, J.; Qi, Y.; Duan, Y.; Huang, Y.-W.; Zhou, Z.; Li, P.; Yao, J.; Huang, B.; Zhang, S.; et al. EZH2 Engages TGFβ Signaling to Promote Breast Cancer Bone Metastasis via Integrin Β1-FAK Activation. Nat. Commun. 2022, 13, 2543. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, P.; Zhou, Y.; Landström, M. The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis. Biomolecules 2022, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Savarese, D.M.F.; Quesenberry, P.J.; Savarese, T.M. Expression of Multiple Angiogenic Cytokines in Cultured Normal Human Prostate Epithelial Cells: Predominance of Vascular Endothelial Growth Factor. Int. J. Cancer 1999, 80, 868–874. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Franzen, C.A.; Pelling, J.C. Apigenin Inhibits TGF-β-Induced VEGF Expression in Human Prostate Carcinoma Cells via a Smad2/3- and Src-Dependent Mechanism. Mol. Carcinog. 2014, 53, 598–609. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Schaer, D.A.; Li, Y.; Castaneda, S.P.; Murphy, M.Y.; Xu, X.; Inigo, I.; Dobkin, J.; Manro, J.R.; Iversen, P.W.; et al. Targeting the TGFβ Pathway with Galunisertib, a TGFβRI Small Molecule Inhibitor, Promotes Anti-Tumor Immunity Leading to Durable, Complete Responses, as Monotherapy and in Combination with Checkpoint Blockade. J. Immunother. Cancer 2018, 6, 47. [Google Scholar] [CrossRef]
- Jank, B.J.; Lenz, T.; Haas, M.; Kadletz-Wanke, L.; Campion, N.J.; Schnoell, J.; Heiduschka, G.; Macfelda, K. Radiosensitizing Effect of Galunisertib, a TGF-ß Receptor I Inhibitor, on Head and Neck Squamous Cell Carcinoma in Vitro. Investig. New Drugs 2022, 40, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Khamoushi, T.; Ahmadi, M.; Ali-Hassanzadeh, M.; Zare, M.; Hesampour, F.; Gharesi-Fard, B.; Amooee, S. Evaluation of Transforming Growth Factor-Β1 and Interleukin-35 Serum Levels in Patients with Placenta Accreta. Lab. Med. 2021, 52, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-C.; Chang, H.-M.; Leung, P.C.K. Transforming Growth Factor-Β1 Inhibits Trophoblast Cell Invasion by Inducing Snail-Mediated Down-Regulation of Vascular Endothelial-Cadherin Protein*. J. Biol. Chem. 2013, 288, 33181–33192. [Google Scholar] [CrossRef] [PubMed]
- Murrieta-Coxca, J.M.; Barth, E.; Fuentes-Zacarias, P.; Gutiérrez-Samudio, R.N.; Groten, T.; Gellhaus, A.; Köninger, A.; Marz, M.; Markert, U.R.; Morales-Prieto, D.M. Identification of Altered MiRNAs and Their Targets in Placenta Accreta. Front. Endocrinol. 2023, 14, 1021640. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Miyahara, Y.; Tanimura, K.; Morita, H.; Kawakami, F.; Itoh, T.; Yamada, H. Expression of Epithelial-Mesenchymal Transition-Related Factors in Adherent Placenta. Int. J. Gynecol. Pathol. 2015, 34, 584–589. [Google Scholar] [CrossRef]
- Shirakawa, T.; Miyahara, Y.; Ebina, Y.; Yamada, H. Expression of Epithelial-Mesenchymal Transition-Related Factors in Placenta Accreta. Placenta 2014, 35, A15. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The Many Faces and Functions of β-Catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, L.; Liu, Z.; Luo, J.; Chen, R.; Yan, J. Expression of β-Catenin in Human Trophoblast and Its Role in Placenta Accreta and Placenta Previa. J. Int. Med. Res. 2019, 47, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.C.; Robbins, D.J.; Ahmed, Y.; Lee, E. Nuclear Regulation of Wnt/β-Catenin Signaling: It’s a Complex Situation. Genes 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Paganelli, F.; Martelli, A.M.; Evangelisti, C. The Role Played by Wnt/β-Catenin Signaling Pathway in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1098. