Assessment of an Anticancer Effect of the Simultaneous Administration of MM-129 and Indoximod in the Colorectal Cancer Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Drug Screening Assay
2.2. Zebrafish Xenograft Injection
2.3. In Vivo Imaging
2.4. RNA Extraction and Quantitative Analysis
2.5. Zebrafish Egg Proliferation Assay
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Flow Cytometry Assessment of Annexin V Binding
2.9. Analysis of Mitochondrial Membrane Potential
2.10. Caspase Activity Assays
2.11. Capillary Protein Separation and Immunodetection
2.12. Statistical Analysis
3. Results
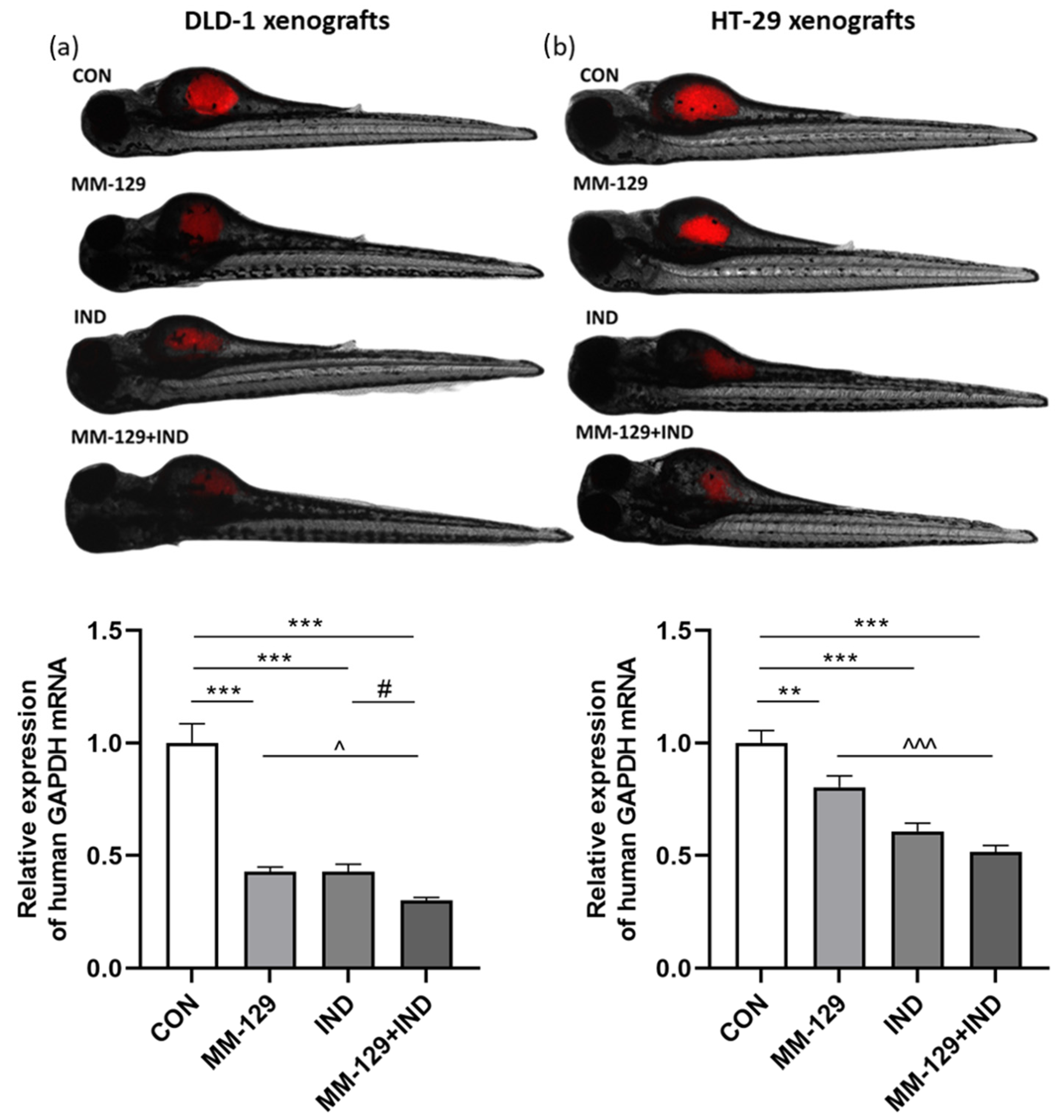
3.1. Compound Combination Had a Favorable Impact on Tumor Growth in Zebrafish Xenografts
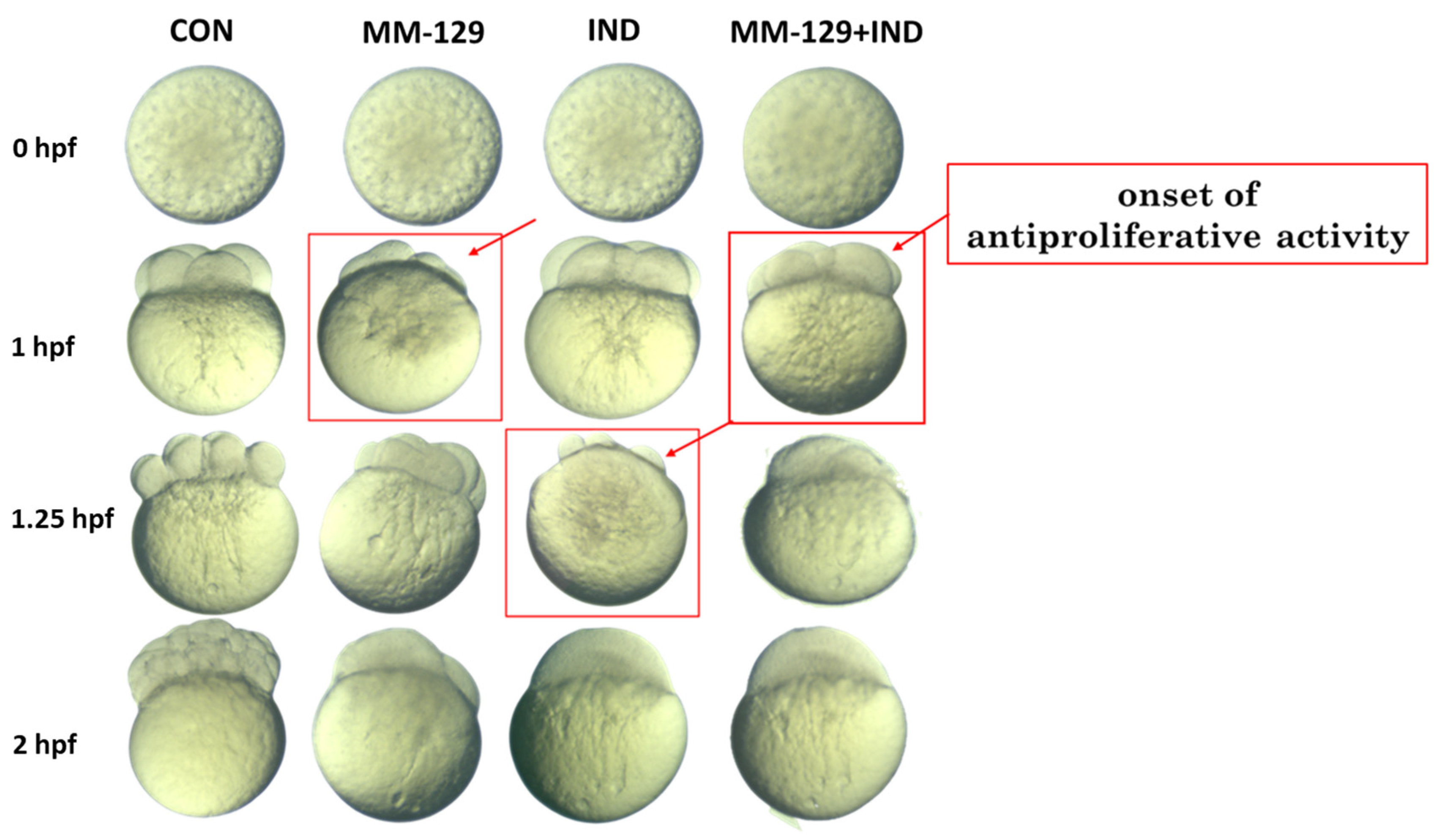
3.2. Antiproliferative Activity of Compound Combination in a Zebrafish Model
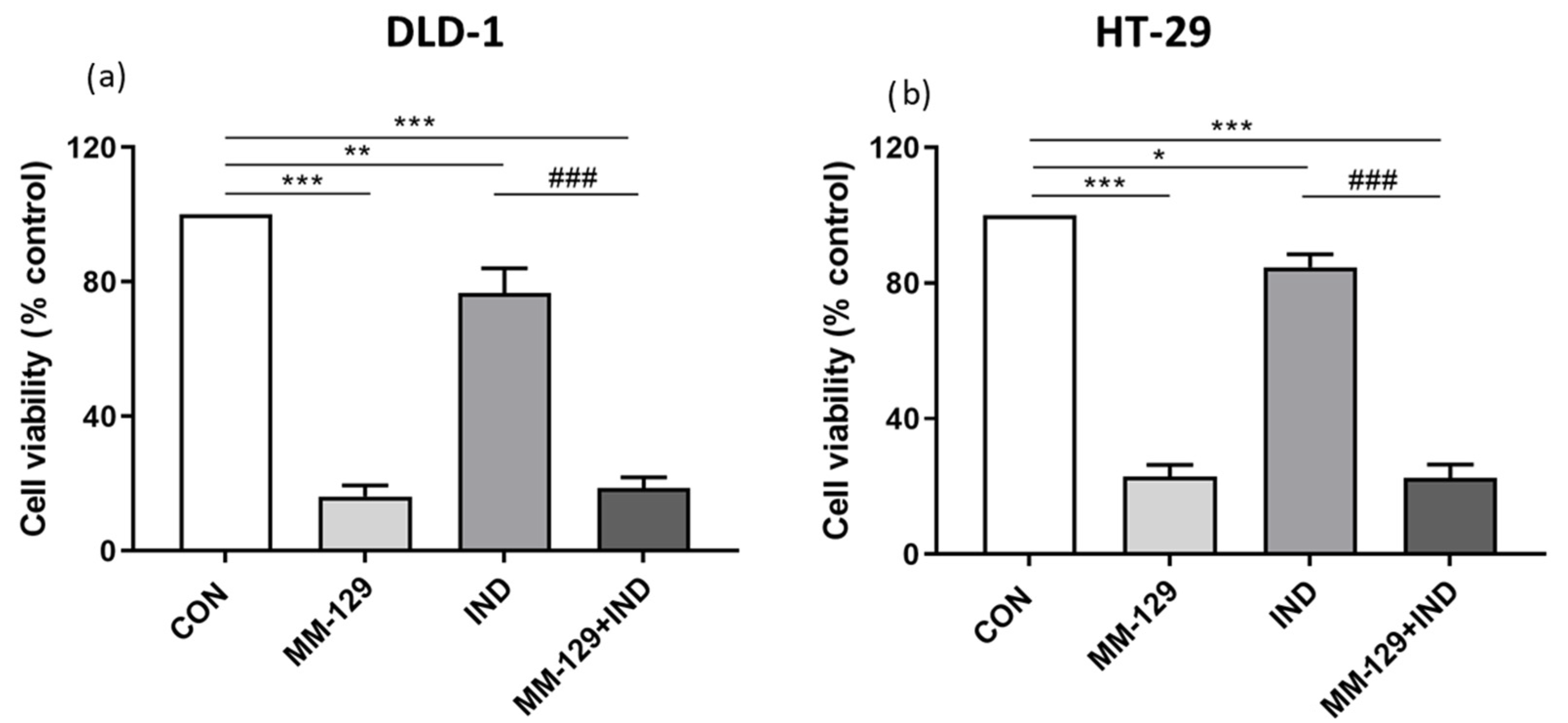
3.3. Antiproliferative Activity of Compound Combination in Colon Cancer Cells
3.4. Compound Combination Enhanced Apoptosis via Decreasing Mitochondrial Membrane Potential and Phosphatidylserine Externalization
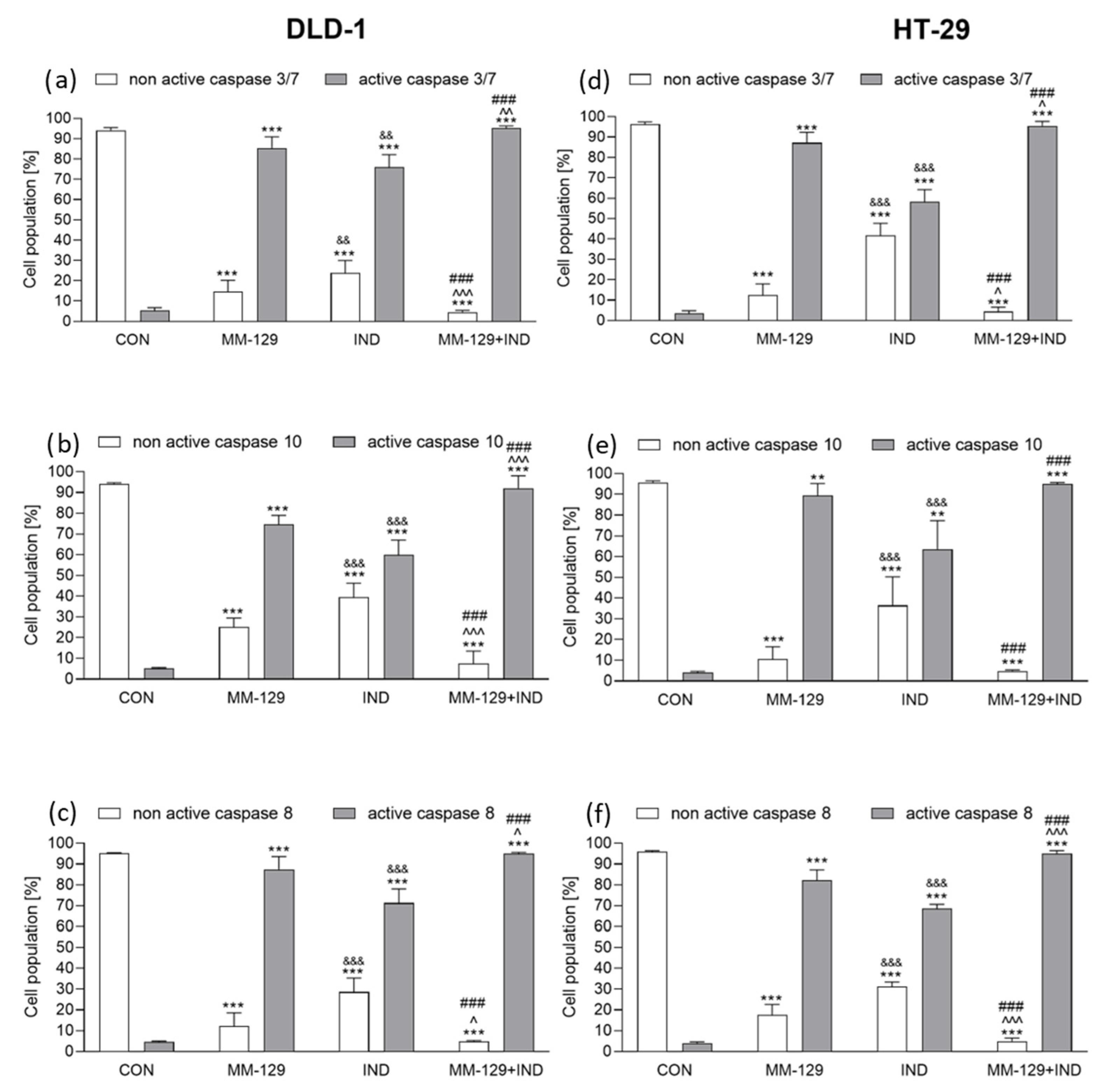
3.5. The Addition of IND Intensified Caspase Activation Exerted by MM-129
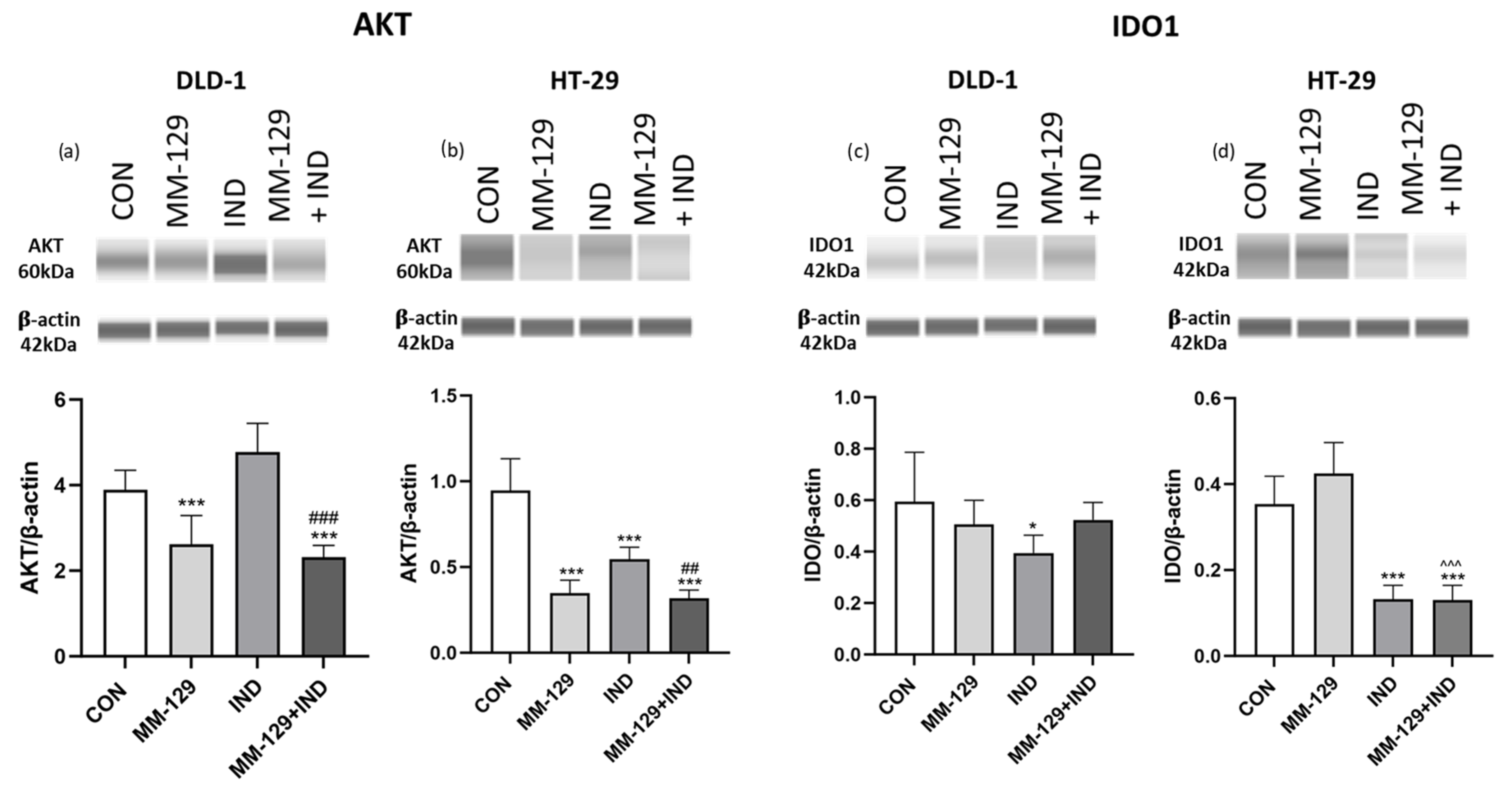
3.6. AKT Signaling Pathway Was Impaired under the Impact of Compound Combination
3.7. Indoximod but Not MM-129 Downregulated IDO1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiyozumi, Y.; Baba, Y.; Okadome, K.; Yagi, T.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Komohara, Y.; et al. IDO1 Expression Is Associated with Immune Tolerance and Poor Prognosis in Patients with Surgically Resected Esophageal Cancer. Ann. Surg. 2019, 269, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Shen, W.; Zhong, Y.; Bast, R.C.; Jazaeri, A.; Sood, A.K.; Liu, J. Expression of B7–H4 and IDO1 Is Associated with Drug Resistance and Poor Prognosis in High-Grade Serous Ovarian Carcinomas. Hum. Pathol. 2021, 113, 20–27. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, N.C.; Rogers, T.J.; Gordon, M.A.; Greene, L.I.; Cochrane, D.R.; Spoelstra, N.S.; Nemkov, T.G.; D’Alessandro, A.; Hansen, K.C.; Richer, J.K. A TDO2-AhR Signaling Axis Facilitates Anoikis Resistance and Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2015, 75, 4651–4664. [Google Scholar] [CrossRef] [PubMed]
