Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Assessment of the Retrieved Articles
2.3. Statistical Analysis
3. Results
3.1. Overview
3.2. Meta-Analysis
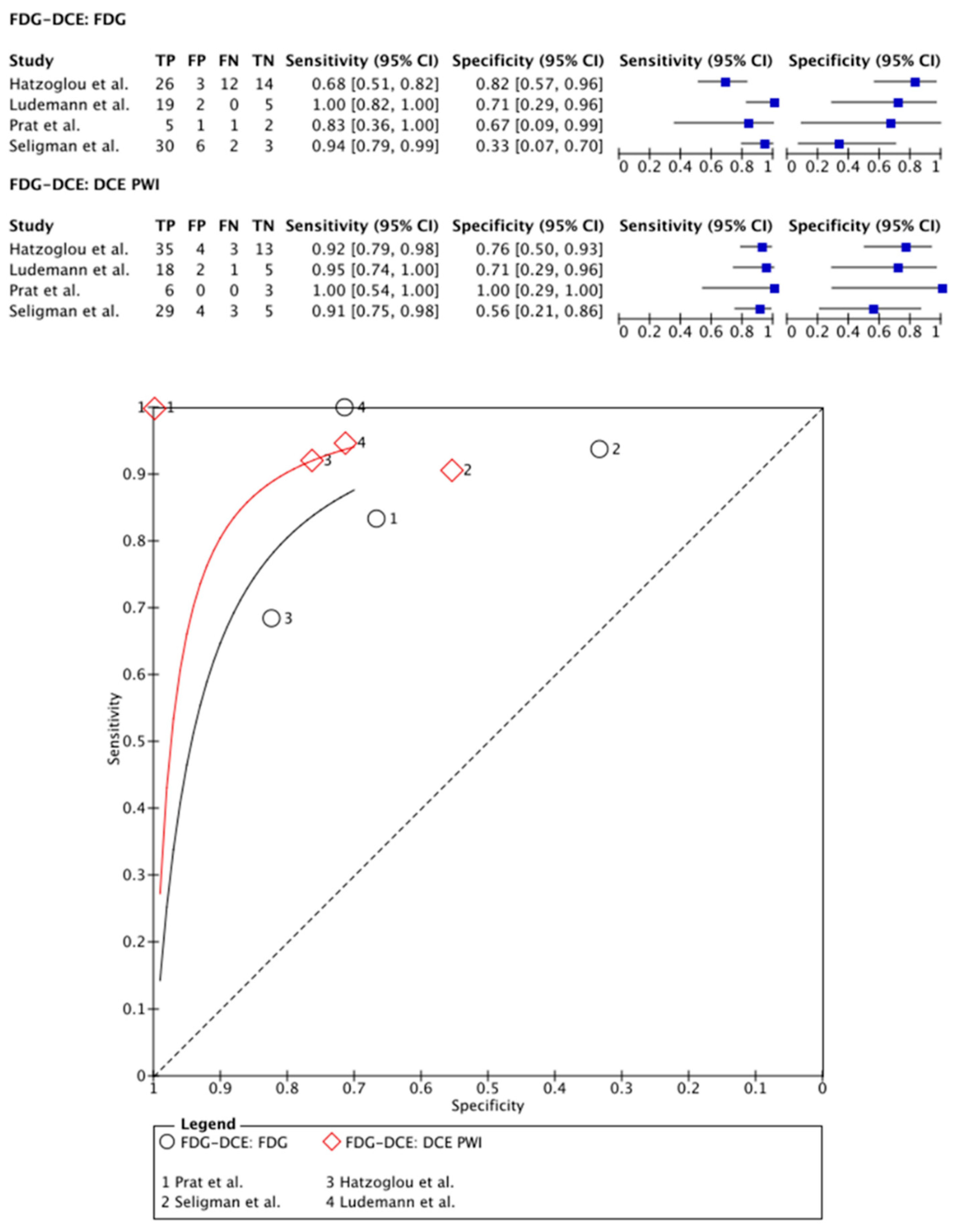
3.2.1. [18F]FDG PET Imaging vs. DCE PWI
3.2.2. [18F]FDG PET Imaging vs. DSC PWI
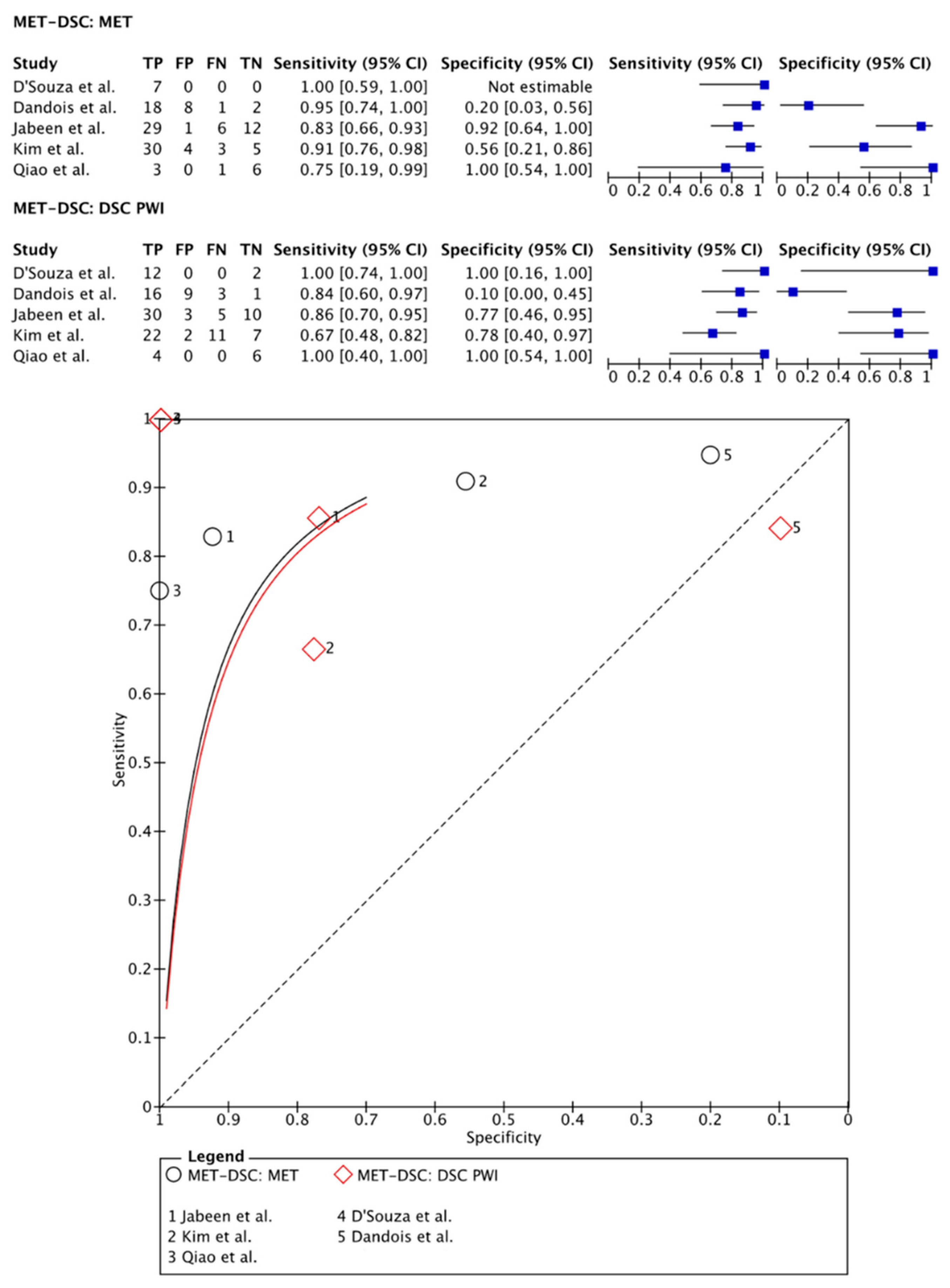
3.2.3. [11C]MET PET Imaging vs. DSC PWI
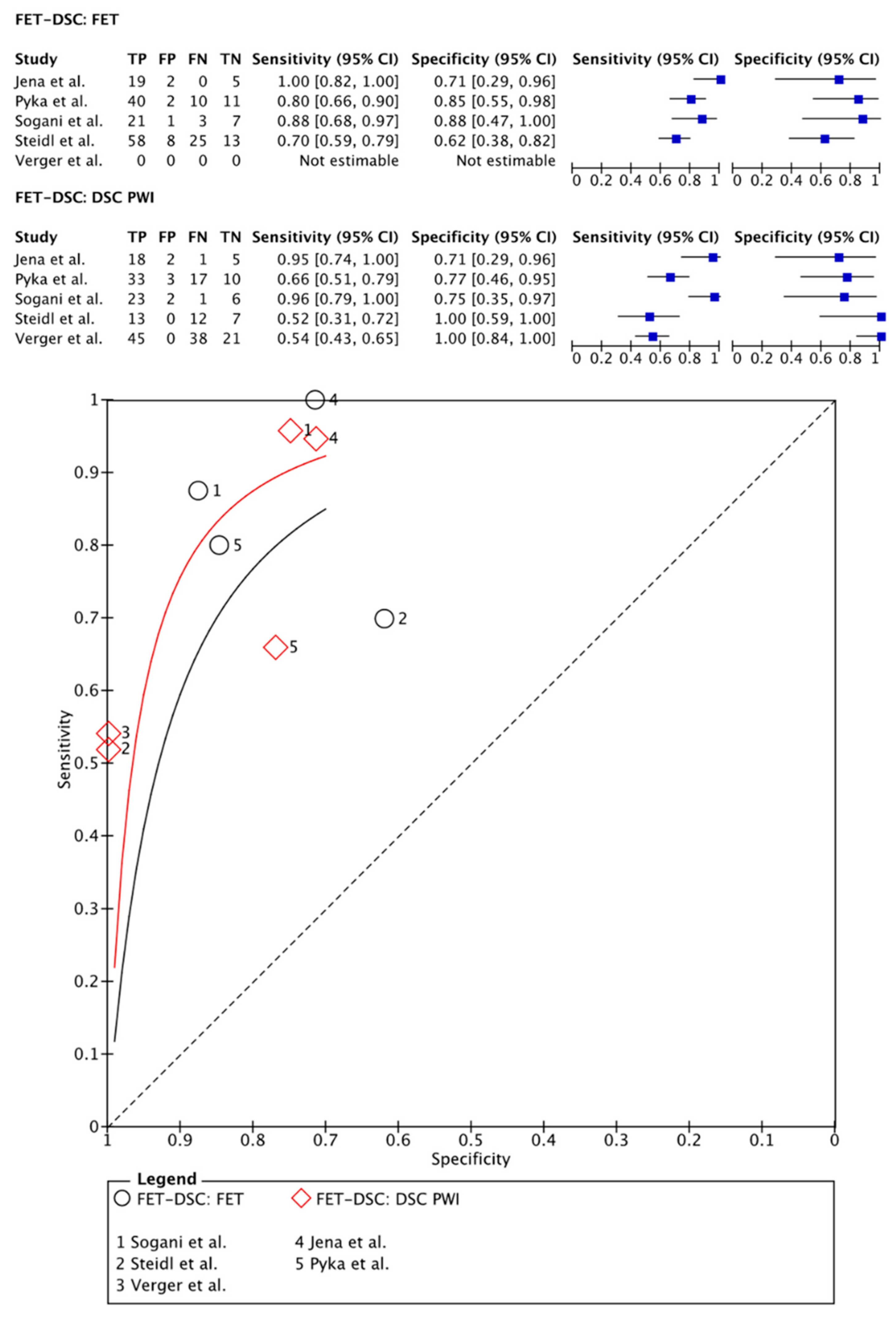
3.2.4. [18F]FET PET Imaging vs. DSC PWI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougere, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Smits, M. MRI biomarkers in neuro-oncology. Nat. Rev. Neurol. 2021, 17, 486–500. [Google Scholar] [CrossRef]
- Santo, G.; Laudicella, R.; Linguanti, F.; Nappi, A.G.; Abenavoli, E.; Vergura, V.; Rubini, G.; Sciagrà, R.; Arnone, G.; Schillaci, O.; et al. The Utility of Conventional Amino Acid PET Radiotracers in the Evaluation of Glioma Recurrence also in Comparison with MRI. Diagnostics 2022, 12, 844. [Google Scholar] [CrossRef]
- Essig, M.; Shiroishi, M.S.; Nguyen, T.B.; Saake, M.; Provenzale, J.M.; Enterline, D.; Anzalone, N.; Dorfler, A.; Rovira, A.; Wintermark, M.; et al. Perfusion MRI: The five most frequently asked technical questions. AJR Am. J. Roentgenol. 2013, 200, 24–34. [Google Scholar] [CrossRef]
- Wang, L.; Wei, L.; Wang, J.; Li, N.; Gao, Y.; Ma, H.; Qu, X.; Zhang, M. Evaluation of perfusion MRI value for tumor progression assessment after glioma radiotherapy: A systematic review and meta-analysis. Medicine 2020, 99, e23766. [Google Scholar] [CrossRef]
- Seeger, A.; Braun, C.; Skardelly, M.; Paulsen, F.; Schittenhelm, J.; Ernemann, U.; Bisdas, S. Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad. Radiol. 2013, 20, 1557–1565. [Google Scholar] [CrossRef]
- Zakhari, N.; Taccone, M.S.; Torres, C.H.; Chakraborty, S.; Sinclair, J.; Woulfe, J.; Jansen, G.H.; Cron, G.O.; Thornhill, R.E.; McInnes, M.D.F.; et al. Prospective comparative diagnostic accuracy evaluation of dynamic contrast-enhanced (DCE) vs. dynamic susceptibility contrast (DSC) MR perfusion in differentiating tumor recurrence from radiation necrosis in treated high-grade gliomas. J. Magn. Reson. Imaging 2019, 50, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.E.; Ahn, K.J.; Choi, H.S.; Jung, S.L.; Kim, B.S.; Jeon, S.S.; Hong, Y.G. DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin. Radiol. 2014, 69, e264–e272. [Google Scholar] [CrossRef]
- Langen, K.J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- de Zwart, P.L.; van Dijken, B.R.J.; Holtman, G.A.; Stormezand, G.N.; Dierckx, R.; Jan van Laar, P.; van der Hoorn, A. Diagnostic Accuracy of PET Tracers for the Differentiation of Tumor Progression from Treatment-Related Changes in High-Grade Glioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2020, 61, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Liu, X.; Tang, G. Carbon-11 and Fluorine-18 Labeled Amino Acid Tracers for Positron Emission Tomography Imaging of Tumors. Front. Chem. 2017, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.; Shayakul, C.; Trotti, D.; Sacchi, V.F.; Harvey, W.R.; Hediger, M.A. Molecular characteristics of mammalian and insect amino acid transporters: Implications for amino acid homeostasis. J. Exp. Biol. 1997, 200, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Ora, M.; Mohindra, N.; Menda, Y.; Bathla, G. Diagnostic Performance of PET and Perfusion-Weighted Imaging in Differentiating Tumor Recurrence or Progression from Radiation Necrosis in Posttreatment Gliomas: A Review of Literature. AJNR Am. J. Neuroradiol. 