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; Graveel, C.R.; Zylstra-Diegel, C.R.; Zhong, Z.; Williams, B.O. Wnt/β-Catenin Signaling in Normal and Cancer Stem Cells. Cancers 2011, 3, 2050–2079. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, X.; Li, Y.; Zhi, J.; Hu, L.; Hou, X.; Shi, X.; Wang, X.; Wang, J.; Ma, W.; et al. KDM1A Promotes Thyroid Cancer Progression and Maintains Stemness through the Wnt/β-Catenin Signaling Pathway. Theranostics 2022, 12, 1500–1517. [Google Scholar] [CrossRef]
- Nalli, M.; Masci, D.; Urbani, A.; La Regina, G.; Silvestri, R. Emerging Direct Targeting β-Catenin Agents. Molecules 2022, 27, 7735. [Google Scholar] [CrossRef]
- Kotolloshi, R.; Gajda, M.; Grimm, M.-O.; Steinbach, D. Wnt/β-Catenin Signalling and Its Cofactor BCL9L Have an Oncogenic Effect in Bladder Cancer Cells. Int. J. Mol. Sci. 2022, 23, 5319. [Google Scholar] [CrossRef]
- Chehrazi-Raffle, A.; Dorff, T.B.; Pal, S.K.; Lyou, Y. Wnt/β-Catenin Signaling and Immunotherapy Resistance: Lessons for the Treatment of Urothelial Carcinoma. Cancers 2021, 13, 889. [Google Scholar] [CrossRef]
- Ren, J.; Yang, Y.; Peng, T.; Xu, D. Predictive Value of β-Catenin in Bladder Cancer: A Systematic Review and Meta-Analysis. Biosci. Rep. 2020, 40, BSR20202127. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Maurya, N. WNT/β-Catenin Signaling in Urothelial Carcinoma of Bladder. World J. Nephrol. 2019, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.C.; Sathe, A.; Guerth, F.; Seitz, A.-K.; Heck, M.M.; Maurer, T.; Schwarzenböck, S.M.; Krause, B.J.; Schulz, W.A.; Stoehr, R.; et al. Wntless Promotes Bladder Cancer Growth and Acts Synergistically as a Molecular Target in Combination with Cisplatin. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 544.e1–544.e10. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, Z.; Moraczewska, J. Cofilin—A Protein Controlling Dynamics of Actin Filaments. Postepy Hig. Med. Doswiadczalnej Online 2017, 71, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Jalali, B.M.; Likszo, P.; Andronowska, A.; Skarzynski, D.J. Alterations in the Distribution of Actin and Its Binding Proteins in the Porcine Endometrium during Early Pregnancy: Possible Role in Epithelial Remodeling and Embryo Adhesion. Theriogenology 2018, 116, 17–27. [Google Scholar] [CrossRef]
- Kanellos, G.; Zhou, J.; Patel, H.; Ridgway, R.A.; Huels, D.; Gurniak, C.B.; Sandilands, E.; Carragher, N.O.; Sansom, O.J.; Witke, W.; et al. ADF and Cofilin1 Control Actin Stress Fibers, Nuclear Integrity, and Cell Survival. Cell Rep. 2015, 13, 1949–1964. [Google Scholar] [CrossRef]
- Ali, M.; Rogers, L.K.; Heyob, K.M.; Buhimschi, C.S.; Buhimschi, I.A. Changes in Vasodilator-Stimulated Phosphoprotein Phosphorylation, Profilin-1, and Cofilin-1 in Accreta and Protection by DHA. Reprod. Sci. 2019, 26, 757–765. [Google Scholar] [CrossRef]
- Sparrow, N.; Manetti, M.E.; Bott, M.; Fabianac, T.; Petrilli, A.; Bates, M.L.; Bunge, M.B.; Lambert, S.; Fernandez-Valle, C. The Actin-Severing Protein Cofilin Is Downstream of Neuregulin Signaling and Is Essential for Schwann Cell Myelination. J. Neurosci. 2012, 32, 5284–5297. [Google Scholar] [CrossRef]
