Performance of Ultra-Rapid Idylla™ EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
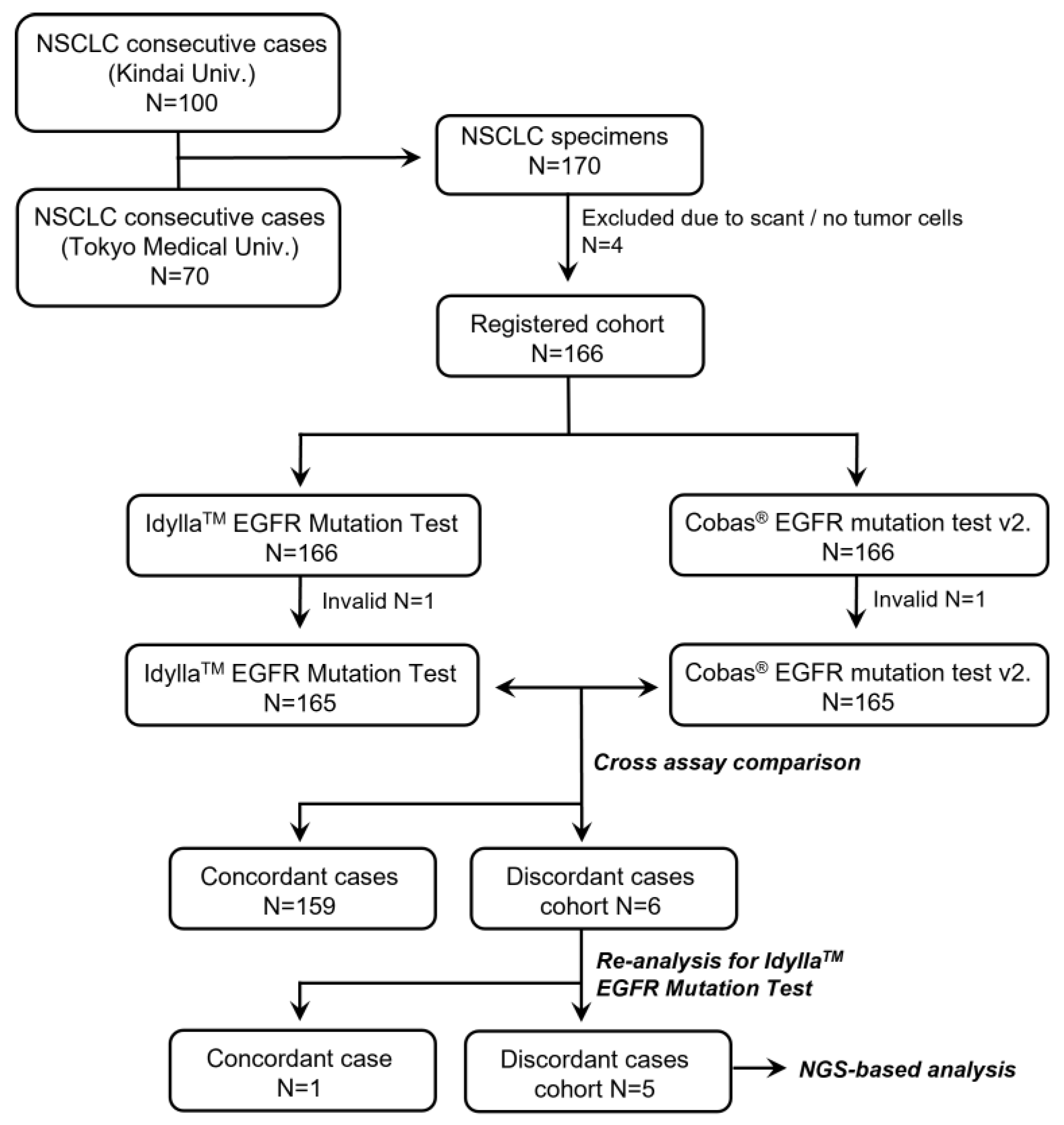
2.2. Study Design
2.3. DNA Extraction and NGS-Based Panel Test
2.4. Statistical Analysis
2.5. Total Expense Calculation for Molecular Testing
3. Results
3.1. Performance of the Idylla EGFR Mutation Test
3.2. Detailed Examination of the Discordant Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A., Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Loong, H.H.; Wong, C.K.H.; Chan, C.P.K.; Chang, A.; Zhou, Z.Y.; Tang, W.; Gibbs, M. Clinical and Economic Impact of Upfront Next-Generation Sequencing for Metastatic NSCLC in East Asia. JTO Clin. Res. Rep. 2022, 3, 100290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- De Luca, C.; Gragnano, G.; Pisapia, P.; Vigliar, E.; Malapelle, U.; Bellevicine, C.; Troncone, G. EGFR mutation detection on lung cancer cytological specimens by the novel fully automated PCR-based Idylla EGFR Mutation Assay. J. Clin. Pathol. 2017, 70, 295–300. [Google Scholar] [CrossRef]
- Lambros, L.; Caumont, C.; Guibourg, B.; Barel, F.; Quintin-Roue, I.; Marcorelles, P.; Merlio, J.P.; Uguen, A. Evaluation of a fast and fully automated platform to diagnose EGFR and KRAS mutations in formalin-fixed and paraffin-embedded non-small cell lung cancer samples in less than one day. J. Clin. Pathol. 2017, 70, 544–549. [Google Scholar] [CrossRef]
- Murakami, S.; Yokose, T.; Shinada, K.; Isaka, T.; Katakura, K.; Ushio, R.; Kondo, T.; Kato, T.; Ito, H.; Saito, H. Comparison of next-generation sequencing and cobas EGFR mutation test v2 in detecting EGFR mutations. Thorac. Cancer 2022, 13, 3217–3224. [Google Scholar] [CrossRef]
- Belardinilli, F.; Capalbo, C.; Buffone, A.; Petroni, M.; Colicchia, V.; Ferraro, S.; Zani, M.; Nicolussi, A.; D’Inzeo, S.; Coppa, A.; et al. Validation of the Ion Torrent PGM sequencing for the prospective routine molecular diagnostic of colorectal cancer. Clin. Biochem. 2015, 48, 908–910. [Google Scholar] [CrossRef]
- Behnke, A.; Cayre, A.; De Maglio, G.; Giannini, G.; Habran, L.; Tarsitano, M.; Chetta, M.; Cappellen, D.; Lespagnol, A.; Le Naoures, C.; et al. FACILITATE: A real-world, multicenter, prospective study investigating the utility of a rapid, fully automated real-time PCR assay versus local reference methods for detecting epidermal growth factor receptor variants in NSCLC. Pathol. Oncol. Res. 2023, 29, 1610707. [Google Scholar] [CrossRef] [PubMed]