- Thaker, A.I.; Rao, M.S.; Bishnupuri, K.S.; Kerr, T.A.; Foster, L.; Marinshaw, J.M.; Newberry, R.D.; Stenson, W.F.; Ciorba, M.A. IDO1 Metabolites Activate β-Catenin Signaling to Promote Cancer Cell Proliferation and Colon Tumorigenesis in Mice. Gastroenterology 2013, 145, 416–425.e4. [Google Scholar] [CrossRef] [PubMed]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.; Ron, D.; Mellor, A.L. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Capuk, O.; Patel, S.M.; Sun, D. The Role of Metabolic Plasticity of Tumor-Associated Macrophages in Shaping the Tumor Microenvironment Immunity. Cancers 2022, 14, 3331. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, K.; Verhoef, E.I.; Smid, M.; Böttcher, R.; Jenster, G.W.; Debets, R.; Van Leenders, G.J.L.H. Epithelial–Mesenchymal Transition in Human Prostate Cancer Demonstrates Enhanced Immune Evasion Marked by IDO1 Expression. Cancer Res. 2018, 78, 4671–4679. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Zhang, Z.; Guo, Y.; Wu, Y.; Wang, R.; Wang, L.; Mao, S.; Yao, X. Overexpression of Indoleamine 2,3-Dioxygenase 1 Promotes Epithelial-Mesenchymal Transition by Activation of the IL-6/STAT3/PD-L1 Pathway in Bladder Cancer. Transl. Oncol. 2019, 12, 485–492. [Google Scholar] [CrossRef]
- Mondal, A.; Smith, C.; DuHadaway, J.B.; Sutanto-Ward, E.; Prendergast, G.C.; Bravo-Nuevo, A.; Muller, A.J. IDO1 Is an Integral Mediator of Inflammatory Neovascularization. EBioMedicine 2016, 14, 74–82. [Google Scholar] [CrossRef]
- Mautino, M.R.; Kumar, S.; Zhuang, H.; Waldo, J.; Jaipuri, F.; Potturi, H.; Brincks, E.; Adams, J.; Marcinowicz, A.; Allen, C.V.; et al. Abstract 4076: A Novel Prodrug of Indoximod with Enhanced Pharmacokinetic Properties. Cancer Res. 2017, 77, 4076. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, J.; Xu, J.; Moharil, P.; Chen, J.; Xu, J.; Zhu, J.; Li, J.; Huang, Y.; Xu, P.; et al. Dual Functional Immunostimulatory Polymeric Prodrug Carrier with Pendent Indoximod for Enhanced Cancer Immunochemotherapy. Acta Biomater. 2019, 90, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Oliver, T.; Rowe, M.; Thomas, S.; Zakharia, Y.; Gilman, P.B.; Muller, A.J.; Prendergast, G.C. Indoximod: An Immunometabolic Adjuvant That Empowers T Cell Activity in Cancer. Front. Oncol. 2018, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Mautino, M.R.; Link, C.J.; Vahanian, N.N.; Adams, J.T.; Allen, C.V.; Sharma, M.D.; Johnson, T.S.; Munn, D. Abstract 5023: Synergistic Antitumor Effects of Combinatorial Immune Checkpoint Inhibition with Anti-PD-1/PD-L Antibodies and the IDO Pathway Inhibitors NLG-919 and Indoximod in the Context of Active Immunotherapy. Cancer Res. 2014, 74, 5023. [Google Scholar] [CrossRef]