2020, 41, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, H.; Healy, M.J. The graphical presentation of a collection of means. J. R. Stat. Soc. Ser. A Stat. Soc. 1995, 158, 175–177. [Google Scholar] [CrossRef]
- Cumming, G.; Finch, S. Inference by eye: Confidence intervals and how to read pictures of data. Am. Psychol. 2005, 60, 170. [Google Scholar] [CrossRef]
- Dandois, V.; Rommel, D.; Renard, L.; Jamart, J.; Cosnard, G. Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: Preliminary results in clinical practice. J. Neuroradiol. 2010, 37, 89–97. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Sharma, R.; Jaimini, A.; Panwar, P.; Saw, S.; Kaur, P.; Mondal, A.; Mishra, A.; Tripathi, R.P. 11C-MET PET/CT and advanced MRI in the evaluation of tumor recurrence in high-grade gliomas. Clin. Nucl. Med. 2014, 39, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhao, X.; Wang, K.; Zhang, Y.; Fan, D.; Yu, T.; Shen, H.; Chen, Q.; Ai, L. Utility of Dynamic Susceptibility Contrast Perfusion-Weighted MR Imaging and (11)C-Methionine PET/CT for Differentiation of Tumor Recurrence from Radiation Injury in Patients with High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2019, 40, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Oh, S.W.; Lim, Y.J.; Park, C.K.; Lee, S.H.; Kang, K.W.; Jung, H.W.; Chang, K.H. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin. Neurol. Neurosurg. 2010, 112, 758–765. [Google Scholar] [CrossRef]
- Hojjati, M.; Badve, C.; Garg, V.; Tatsuoka, C.; Rogers, L.; Sloan, A.; Faulhaber, P.; Ros, P.R.; Wolansky, L.J. Role of FDG-PET/MRI, FDG-PET/CT, and Dynamic Susceptibility Contrast Perfusion MRI in Differentiating Radiation Necrosis from Tumor Recurrence in Glioblastomas. J. Neuroimaging 2018, 28, 118–125. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Jha, A.; Damesha, N.K.; Negi, P.; Jadhav, G.K.; Verma, S.M.; Sogani, S.K. Multiparametric Evaluation in Differentiating Glioma Recurrence from Treatment-Induced Necrosis Using Simultaneous (18)F-FDG-PET/MRI: A Single-Institution Retrospective Study. AJNR Am. J. Neuroradiol. 2017, 38, 899–907. [Google Scholar] [CrossRef]
- Ozsunar, Y.; Mullins, M.E.; Kwong, K.; Hochberg, F.H.; Ament, C.; Schaefer, P.W.; Gonzalez, R.G.; Lev, M.H. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad. Radiol. 2010, 17, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016, 18, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lundemann, M.; Munck Af Rosenschold, P.; Muhic, A.; Larsen, V.A.; Poulsen, H.S.; Engelholm, S.A.; Andersen, F.L.; Kjaer, A.; Larsson, H.B.W.; Law, I.; et al. Feasibility of multi-parametric PET and MRI for prediction of tumour recurrence in patients with glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 603–613. [Google Scholar] [CrossRef]
- Prat, R.; Galeano, I.; Lucas, A.; Martinez, J.C.; Martin, M.; Amador, R.; Reynes, G. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J. Clin. Neurosci. 2010, 17, 50–53. [Google Scholar] [CrossRef]