- Arcila, M.E.; Yang, S.R.; Momeni, A.; Mata, D.A.; Salazar, P.; Chan, R.; Elezovic, D.; Benayed, R.; Zehir, A.; Buonocore, D.J.; et al. Ultrarapid EGFR Mutation Screening Followed by Comprehensive Next-Generation Sequencing: A Feasible, Informative Approach for Lung Carcinoma Cytology Specimens with a High Success Rate. JTO Clin. Res. Rep. 2020, 1, 100077. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Salazar, P.; Zheng, T.; Mensah, N.; Rijo, I.; Dogan, S.; Yao, J.; Moung, C.; Vanderbilt, C.; Benhamida, J.; et al. Rapid EGFR Mutation Detection Using the Idylla Platform: Single-Institution Experience of 1200 Cases Analyzed by an In-House Developed Pipeline and Comparison with Concurrent Next-Generation Sequencing Results. J. Mol. Diagn. 2021, 23, 310–322. [Google Scholar] [CrossRef]
- Suda, K.; Mitsudomi, T.; Shintani, Y.; Okami, J.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.I.; Asamura, H.; et al. Clinical Impacts of EGFR Mutation Status: Analysis of 5780 Surgically Resected Lung Cancer Cases. Ann. Thorac. Surg. 2021, 111, 269–276. [Google Scholar] [CrossRef]
- O’Sullivan, H.; d’Arienzo, P.D.; Yousaf, N.; Cui, W.; Popat, S. Inadequacy of PCR genotyping in advanced non-small cell lung cancer: EGFR L747_A755delinsSS exon 19 deletion is not detected by the real-time PCR Idylla EGFR mutation test but is detected by ctDNA next generation sequencing and responds to osimertinib. Eur. J. Cancer 2022, 166, 38–40. [Google Scholar] [CrossRef]
- Nkosi, D.; Casler, V.L.; Syposs, C.R.; Oltvai, Z.N. Utility of Select Gene Mutation Detection in Tumors by the Idylla Rapid Multiplex PCR Platform in Comparison to Next-Generation Sequencing. Genes 2022, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csoszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Pisters, K.; Kris, M.G.; Gaspar, L.E.; Ismaila, N.; Adjuvant Systemic, T.; Adjuvant Radiation Therapy for Stage, I.t.I.N.G.E.P. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I-IIIA Completely Resected Non-Small-Cell Lung Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 1127–1129. [Google Scholar] [CrossRef]
- Passaro, A.; Leighl, N.; Blackhall, F.; Popat, S.; Kerr, K.; Ahn, M.J.; Arcila, M.E.; Arrieta, O.; Planchard, D.; de Marinis, F.; et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann. Oncol. 2022, 33, 466–487. [Google Scholar] [CrossRef]
- Khalifa, E.; Chapusot, C.; Tournier, B.; Sentis, J.; Marion, E.; Remond, A.; Aubry, M.; Pioche, C.; Bergeron, A.; Primois, C.; et al. Idylla EGFR assay on extracted DNA: Advantages, limits and place in molecular screening according to the latest guidelines for non-small-cell lung cancer (NSCLC) patients. J. Clin. Pathol. 2022. [Google Scholar] [CrossRef]
- Kunimasa, K.; Sugimoto, N.; Kawamura, T.; Yamasaki, T.; Honma, K.; Nagata, S.; Kukita, Y.; Fujisawa, F.; Inoue, T.; Yamaguchi, Y.; et al. Clinical application of comprehensive genomic profiling panel to thoracic malignancies: A single-center retrospective study. Thorac. Cancer 2022, 13, 2970–2977. [Google Scholar] [CrossRef]