- Hermanowicz, J.M.; Szymanowska, A.; Sieklucka, B.; Czarnomysy, R.; Pawlak, K.; Bielawska, A.; Bielawski, K.; Kalafut, J.; Przybyszewska, A.; Surazynski, A.; et al. Exploration of Novel Heterofused 1,2,4-Triazine Derivative in Colorectal Cancer. J. Enzym. Inhib. Med. Chem. 2021, 36, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, J.M.; Pawlak, K.; Sieklucka, B.; Czarnomysy, R.; Kwiatkowska, I.; Kazberuk, A.; Surazynski, A.; Mojzych, M.; Pawlak, D. MM-129 as a Novel Inhibitor Targeting PI3K/AKT/mTOR and PD-L1 in Colorectal Cancer. Cancers 2021, 13, 3203. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, J.M.; Kalaska, B.; Pawlak, K.; Sieklucka, B.; Miklosz, J.; Mojzych, M.; Pawlak, D. Preclinical Toxicity and Safety of MM-129—First-in-Class BTK/PD-L1 Inhibitor as a Potential Candidate against Colon Cancer. Pharmaceutics 2021, 13, 1222. [Google Scholar] [CrossRef]
- Avci, M.E.; Keskus, A.G.; Targen, S.; Isilak, M.E.; Ozturk, M.; Atalay, R.C.; Adams, M.M.; Konu, O. Development of a Novel Zebrafish Xenograft Model in Ache Mutants Using Liver Cancer Cell Lines. Sci. Rep. 2018, 8, 1570. [Google Scholar] [CrossRef]
- Al-Samadi, A.; Tuomainen, K.; Kivimäki, A.; Salem, A.; Al-Kubati, S.; Hyytiäinen, A.; Parikka, M.; Mesimäki, K.; Wilkman, T.; Mäkitie, A.; et al. PCR-Based Zebrafish Model for Personalised Medicine in Head and Neck Cancer. J. Transl. Med. 2019, 17, 235. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Shvedova, A.A.; Kawai, K.; Tyurin, V.A.; Kommineni, C.; Quinn, P.J.; Schor, N.F.; Fabisiak, J.P.; Kagan, V.E. Phospholipid Signaling in Apoptosis: Peroxidation and Externalization of Phosphatidylserine. Toxicology 2000, 148, 93–101. [Google Scholar] [CrossRef]
- Kwiatkowska, I.; Hermanowicz, J.M.; Iwinska, Z.; Kowalczuk, K.; Iwanowska, J.; Pawlak, D. Zebrafish-An Optimal Model in Experimental Oncology. Molecules 2022, 27, 4223. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Emergence of Zebrafish Models in Oncology for Validating Novel Anticancer Drug Targets and Nanomaterials. Drug Discov. Today 2013, 18, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Nagaraju, R.; Hurlstone, A. Zebrafish: A New Companion for Translational Research in Oncology. Clin. Cancer Res. 2015, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Bergamo, A.; Carpi, S.; Donnini, S.; Notarbartolo Di Villarosa, M.; Serpe, L.; Lisi, L. Preclinical Models in Oncological Pharmacology: Limits and Advantages. Pharmadvances 2021, 3, 402–420. [Google Scholar] [CrossRef]