- Seligman, L.; Kovanlikaya, I.; Pisapia, D.J.; Naeger, D.M.; Magge, R.; Fine, H.A.; Chiang, G.C. Integrated PET-MRI for Glioma Surveillance: Perfusion-Metabolism Discordance Rate and Association with Molecular Profiling. AJR Am. J. Roentgenol. 2019, 212, 883–891. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Gambhir, A.; Mishra, A.K.; D’Souza, M.M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jhadav, G.K.; Sogani, S.K. Glioma Recurrence Versus Radiation Necrosis: Single-Session Multiparametric Approach Using Simultaneous O-(2-18F-Fluoroethyl)-L-Tyrosine PET/MRI. Clin. Nucl. Med. 2016, 41, e228–e236. [Google Scholar] [CrossRef] [PubMed]
- Pyka, T.; Hiob, D.; Preibisch, C.; Gempt, J.; Wiestler, B.; Schlegel, J.; Straube, C.; Zimmer, C. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 2018, 103, 32–37. [Google Scholar] [CrossRef]
- Sogani, S.K.; Jena, A.; Taneja, S.; Gambhir, A.; Mishra, A.K.; D’Souza, M.M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jadhav, G.K. Potential for differentiation of glioma recurrence from radionecrosis using integrated (18)F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: A prospective evaluation. Neurol. India 2017, 65, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Kops, E.R.; Galldiks, N.; Fink, G.R.; Shah, N.J.; et al. Comparison of O-(2-(18)F-Fluoroethyl)-L-Tyrosine Positron Emission Tomography and Perfusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Patients with Progressive and Recurrent Glioma: A Hybrid Positron Emission Tomography/Magnetic Resonance Study. World Neurosurg. 2018, 113, e727–e737. [Google Scholar] [CrossRef] [PubMed]
- Steidl, E.; Langen, K.J.; Hmeidan, S.A.; Polomac, N.; Filss, C.P.; Galldiks, N.; Lohmann, P.; Keil, F.; Filipski, K.; Mottaghy, F.M.; et al. Sequential implementation of DSC-MR perfusion and dynamic [(18)F]FET PET allows efficient differentiation of glioma progression from treatment-related changes. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1956–1965. [Google Scholar] [CrossRef]
- Fraioli, F.; Shankar, A.; Hyare, H.; Ferrazzoli, V.; Militano, V.; Samandouras, G.; Mankad, K.; Solda, F.; Zaccagna, F.; Mehdi, E.; et al. The use of multiparametric 18F-fluoro-L-3,4-dihydroxy-phenylalanine PET/MRI in post-therapy assessment of patients with gliomas. Nucl. Med. Commun. 2020, 41, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, A.; Khalifé, M.; Sanson, M.; Rozenblum-Beddok, L.; Bertaux, M.; Soret, M.; Galanaud, D.; Dormont, D.; Kas, A.; Pyatigorskaya, N. Simultaneously acquired PET and ASL imaging biomarkers may be helpful in differentiating progression from pseudo-progression in treated gliomas. Eur. Radiol. 2021, 31, 7395–7405. [Google Scholar] [CrossRef]
- Jabeen, S.; Arbind, A.; Kumar, D.; Singh, P.K.; Saini, J.; Sadashiva, N.; Krishna, U.; Arimappamagan, A.; Santosh, V.; Nagaraj, C. Combined amino acid PET-MRI for identifying recurrence in post-treatment gliomas: Together we grow. Eur. J. Hybrid Imaging 2021, 5, 15. [Google Scholar] [CrossRef]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro Oncol. 2017, 19, 118–127. [Google Scholar] [CrossRef]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef]