- Dumpich, M.; Mannherz, H.G.; Theiss, C. VEGF Signaling Regulates Cofilin and the Arp2/3-Complex within the Axonal Growth Cone. Curr. Neurovasc. Res. 2015, 12, 293–307. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- van Rheenen, J.; Condeelis, J.; Glogauer, M. A Common Cofilin Activity Cycle in Invasive Tumor Cells and Inflammatory Cells. J. Cell Sci. 2009, 122, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, S.; Eremina, L.; Pashintseva, N.; Kovalev, L.; Kovaleva, M. Cofilin-1 and Other ADF/Cofilin Superfamily Members in Human Malignant Cells. Int. J. Mol. Sci. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, J.; Yan, M.; Zhang, Y.; Zhao, Y.; Yue, C.; Xu, J.; Zheng, F.; Chen, J.; Kang, Z.; et al. The Mitotic Kinase Aurora-A Induces Mammary Cell Migration and Breast Cancer Metastasis by Activating the Cofilin-F-Actin Pathway. Cancer Res. 2010, 70, 9118–9128. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, Y.; Jie, T.; Jing, Z.; Changwen, W.; Pan, Y.; Chen, C.; Tao, H. Aurora Kinase a Induces Papillary Thyroid Cancer Lymph Node Metastasis by Promoting Cofilin-1 Activity. Biochem. Biophys. Res. Commun. 2016, 473, 212–218. [Google Scholar] [CrossRef]
- Howard, J.; Goh, C.Y.; Gorzel, K.W.; Higgins, M.; McCann, A. The Potential Role of Cofilin-1 in Promoting Triple Negative Breast Cancer (TNBC) Metastasis via the Extracellular Vesicles (EVs). Transl. Oncol. 2022, 15, 101247. [Google Scholar] [CrossRef]
- Wang, W.; Eddy, R.; Condeelis, J. The Cofilin Pathway in Breast Cancer Invasion and Metastasis. Nat. Rev. Cancer 2007, 7, 429–440. [Google Scholar] [CrossRef]
- Bravo-Cordero, J.J.; Magalhaes, M.A.O.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of Cofilin in Cell Locomotion and Invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.; Li, Z.; Chang, A.; Chen, Y.; Zhao, T.; Li, Y.; Wang, X.; Zhang, W.; Wang, Z.; et al. Tumour-Derived Interleukin 35 Promotes Pancreatic Ductal Adenocarcinoma Cell Extravasation and Metastasis by Inducing ICAM1 Expression. Nat. Commun. 2017, 8, 14035. [Google Scholar] [CrossRef]
- Yazdani, Z.; Rafiei, A.; Golpour, M.; Zafari, P.; Moonesi, M.; Ghaffari, S. IL-35, a Double-Edged Sword in Cancer. J. Cell. Biochem. 2020, 121, 2064–2076. [Google Scholar] [CrossRef]
- Liu, K.; Huang, A.; Nie, J.; Tan, J.; Xing, S.; Qu, Y.; Jiang, K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021, 12, 683332. [Google Scholar] [CrossRef]
- Lombardelli, L.; Logiodice, F.; Kullolli, O.; Haller, H.; Agostinis, C.; Bulla, R.; Rukavina, D.; Piccinni, M.-P. At Embryo Implantation Site IL-35 Secreted by Trophoblast, Polarizing T Cells towards IL-35+ IL-10+ IL-4+ Th2-Type Cells, Could Favour Fetal Allograft Tolerance and Pregnancy Success. Int. J. Mol. Sci. 2022, 23, 4926. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Zhang, B.; Ying, C. Elevated Serum Level of IL-35 Associated with the Maintenance of Maternal-Fetal Immune Tolerance in Normal Pregnancy. PLoS ONE 2015, 10, e0128219. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dong, H.; Li, W.; Lv, X.; Shi, B.; Gao, P. The Molecular Role of IL-35 in Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Liao, K.-L.; Friedman, A.; Liao, K.-L. The Role of the Cytokines IL-27 and IL-35 in Cancer. Math. Biosci. Eng. 2015, 12, 1203–1217. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lin, J.-C.; Hwang, W.-L.; Kuo, Y.-J.; Chen, H.-K.; Tai, S.-K.; Lin, C.-C.; Yang, M.-H. Macrophage-Secreted Interleukin-35 Regulates Cancer Cell Plasticity to Facilitate Metastatic Colonization. Nat. Commun. 2018, 9, 3763. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Li, D.; Zhang, H.; Guo, Z.; Yang, X. Interleukin-35 Promotes Progression of Prostate Cancer and Inhibits Anti-Tumour Immunity. Cancer Cell Int. 2020, 20, 487. [Google Scholar] [CrossRef]