- Brincks, E.L.; Adams, J.; Wang, L.; Turner, B.; Marcinowicz, A.; Ke, J.; Essmann, M.; Mautino, L.M.; Allen, C.V.; Kumar, S.; et al. Indoximod Opposes the Immunosuppressive Effects Mediated by IDO and TDO via Modulation of AhR Function and Activation of mTORC1. Oncotarget 2020, 11, 2438–2461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jaipuri, F.A.; Waldo, J.P.; Potturi, H.; Marcinowicz, A.; Adams, J.; Van Allen, C.; Zhuang, H.; Vahanian, N.; Link, C.; et al. Discovery of Indoximod Prodrugs and Characterization of Clinical Candidate NLG802. Eur. J. Med. Chem. 2020, 198, 112373. [Google Scholar] [CrossRef] [PubMed]
- Frydrychowicz, M.; Boruczkowski, M.; Kolecka-Bednarczyk, A.; Dworacki, G. The Dual Role of Treg in Cancer. Scand. J. Immunol. 2017, 86, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhuri, R.; Eil, R.L.; Restifo, N.P. The Interplay of Effector and Regulatory T Cells in Cancer. Curr. Opin. Immunol. 2015, 33, 101–111. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T Cells in Cancer Immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Kwiatkowska, I.; Hermanowicz, J.M.; Przybyszewska-Podstawka, A.; Pawlak, D. Not Only Immune Escape—The Confusing Role of the TRP Metabolic Pathway in Carcinogenesis. Cancers 2021, 13, 2667. [Google Scholar] [CrossRef]
- Dee, C.T.; Nagaraju, R.T.; Athanasiadis, E.I.; Gray, C.; Fernandez Del Ama, L.; Johnston, S.A.; Secombes, C.J.; Cvejic, A.; Hurlstone, A.F.L. CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell–like Populations and Diverse Mononuclear Phagocytes. J. Immunol. 2016, 197, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Hammarén, M.M.; Oksanen, K.E.; Nisula, H.M.; Luukinen, B.V.; Pesu, M.; Rämet, M.; Parikka, M. Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish. PLoS Pathog. 2014, 10, e1004190. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; Houseright, R.A.; Graves, A.L.; Golenberg, N.; Korte, B.G.; Miskolci, V.; Huttenlocher, A. Metformin Modulates Innate Immune-Mediated Inflammation and Early Progression of NAFLD-Associated Hepatocellular Carcinoma in Zebrafish. J. Hepatol. 