- European Imaging Biomarkers Alliance—EIBALL. Available online: https://www.myesr.org/research/european-imaging-biomarkers-alliance-eiball (accessed on 9 May 2022).
- Quantitative Imaging Biomarkers Alliance. Available online: https://www.rsna.org/research/quantitative-imaging-biomarkers-alliance (accessed on 9 May 2022).
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.J. (18)F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Mathilde Jacobsen, S.; Molby Henriksen, O.; Broholm, H.; Urup, T.; Grunnet, K.; Andree Larsen, V.; Moller, S.; Skjoth-Rasmussen, J.; Skovgaard Poulsen, H.; et al. Recurrent glioblastoma versus late posttreatment changes: Diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 2019, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.P.; Akkurt, B.H.; Musigmann, M.; Mammadov, O.; Blömer, D.A.; Kasap, D.N.G.; Henssen, D.J.H.A.; Nacul, N.G.; Sartoretti, E.; Sartoretti, T.; et al. Pseudoprogression prediction in high grade primary CNS tumors by use of radiomics. Sci. Rep. 2022, 12, 5915. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, O.; Akkurt, B.H.; Musigmann, M.; Ari, A.P.; Blömer, D.A.; Kasap, D.N.G.; Henssen, D.J.H.A.; Nacul, N.G.; Sartoretti, E.; Sartoretti, T.; et al. Radiomics for pseudoprogression prediction in high grade gliomas: Added value of MR contrast agent. Heliyon 2022, 8, e10023. [Google Scholar] [CrossRef]
- Jang, B.S.; Park, A.J.; Jeon, S.H.; Kim, I.H.; Lim, D.H.; Park, S.H.; Lee, J.H.; Chang, J.H.; Cho, K.H.; Kim, J.H.; et al. Machine Learning Model to Predict Pseudoprogression Versus Progression in Glioblastoma Using MRI: A Multi-Institutional Study (KROG 18-07). Cancers 2020, 12, 2706. [Google Scholar] [CrossRef]
- Kebir, S.; Schmidt, T.; Weber, M.; Lazaridis, L.; Galldiks, N.; Langen, K.J.; Kleinschnitz, C.; Hattingen, E.; Herrlinger, U.; Lohmann, P.; et al. A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma. Cancers 2020, 12, 3080. [Google Scholar] [CrossRef]


| PubMed |
|---|
| (“Glioma”[Title/Abstract] OR “glioblastoma”[Title/Abstract]) AND (“tumor recurrence”[Title/Abstract] OR “pseudoprogression”[Title/Abstract] OR “progression”[Title/Abstract]) AND (“PET”[Title/Abstract] OR “positron emission tomography”[Title/Abstract] OR “Positron Emission Tomography Computed Tomography”[MeSH Terms]) AND (“dynamic susceptibility contrast”[Title/Abstract] OR “dynamic contrast enhancement”[Title/Abstract] OR “arterial spin labeling”[Title/Abstract] OR (“Magnetic Resonance Imaging”[MeSH Terms] OR “Multiparametric Magnetic Resonance Imaging”[MeSH Terms] OR (“Magnetic Resonance Imaging”[Title/Abstract] OR “MRI”[Title/Abstract]))) |
| EMBASE |
| (glioma or glioblastoma).m_titl.OR ((tumor recurrence or pseudoprogression or recurrence).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] OR (tumor recurrence or pseudoprogression or recurrence).m_titl.)) AND ((PET or positron emission tomography).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] OR (PET or positron emission tomography).m_titl) AND ((MRI perfusion.m_titl. OR MRI perfusion.mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word]) OR (dynamic contrast enhancement or dynamic susceptibility contrast or arterial spin labeling).m_titl. OR (dynamic contrast enhancement or dynamic susceptibility contrast or arterial spin labeling).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word]) |