- Xue, W.; Yan, D.; Kan, Q. Interleukin-35 as an Emerging Player in Tumor Microenvironment. J. Cancer 2019, 10, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Das, S.; Handler, J.S.; Hajdu, C.H.; Coffre, M.; Koralov, S.B.; Bar-Sagi, D. IL-35 Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016, 6, 247–255. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.-H. Mechanisms of TGFβ-Induced Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular Mechanisms of Blood Vessel Growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Mansouri, S.; Heylmann, D.; Stiewe, T.; Kracht, M.; Savai, R. Cancer Genome and Tumor Microenvironment: Reciprocal Crosstalk Shapes Lung Cancer Plasticity. eLife 2022, 11, e79895. [Google Scholar] [CrossRef] [PubMed]
| Biomolecule | Metastasis | PAS | References | ||
|---|---|---|---|---|---|
| PlGF | ↑ | increased level | ↑ | increased level | [14,19,20,21,22,23,24,25,26,27,28] |
| VEGF | ↑ | overexpression | ↓ | decreased level | [16,19,33,34,38] |
| CDH1 | ↓ | decreased level | ↓ | decreased level | [43,45,46,50,51,57,58] |
| sFLT-1 | ↓ | decreased level | ↑ | increased level | [16,19,27,28,31,38] |
| LAMC2 | ↑ | overexpression | ↑ | overexpression | [59,63,68] |
| ZEB1 | ↑ | ZEB1 promotes metastasis in various cancers | ↑ | overexpression | [77,78,79,83,84] |
| ZEB2 | ↑ | overexpression | ↑ | ZEB 2 promotes EMT thus cytotrophoblast differentiation into invasive EVT | [72,75,82] |
| αVβ3 integrin | ↑ | overexpression | ↑ | overexpression | [6,85,87,88,89,90,91] |
| TGF-β | ≠ | TGF-β enhances metastasis in later-stage cancer, represses in early-stage cancer | ≠ | Increased/decreased | [58,77,96,97,98,99,102,103,104,107,109] |
| β-Catenin | ↑ | overexpression | ↓ | decreased expression | [46,58,62,113,116,121] |
| CFL-1 | ↑ | overexpression | ↑ | increased levels | [127,131,135,136] |
| IL-35 | ↑ | overexpression | ↑ | increased levels | [107,138,139,140,146,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekowska, A.K.; Obuchowska, K.; Bartosik, M.; Kimber-Trojnar, Ż.; Słodzińska, M.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Biomolecules Involved in Both Metastasis and Placenta Accreta Spectrum—Does the Common Pathophysiological Pathway Exist? Cancers 2023, 15, 2618. https://doi.org/10.3390/cancers15092618
Rekowska AK, Obuchowska K, Bartosik M, Kimber-Trojnar Ż, Słodzińska M, Wierzchowska-Opoka M, Leszczyńska-Gorzelak B. Biomolecules Involved in Both Metastasis and Placenta Accreta Spectrum—Does the Common Pathophysiological Pathway Exist? Cancers. 2023; 15(9):2618. https://doi.org/10.3390/cancers15092618
Chicago/Turabian StyleRekowska, Anna K., Karolina Obuchowska, Magdalena Bartosik, Żaneta Kimber-Trojnar, Magdalena Słodzińska, Magdalena Wierzchowska-Opoka, and Bożena Leszczyńska-Gorzelak. 2023. "Biomolecules Involved in Both Metastasis and Placenta Accreta Spectrum—Does the Common Pathophysiological Pathway Exist?" Cancers 15, no. 9: 2618. https://doi.org/10.3390/cancers15092618
APA StyleRekowska, A. K., Obuchowska, K., Bartosik, M., Kimber-Trojnar, Ż., Słodzińska, M., Wierzchowska-Opoka, M., & Leszczyńska-Gorzelak, B. (2023). Biomolecules Involved in Both Metastasis and Placenta Accreta Spectrum—Does the Common Pathophysiological Pathway Exist? Cancers, 15(9), 2618. https://doi.org/10.3390/cancers15092618