2019, 70, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, V.; Rebelo De Almeida, C.; Maia-Gil, M.; Sobral, D.; Domingues, M.; Martinez-Lopez, M.; De Almeida Fuzeta, M.; Silva, C.; Grosso, A.R.; Fior, R. Innate Immune Evasion Revealed in a Colorectal Zebrafish Xenograft Model. Nat. Commun. 2021, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The Use of Zebrafish to Understand Immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.M.; Loynes, C.A.; Whyte, M.K.B.; Renshaw, S.A. Zebrafish as a Model for the Study of Neutrophil Biology. J. Leukoc. Biol. 2013, 94, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Fedelis, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-Catabolizing Enzymes—Party of Three. Front. Immunol. 2014, 5, 485. [Google Scholar] [CrossRef]
- Majewski, M.; Kasica, N.; Jakimiuk, A.; Podlasz, P. Toxicity and Cardiac Effects of Acute Exposure to Tryptophan Metabolites on the Kynurenine Pathway in Early Developing Zebrafish (Danio Rerio) Embryos. Toxicol. Appl. Pharmacol. 2018, 341, 16–29. [Google Scholar] [CrossRef]
- Giacomini, A.C.V.V.; Piassetta, A.S.; Genario, R.; Bonan, C.D.; Piato, A.; Barcellos, L.J.G.; De Abreu, M.S. Tryptophan Alleviates Neuroendocrine and Behavioral Responses to Stress in Zebrafish. Behav. Brain Res. 2020, 378, 112264. [Google Scholar] [CrossRef]
- Siddiqui, T.; Bhattarai, P.; Popova, S.; Cosacak, M.I.; Sariya, S.; Zhang, Y.; Mayeux, R.; Tosto, G.; Kizil, C. KYNA/Ahr Signaling Suppresses Neural Stem Cell Plasticity and Neurogenesis in Adult Zebrafish Model of Alzheimer’s Disease. Cells 2021, 10, 2748. [Google Scholar] [CrossRef]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of Indoleamine 2,3-Dioxygenase, an Immunoregulatory Target of the Cancer Suppression Gene Bin1, Potentiates Cancer Chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Fan, H.; Shi, L.; Deng, Y.; Zhao, L.; Xiang, M.; Xu, Y.; Jiang, X.; Wang, G.; et al. IDO-Inhibitor Potentiated Immunogenic Chemotherapy Abolishes Primary Tumor Growth and Eradicates Metastatic Lesions by Targeting Distinct Compartments within Tumor Microenvironment. Biomaterials 2021, 269, 120388. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Xu, Z.; Chen, X.; Zhu, G. A Cisplatin-Loaded Immunochemotherapeutic Nanohybrid Bearing Immune Checkpoint Inhibitors for Enhanced Cervical Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 3426–3430. [Google Scholar] [CrossRef]