| Cochrane Library |
| (((“glioblastoma”):ti,ab,kw OR (“glioma”):ti,ab,kw) OR ((“pseudoprogression”):ti,ab,kw OR (“tumor recurrence”):ti,ab,kw AND (“tumor progression”):ti,ab,kw)) AND (“positron emission tomography”):ti,ab,kw AND ((“perfusion weighted magnetic resonance imaging”):ti,ab,kw” OR (“dynamic susceptibility contrast”):ti,ab,kw OR (“dynamic contrast enhancement”):ti,ab,kw OR (“arterial spin labeling”):ti,ab,kw) |
| Study | Patients (n) | M/F (n) | Age (Years) | WHO Classification and Grade of Glioma | Lesions (n) | PET-Tracer | PET-CT vs. PET-MRI | Dose | Sens | Spec | PWI Technique | Sens | Spec | Lesion Diagnosis (Gold Standard) | Parameter PET | Cut-Off | Parameter PWI | Cut-Off |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dandois et al. (2010) [20] | 28 | 16/12 | mean 51 (range 25–74) | WHO grade 3 astrocytoma (9); WHO grade 3 oligodendroglioma (5); WHO grade 4 glioblastoma (14) | 28 | [11C]MET | PET-CT | 740 MBq | 100 | N/E | DSC | 67 | 100 | Histological assessment after biopsy | Qualitative assessment | rCBV | >1.82 | |
| Kim et al. (2010); part 1 [23] | 10 | 8/2 | mean age: 46.1 years | WHO grade 3 astrocytoma (3); WHO grade 3 oligodendroglioma (2); WHO grade 4 (5) | 10 | [18F]FDG | PET-CT | NR | 100 | 75 | DSC | 100 | 100 | Histological assessment after biopsy and/or radiological/clinical follow-up | Uptake ratios Lmax/Rmax | 2.64 | L/R ratio from rCBV | >3.69 |
| Kim et al. (2010); part 2 [23] | 10 | 8/2 | mean age: 46.1 years | WHO grade 3 astrocytoma (3); WHO grade 3 oligodendroglioma (2); WHO grade 4 (5) | 10 | [11C]MET | PET-CT | NR | 75 | 100 | DSC | 100 | 100 | Histological assessment after biopsy and/or radiological/clinical follow-up | Uptake ratios Lmax/Rmax | 2.64 | L/R ratio from rCBV | >3.69 |
| Ozsunar et al. (2010); part 1 [26] | 30 | 22/8 | mean 42 (SD 11) | WHO grade 2 (7); WHO grade 3 (9); WHO grade 4 (19) | 30 | [18F]FDG | PET-CT | 185–370 MBq | 81 | 90 | DSC | 71 | 40 | Histological assessment after biopsy | Qualitative assessment | normalized rCBV | >1.5 | |
| Ozsunar et al. (2010); part 2 [26] | 30 | 22/8 | mean 42 (SD 11) | WHO grade 2 (7); WHO grade 3 (9); WHO grade 4 (19) | 30 | [18F]FDG | PET-CT | 185–370 MBq | 81 | 90 | ASL | 94 | 52 | Histological assessment after biopsy | Qualitative assessment | normalized rCBV | >1.3 | |
| Prat et al. (2010) [29] | 9 | 5/4 | 44.5 (16.3) | WHO grade 2 astrocytoma (3); WHO grade 2 oligodendroglioma (1); WHO grade 3 astrocytoma (5); WHO grade 3 oligodendroglioma (3); WHO grade 4 (11) | 9 | [18F]FDG | PET-CT | NR | 83 | 67 | DCE | 100 | 100 | Histological assessment after biopsy and/or radiological/clinical follow-up | Qualitative assessment | Qualitative assessment | ||
| D’Souza et al. (2014) [21] | 29 | 24/17 | NR | WHO grade 3 astrocytoma (16); WHO grade 4 glioblastoma (13) | 29 | [11C]MET | PET-CT | 6 MBq/kg | 95 | 20 | DSC | 84 | 90 | Histological assessment after biopsy | L/R ratio from SUVmean | >1.58 | rCBV | >1.82 |