- Shepherd, P.; Sheath, K.L.; Tin, S.T.; Khwaounjoo, P.; Aye, P.S.; Li, A.; Laking, G.R.; Kingston, N.J.; Lewis, C.A.; Elwood, J.M.; et al. Lung cancer mutation testing: A clinical retesting study of agreement between a real-time PCR and a mass spectrometry test. Oncotarget 2017, 8, 101437–101451. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.K.; Ko, J.C.; Yang, J.C.; Shih, J.Y. Afatinib is effective in the treatment of lung adenocarcinoma with uncommon EGFR p.L747P and p.L747S mutations. Lung Cancer 2019, 133, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Robichaux, J.P.; Le, X.; Vijayan, R.S.K.; Hicks, J.K.; Heeke, S.; Elamin, Y.Y.; Lin, H.Y.; Udagawa, H.; Skoulidis, F.; Tran, H.; et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021, 597, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Zhai, Y.; Wang, J. Non-small cell lung cancer harboring a rare EGFR L747P mutation showing intrinsic resistance to both gefitinib and osimertinib (AZD9291): A case report. Thorac. Cancer 2018, 9, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhou, X.; Li, P.; Qi, C.; Ling, Y. EGFR L747P mutation in one lung adenocarcinoma patient responded to afatinib treatment: A case report. J. Thorac. Dis. 2018, 10, E802–E805. [Google Scholar] [CrossRef]
- He, M.; Capelletti, M.; Nafa, K.; Yun, C.H.; Arcila, M.E.; Miller, V.A.; Ginsberg, M.S.; Zhao, B.; Kris, M.G.; Eck, M.J.; et al. EGFR exon 19 insertions: A new family of sensitizing EGFR mutations in lung adenocarcinoma. Clin. Cancer Res. 2012, 18, 1790–1797. [Google Scholar] [CrossRef]
- Janning, M.; Suptitz, J.; Albers-Leischner, C.; Delpy, P.; Tufman, A.; Velthaus-Rusik, J.L.; Reck, M.; Jung, A.; Kauffmann-Guerrero, D.; Bonzheim, I.; et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM). Ann. Oncol. 2022, 33, 602–615. [Google Scholar] [CrossRef]
- Leone, A.; Muscarella, L.A.; Graziano, P.; Tornese, A.; Grillo, L.R.; Di Lorenzo, A.; Bronzini, M.; Scarpino, S.; Sparaneo, A.; Rossi, G. Robust Performance of the Novel Research-Use-Only Idylla GeneFusion Assay Using a Diverse Set of Pathological Samples with a Proposed 1-Day Workflow for Advanced NSCLC Evaluation. Cancers 2022, 15, 292. [Google Scholar] [CrossRef]
- Hofman, V.; Heeke, S.; Bontoux, C.; Chalabreysse, L.; Barritault, M.; Bringuier, P.P.; Fenouil, T.; Benzerdjeb, N.; Begueret, H.; Merlio, J.P.; et al. Ultrafast Gene Fusion Assessment for Nonsquamous NSCLC. JTO Clin. Res. Rep. 2023, 4, 100457. [Google Scholar] [CrossRef] [PubMed]
- Tsongalis, G.J.; Al Turkmani, M.R.; Suriawinata, M.; Babcock, M.J.; Mitchell, K.; Ding, Y.; Scicchitano, L.; Tira, A.; Buckingham, L.; Atkinson, S.; et al. Comparison of Tissue Molecular Biomarker Testing Turnaround Times and Concordance Between Standard of Care and the Biocartis Idylla Platform in Patients with Colorectal Cancer. Am. J. Clin. Pathol. 2020, 154, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Makutani, Y.; Sakai, K.; Yamada, M.; Wada, T.; Chikugo, T.; Satou, T.; Iwasa, Y.; Yamamoto, H.; de Velasco, M.A.; Nishio, K.; et al. Performance of Idylla() RAS-BRAF mutation test for formalin-fixed paraffin-embedded tissues of colorectal cancer. Int. J. Clin. Oncol. 2022, 27, 1180–1187. [Google Scholar] [CrossRef]