- Santhanam, S.; Alvarado, D.M.; Ciorba, M.A. Therapeutic Targeting of Inflammation and Tryptophan Metabolism in Colon and Gastrointestinal Cancer. Transl. Res. 2016, 167, 67–79. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Huang, S.; Du, J.; Huang, C. Screening of Anti-Cancer Agent Using Zebrafish: Comparison with the MTT Assay. Biochem. Biophys. Res. Commun. 2012, 422, 85–90. [Google Scholar] [CrossRef]
- Hill, M.; Pereira, V.; Chauveau, C.; Zagani, R.; Remy, S.; Tesson, L.; Mazal, D.; Ubillos, L.; Brion, R.; Ashgar, K.; et al. Heme Oxygenase-1 Inhibits Rat and Human Breast Cancer Cell Proliferation: Mutual Cross Inhibition with Indoleamine 2,3-dioxygenase. FASEB J. 2005, 19, 1957–1968. [Google Scholar] [CrossRef]
- Maletzki, C.; Scheinpflug, P.; Witt, A.; Klar, E.; Linnebacher, M. Targeting Immune-Related Molecules in Cancer Therapy: A Comprehensive In Vitro Analysis on Patient-Derived Tumor Models. BioMed Res. Int. 2019, 2019, 4938285. [Google Scholar] [CrossRef]
- Xu, J.; Ren, X.; Guo, T.; Sun, X.; Chen, X.; Patterson, L.H.; Li, H.; Zhang, J. NLG919/Cyclodextrin Complexation and Anti-Cancer Therapeutic Benefit as a Potential Immunotherapy in Combination with Paclitaxel. Eur. J. Pharm. Sci. 2019, 138, 105034. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van Den Eynde, B.J. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Narayanankutty, A. PI3K/Akt/mTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. CDT 2019, 20, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Alvarado, D.; Khouri, A.; Dieckgraefe, B.; Bishnupuri, K.; Ciorba, M. PD-236 Defining the Signaling Pathways and Functional Role for Kynurenine Metabolites in the Normal and Neoplastic Colon Epithelium. Inflamm. Bowel Dis. 2017, 23, S77–S78. [Google Scholar] [CrossRef]
- Maleki Vareki, S.; Chen, D.; Di Cresce, C.; Ferguson, P.J.; Figueredo, R.; Pampillo, M.; Rytelewski, M.; Vincent, M.; Min, W.; Zheng, X.; et al. IDO Downregulation Induces Sensitivity to Pemetrexed, Gemcitabine, FK866, and Methoxyamine in Human Cancer Cells. PLoS ONE 2015, 10, e0143435. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.; He, Y.; Yi, Y.; Liao, S.; Wang, Z.; Du, J. Curcumin inhibiting the expression of indoleamine 2,3-dioxygenase induced by IFN-gamma in cancer cells. Zhong Yao Cai 2008, 31, 1207–1211. [Google Scholar]