| Hatzoglou et al. (2015) [27] | 53 | 35/18 | mean 57 (range 19–81) | WHO grade 2 astrocytoma (2); WHO grade 3 astrocytoma (6); WHO grade 2 oligodendroglioma (1); WHO grade 3 oligodendroglioma (2); WHO grade 4 glioblastoma (18); 24 metastases | 29 | [18F]FDG | PET-CT | 370 MBq | 68 | 82 | DCE | 92 | 77 | Histological assessment after biopsy and/or radiological/clinical follow-up | SUV ratio | >1.2 | Vp ratio | >2.1 |
| Jena et al. (2016) [31] | 26 | 21/5 | mean 51.6 (SD 16.0) | NR | 32 | [18F]FET | PET-MRI | 352.12 ± 64.26 | 100 | 71.4 | DSC | 96 | 71.4 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >2.11 | rCBV mean | >1.89 |
| Jena et al. (2017) [25] | 35 | 29/6 | mean 50 (SD 12.0) | WHO grade 2 (9); WHO grade 3 (13); WHO grade 4 (19) | 41 | [18F]FDG | PET-MRI | 222 ± 30 MBq | 90 | 81.8 | DSC | 83 | 63.6 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmean | >1.18 | rCBVmean | >1.7 |
| Sogani et al. (2017); part 1 [33] | 32 | 25/7 | 52.3 (17–80) | NR | 32 | [18F]FET | PET-MRI | 207.2 ± 25 MBq | 89 | 86,2 | DSC | 95 | 72 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >2.09 | rCBVmean | >1.78 |
| Sogani et al. (2017); part 2 [33] | 32 | 25/7 | 52.3 (17–80) | NR | 32 | [18F]FET | PET-MRI | 207.2 ± 25 MBq | 89 | 86.2 | DSC | 95 | 72 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmean | >1.52 | rCBVmean | >1.78 |
| Hojjati et al. (2018); part 1 [24] | 24 | 16/8 | mean 57.5 (range 34–81) | WHO grade 4 (24) | 23 | [18F]FDG | PET-MRI | 440 MBq | 100 | 80 | DSC | 100 | 75 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmean | >1.31 | rCBVmax | >3.32 |
| Hojjati et al. (2018); part 2 [24] | 24 | 16/8 | mean 57.5 (range 34–81) | WHO grade 4 (24) | 23 | [18F]FDG | PET-CT | 440 MBq | 83 | 80 | DSC | 100 | 75 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmean | >1.47 | rCBVmax | >3.32 |
| Pyka et al. (2018) [32] | 47 | 22/25 | mean 54 (SD 11) | WHO grade 2 astrocytoma (2); WHO grade 2 oligodendroglioma (1); WHO grade 3 astrocytoma (13); WHO grade 3 oligodendroglioma (3); WHO grade 4 (27) | 63 | [18F]FET | PET-MRI | 190 MBq | 80 | 85 | DSC | 66 | 77 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >2.07 | rCBVmean | >3.35 |
| Qiao et al. (2018) [22] | 42 | 28/14 | mean 47.2 (SD 10.5) | WHO grade 3 astrocytoma (12); WHO grade 3 oligodendroglioma (7); WHO grade 4 (23) | 42 | [11C]MET | PET-CT | 370–738.8 MBq | 91 | 56 | DSC | 67 | 77.8 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >1.85 | rCBVmean | >1.83 |
| Verger et al. (2018) [34] | 32 | 17/15 | mean age, 52 (SD 13.4) | WHO grade 2 astrocytoma (1); WHO grade 2 oligodendroglioma (1); WHO grade 3 astrocytoma (2); WHO grade 3 oligodendroglioma (1); WHO grade 4 (27) | 32 | [18F]FET | PET-MRI | 3 MBq/kg | 80 | 86 | DSC | 52 | 0 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >2.61 | rCBVmean | NR |
| Lundemann et al. (2019); part1 [28] | 9 | 7/2 | mean 58.7 (SD 12.1) | WHO grade 4 (9) | 9 | [18F]FDG | PET-MRI | 200 MBq | 100 | 71.4 | DCE | 96 | 71.4 | Histological assessment after biopsy and/or radiological/clinical follow-up | Qualitative assessment | Qualitative assessment | ||
| Lundemann et al. (2019); part 2 [28] | 9 | 7/2 | mean 58.7 (SD 12.1) | WHO grade 4 (9) | 9 | [18F]FET | PET-CT | 200 MBq | 90 | 81.8 | DCE | 83 | 63.6 | Histological assessment after biopsy and/or radiological/clinical follow-up | Qualitative assessment | Qualitative assessment | ||