- Long-Mira, E.; Picard-Gauci, A.; Lassalle, S.; Hofman, V.; Lalvee, S.; Tanga, V.; Zahaf, K.; Bonnetaud, C.; Lespinet, V.; Camuzard, O.; et al. Comparison of Two Rapid Assays for the Detection of BRAF V600 Mutations in Metastatic Melanoma including Positive Sentinel Lymph Nodes. Diagnostics 2022, 12, 751. [Google Scholar] [CrossRef] [PubMed]


| Factors | Registered Cases (N = 166) | |
|---|---|---|
| Sex | Female | 56 (34%) |
| Male | 110 (66%) | |
| Age | Median (range) | 72-year-old (35–90) |
| Histology | Adenocarcinoma | 122 (73%) |
| Adenosquamous carcinoma | 6 (4%) | |
| Squamous cell carcinoma | 35 (21%) | |
| Pleomorphic carcinoma | 3 (2%) | |
| pStage (8th Edition) | IA1-3 | 100 (60%) |
| IB | 20 (12%) | |
| II-III | 37 (22%) | |
| IV | 9 (5%) | |
| Macro-dissection | Yes | 141 (85%) |
| No | 25 (15%) | |
| Proportion of tumor cells | Median (range) | 25% (20–70) |
| Detected Mutation | Idylla | ||||||
|---|---|---|---|---|---|---|---|
| Exon 19 del | L858R | G719X | Exon 20 ins | L861Q | Wild-Type | ||
| Cobas | Exon 19 del | 21 * | - | - | - | - | 4 ** |
| L858R | - | 28 | - | - | - | - | |
| G719X | - | - | 2 | - | - | - | |
| Exon 20 ins | - | - | - | - | - | - | |
| L861Q | - | - | - | - | 1 | - | |
| Wild-type | - | 1 # | - | 1 | - | 107 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suda, K.; Sakai, K.; Ohira, T.; Chikugo, T.; Satou, T.; Matsubayashi, J.; Nagao, T.; Ikeda, N.; Tsutani, Y.; Mitsudomi, T.; et al. Performance of Ultra-Rapid Idylla™ EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening. Cancers 2023, 15, 2648. https://doi.org/10.3390/cancers15092648
Suda K, Sakai K, Ohira T, Chikugo T, Satou T, Matsubayashi J, Nagao T, Ikeda N, Tsutani Y, Mitsudomi T, et al. Performance of Ultra-Rapid Idylla™ EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening. Cancers. 2023; 15(9):2648. https://doi.org/10.3390/cancers15092648
Chicago/Turabian StyleSuda, Kenichi, Kazuko Sakai, Tatsuo Ohira, Takaaki Chikugo, Takao Satou, Jun Matsubayashi, Toshitaka Nagao, Norihiko Ikeda, Yasuhiro Tsutani, Tetsuya Mitsudomi, and et al. 2023. "Performance of Ultra-Rapid Idylla™ EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening" Cancers 15, no. 9: 2648. https://doi.org/10.3390/cancers15092648
APA StyleSuda, K., Sakai, K., Ohira, T., Chikugo, T., Satou, T., Matsubayashi, J., Nagao, T., Ikeda, N., Tsutani, Y., Mitsudomi, T., & Nishio, K. (2023). Performance of Ultra-Rapid Idylla™ EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening. Cancers, 15(9), 2648. https://doi.org/10.3390/cancers15092648