- Zheng, Q.; Gan, G.; Gao, X.; Luo, Q.; Chen, F. Targeting the IDO-BCL2A1-Cytochrome c Pathway Promotes Apoptosis in Oral Squamous Cell Carcinoma. OTT 2021, 14, 1673–1687. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Zhang, X.; Ding, Y.; Du, Q.; Hu, R. 1-L-MT, an IDO Inhibitor, Prevented Colitis-associated Cancer by Inducing CDC20 Inhibition-mediated Mitotic Death of Colon Cancer Cells. Int. J. Cancer 2018, 143, 1516–1529. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, J.; Lin, Y.; Luo, X.; Lin, H.; Lin, H.; Gao, J. A Dual-Responsive Doxorubicin–Indoximod Conjugate for Programmed Chemoimmunotherapy. RSC Chem. Biol. 2022, 3, 853–858. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. PSF Knockdown Enhances Apoptosis via Downregulation of LC3B in Human Colon Cancer Cells. BioMed Res. Int. 2013, 2013, 204973. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Huang, C.-Y.; Hung, C.-S.; Chen, W.-Y.; Wei, P.-L. GRP78 Mediates the Therapeutic Efficacy of Curcumin on Colon Cancer. Tumor Biol. 2015, 36, 633–641. [Google Scholar] [CrossRef]
- Baartzes, N.; Szabo, C.; Cenariu, M.; Imre-Lucaci, F.; Dorneanu, S.A.; Fischer-Fodor, E.; Smith, G.S. In Vitro Antitumour Activity of Two Ferrocenyl Metallodendrimers in a Colon Cancer Cell Line. Inorg. Chem. Commun. 2018, 98, 75–79. [Google Scholar] [CrossRef]
- Liou, J.-Y.; Aleksic, N.; Chen, S.-F.; Han, T.-J.; Shyue, S.-K.; Wu, K.K. Mitochondrial Localization of Cyclooxygenase-2 and Calcium-Independent Phospholipase A2 in Human Cancer Cells: Implication in Apoptosis Resistance. Exp. Cell Res. 2005, 306, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cesario, A.; Rocca, B.; Rutella, S. The Interplay between Indoleamine 2,3-Dioxygenase 1 (IDO1) and Cyclooxygenase (COX)-2 In Chronic Inflammation and Cancer. CMC 2011, 18, 2263–2271. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Nik, M.E.; Malaekeh-Nikouei, B.; Amin, M.; Hatamipour, M.; Teymouri, M.; Sadeghnia, H.R.; Iranshahi, M.; Jaafari, M.R. Liposomal Formulation of Galbanic Acid Improved Therapeutic Efficacy of Pegylated Liposomal Doxorubicin in Mouse Colon Carcinoma. Sci. Rep. 2019, 9, 9527. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Porav, S.; Banciu, M.; Porfire, A. Co-Delivery of Curcumin and Doxorubicin in PEGylated Liposomes Favored the Antineoplastic C26 Murine Colon Carcinoma Microenvironment. Drug Deliv. Transl. Res. 2019, 9, 260–272. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer Nanoparticles for Colorectal Cancer Applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Wu, P.; Yao, S.; Wang, X.; Yang, L.; Wang, S.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, S.; et al. Oral Administration of Nanoformulated Indoximod Ameliorates Ulcerative Colitis by Promoting Mitochondrial Function and Mucosal Healing. Int. J. Pharm. 2023, 637, 122813. [Google Scholar] [CrossRef]