| Seligman et al. (2019) [30] | 41 | NR | median 53 (21–79) | WHO grade 3 (21); WHO grade 4 (20) | 41 | [18F]FDG | PET-MRI | NR | 94 | 33 | DCE | 91 | 56 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmean (Whole-tumor SUVmean divided by SUVmean of normal WM) | >0.75 | Ktransmean (Mean Ktrans of whole tumor divided by mean Ktrans of contralateral brain) | >4.5 |
| Fraioli et al. (2020) [36] | 40 | 23/17 | median 34 years (range 5–65) | WHO grade 1 (3); WHO grade 2 (12); WHO grade 3 (14); WHO grade 4 (11); glioblastoma (11); astrocytoma (23); oligodendroglioma (6) | 40 | [18F] FDOPA | PET-MRI | 250– 370 MBq | 100 | 100 | DSC | 99 | 25 | Histological assessment after biopsy and/or radiological/clinical follow-up | Qualitative assessment | Qualitative assessment | ||
| Steidl et al. (2021) [35] | 104 | 68/36 | median age of 52 (range 20–78) | WHO grade 2 (9); WHO grade 3 (24); WHO grade 4 (71) | 104 | [18F]FET | PET-CT | 3 MBq/kg | 70 | 60 | DSC | 54 | 100 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >1.95 | rCBVmax | >2.85 |
| Pellerin et al. (2021) [37] | 58 | 34/24 | mean age 53.1 ± 14.3 | WHO grade 2 (10); WHO grade 3 (21); WHO grade 4 (27) | 58 | [18F] FDOPA | PET-MRI | 2 MBq/kg | 94.1 | 79.2 | ASL | 64.7 | 100 | Histological assessment after biopsy and/or radiological/clinical follow-up | L/R 2 sample t-test | t > 6.36 | L/R 2 sample t-test | t > 3.25 |
| Jabeen et al. (2021) [38] | 48 | 31/17 | mean age 39.9 ± 12.5 | WHO grade 2 (3); WHO grade 3 (28); WHO grade 4 (17) | 48 | [11C]MET | PET-MRI | 360–378 MBq | 81.8 | 92.3 | DSC | 84.8 | 76.9 | Histological assessment after biopsy and/or radiological/clinical follow-up | TBRmax | >1.23 | rCBVradio | >1.38 |
| Study | Prospective? | Patient and Treatment Characteristics Compared? | Adequately Described the Treatment Protocol? | Potentially Confounding Adjuvant Treatments? | Did the Study Avoid Inappropriate Exclusions? | Interval between the Completion of Treatment and Imaging Documented? | PET Imaging Results Interpreted without Knowledge of the Results of PW Imaging and Vice Versa | Post-Processing Techniques Reproducible as Described? | Did More than One Investigator Process the Imaging Data? Was There an Evaluation of Inter-Rater Reliability? | Were Histological Criteria Defined? | Reference Standard Adequately Defined When Pathology Was Unavailable? | Pathology Interpreted without Knowledge of the Results of PET/PW Imaging Outcomes? | Did All Patients Receive the Same Reference Test? | Were All Patients Included in the Analysis? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dandois et al. (2010) [20] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| D’Souza et al. (2014) [21] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Fraioli et al. (2020) [36] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Hatzoglou et al. (2015) [29] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Hojjati et al. (2018); part1 [24] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Hojjati et al. (2018); part2 [24] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Jena et al. (2016) [31] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Jena et al. (2017) [25] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Lundemann