- Calleja, P.; Irache, J.M.; Zandueta, C.; Martínez-Oharriz, C.; Espuelas, S. A Combination of Nanosystems for the Delivery of Cancer Chemoimmunotherapeutic Combinations: 1-Methyltryptophan Nanocrystals and Paclitaxel Nanoparticles. Pharmacol. Res. 2017, 126, 77–83. [Google Scholar] [CrossRef]
- Zang, X.; Song, J.; Yi, X.; Piyu, J. Polymeric Indoximod Based Prodrug Nanoparticles with Doxorubicin Entrapment for Inducing Immunogenic Cell Death and Improving the Immunotherapy of Breast Cancer. J. Mater. Chem. B 2022, 10, 2019–2027. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, I.; Hermanowicz, J.M.; Czarnomysy, R.; Surażyński, A.; Kowalczuk, K.; Kałafut, J.; Przybyszewska-Podstawka, A.; Bielawski, K.; Rivero-Müller, A.; Mojzych, M.; et al. Assessment of an Anticancer Effect of the Simultaneous Administration of MM-129 and Indoximod in the Colorectal Cancer Model. Cancers 2024, 16, 122. https://doi.org/10.3390/cancers16010122
Kwiatkowska I, Hermanowicz JM, Czarnomysy R, Surażyński A, Kowalczuk K, Kałafut J, Przybyszewska-Podstawka A, Bielawski K, Rivero-Müller A, Mojzych M, et al. Assessment of an Anticancer Effect of the Simultaneous Administration of MM-129 and Indoximod in the Colorectal Cancer Model. Cancers. 2024; 16(1):122. https://doi.org/10.3390/cancers16010122
Chicago/Turabian StyleKwiatkowska, Iwona, Justyna Magdalena Hermanowicz, Robert Czarnomysy, Arkadiusz Surażyński, Krystyna Kowalczuk, Joanna Kałafut, Alicja Przybyszewska-Podstawka, Krzysztof Bielawski, Adolfo Rivero-Müller, Mariusz Mojzych, and et al. 2024. "Assessment of an Anticancer Effect of the Simultaneous Administration of MM-129 and Indoximod in the Colorectal Cancer Model" Cancers 16, no. 1: 122. https://doi.org/10.3390/cancers16010122
APA StyleKwiatkowska, I., Hermanowicz, J. M., Czarnomysy, R., Surażyński, A., Kowalczuk, K., Kałafut, J., Przybyszewska-Podstawka, A., Bielawski, K., Rivero-Müller, A., Mojzych, M., & Pawlak, D. (2024). Assessment of an Anticancer Effect of the Simultaneous Administration of MM-129 and Indoximod in the Colorectal Cancer Model. Cancers, 16(1), 122. https://doi.org/10.3390/cancers16010122