et al. (2019); part1 [28] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Lundemann et al. (2019); part 2 [28] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Ozsunar et al. (2010); part 1 [26] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Ozsunar et al. (2010); part 2 [26] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Pyka et al. (2018) [32] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Qiao et al. (2018) [22] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Sogani et al. (2017); part 1 [33] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Sogani et al. (2017); part 2 [33] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Kim et al. (2010); part 1 [23] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Kim et al. (2010); part 2 [23] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Prat et al. (2010) [29] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Seligman et al. (2019) [28] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Verger et al. (2018) [34] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Steidl et al. (2021) [35] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Pellerin et al. (2021) [37] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Jabeen et al. (2021) [38] | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Technique | References | Patients (n) | Pooled Sensitivity | 95% CI | Pooled Specificity | 95% CI |
|---|---|---|---|---|---|---|
| DCE PWI | [27,28,29,30] | 112 | 90% | 84–94% | 70% | 56–82% |
| DSC PWI | [20,21,22,23,24,25,26,31,32,33,34,35,36,38] | 497 | 90% | 80–95% | 77% | 61–88% |
| ASL PWI | [26,37] | 56 | 84% | 31–98% | 85% | 11–100% |
| [18F]FDG PET | [23,24,25,26,27,28,29,30] | 192 | 89% | 80–94% | 78% | 65–87% |
| [11C]MET PET | [20,21,22,23,38] | 157 | 89% | 78–95% | 72% | 25–95% |
| [18F]FET PET | [28,31,32,33,34,35] | 250 | 84% | 75–90% | 80% | 67–88% |
| [18F]FDOPA | [36,37] | 98 | 94% | 86–98% | 78% | 58–90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henssen, D.; Leijten, L.; Meijer, F.J.A.; van der Kolk, A.; Arens, A.I.J.; ter Laan, M.; Smeenk, R.J.; Gijtenbeek, A.; van de Giessen, E.M.; Tolboom, N.; et al. Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma. Cancers 2023, 15, 2631. https://doi.org/10.3390/cancers15092631
Henssen D, Leijten L, Meijer FJA, van der Kolk A, Arens AIJ, ter Laan M, Smeenk RJ, Gijtenbeek A, van de Giessen EM, Tolboom N, et al. Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma. Cancers. 2023; 15(9):2631. https://doi.org/10.3390/cancers15092631
Chicago/Turabian StyleHenssen, Dylan, Lars Leijten, Frederick J. A. Meijer, Anja van der Kolk, Anne I. J. Arens, Mark ter Laan, Robert J. Smeenk, Anja Gijtenbeek, Elsmarieke M. van de Giessen, Nelleke Tolboom, and et al. 2023. "Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma" Cancers 15, no. 9: 2631. https://doi.org/10.3390/cancers15092631
APA StyleHenssen, D., Leijten, L., Meijer, F. J. A., van der Kolk, A., Arens, A. I. J., ter Laan, M., Smeenk, R. J., Gijtenbeek, A., van de Giessen, E. M., Tolboom, N., Oprea-Lager, D. E., Smits, M., & Nagarajah, J. (2023). Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma. Cancers, 15(9), 2631. https://doi.org/10.3390/cancers15092631






