Antibiotic Exposure Concurrently with Anti-PD1 Blockade Therapy Reduces Overall Survival in Patients with Child–Pugh Class A Advanced Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Variables and Outcomes
2.3. Statistical Analysis
3. Results
- Patient characteristics:
- Treatment interventions:
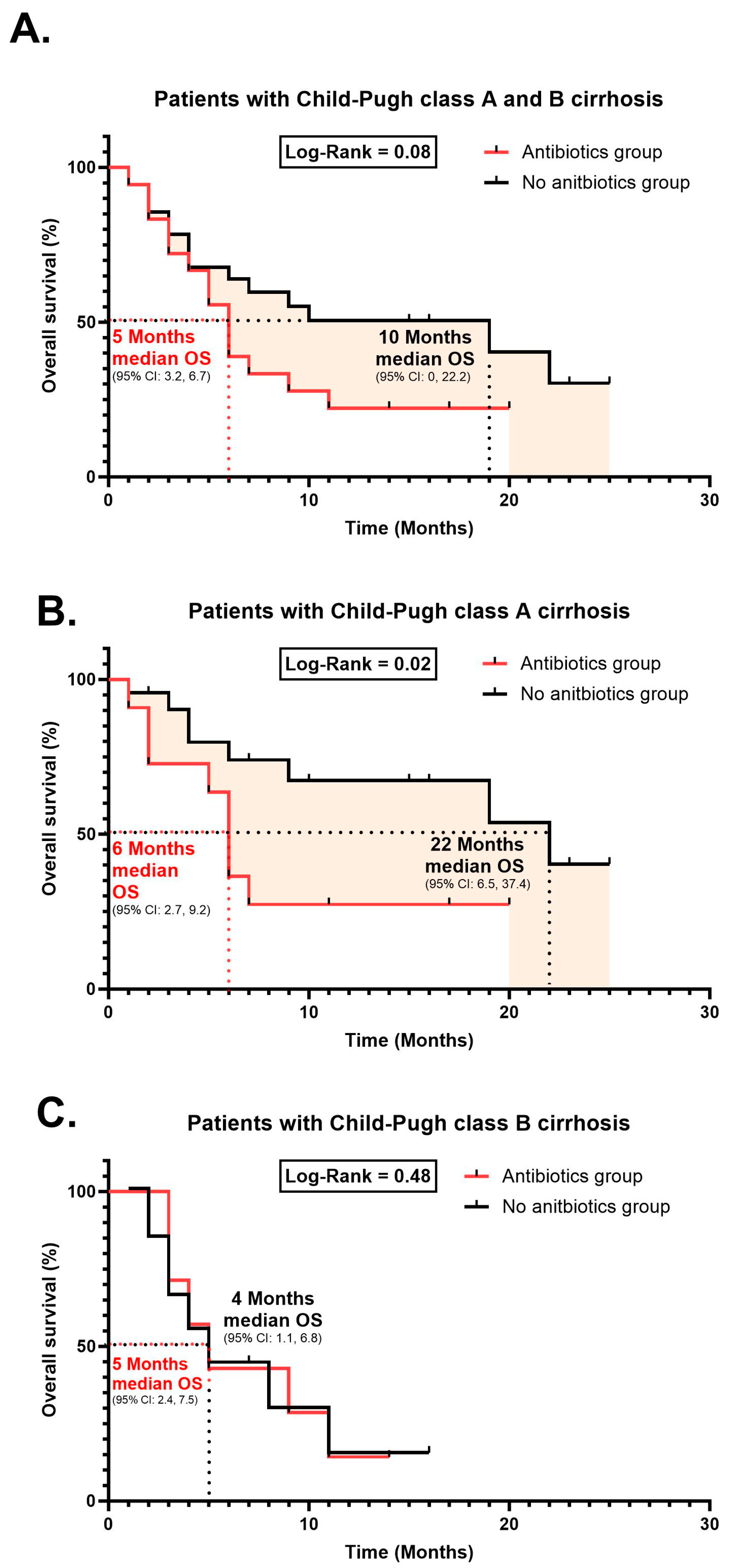
- Treatment response and survival curves
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Pierrard, J.; Seront, E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy—A systematic review. Curr. Oncol. 2019, 26, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hayase, E.; Jenq, R.R. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.; Zheng, B.; Qiu, X.; Wang, H.; Chen, L. Modulation of gut microbiota to enhance effect of checkpoint inhibitor immunotherapy. Front. Immunol. 2021, 12, 669150. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; Gnoni, A.; Licchetta, A.; Delcuratolo, S.; Memeo, R.; Solimando, A.G.; Argentiero, A. Emerging role of immune checkpoint inhibitors in hepatocellular carcinoma. Medicina 2019, 55, 698. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993. [Google Scholar] [PubMed]
- Sperandio, R.C.; Pestana, R.C.; Miyamura, B.V.; Kaseb, A.O. Hepatocellular carcinoma immunotherapy. Annu. Rev. Med. 2022, 73, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- de Castria, T.B.; Khalil, D.N.; Harding, J.J.; O’Reilly, E.M.; Abou-Alfa, G.K. Tremelimumab and durvalumab in the treatment of unresectable, advanced hepatocellular carcinoma. Future Oncol. 2022, 18, 3769–3782. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Bouattour, M.; Lim, H.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; Yau, T. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Enrico, D.; Paci, A.; Chaput, N.; Karamouza, E.; Besse, B. Antidrug antibodies against immune checkpoint blockers: Impairment of drug efficacy or indication of immune activation? Clin. Cancer Res. 2020, 26, 787–792. [Google Scholar] [CrossRef]
- Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Response prediction in immune checkpoint inhibitor immunotherapy for advanced hepatocellular carcinoma. Cancers 2021, 13, 1607. [Google Scholar] [CrossRef]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.; Cerdà-Cuéllar, M.; Martínez, V. Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes 2015, 6, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Giordan, Q.; Salleron, J.; Vallance, C.; Moriana, C.; Clement-Duchene, C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front. Immunol. 2021, 12, 716317. [Google Scholar] [CrossRef]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.-A. NSCLC immunotherapy efficacy and antibiotic use: A systematic review and meta-analysis. J. Thorac. Oncol. 2020, 15, 1147–1159. [Google Scholar] [CrossRef]
- Chambers, L.M.; Michener, C.M.; Rose, P.G.; Reizes, O.; Yao, M.; Vargas, R. Impact of antibiotic treatment on immunotherapy response in women with recurrent gynecologic cancer. Gynecol. Oncol. 2021, 161, 211–220. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Impact of antibiotic therapy and metabolic parameters in non-small cell lung cancer patients receiving checkpoint inhibitors. J. Clin. Med. 2021, 10, 1251. [Google Scholar] [CrossRef]
- Cheung, K.S.; Lam, L.K.; Seto, W.K.; Leung, W.K. Use of antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in hepatocellular carcinoma. Liver Cancer 2021, 10, 606–614. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Gao, P.; Song, Y.-X.; Xu, Y.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Wang, Z.-N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: A pooled analysis of 2740 cancer patients. Oncoimmunology 2019, 8, e1665973. [Google Scholar] [CrossRef]
- Chakraborty, R.; Lam, V.; Kommineni, S.; Stromich, J.; Hayward, M.; Kristich, C.J.; Salzman, N.H. Ceftriaxone administration disrupts intestinal homeostasis, mediating noninflammatory proliferation and dissemination of commensal enterococci. Infect. Immun. 2018, 86, e00674-18. [Google Scholar] [CrossRef]
- Bhalodi, A.A.; van Engelen, T.S.; Virk, H.S.; Wiersinga, W.J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 2019, 74, i6–i15. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, M.M.; Gent, J.F.; Kong, Y.; Halpin, A.L.; Pineles, L.; Harris, A.D.; Johnson, J.K. Gastrointestinal microbiota disruption and risk of colonization with carbapenem-resistant Pseudomonas aeruginosa in intensive care unit patients. Clin. Infect. Dis. 2019, 69, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Bergan, T.; Nord, C.; Thorsteinsson, S. Effect of meropenem on the intestinal microflora. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Lekang, K.; Shekhar, S.; Berild, D.; Petersen, F.C.; Winther-Larsen, H.C. Effects of different amoxicillin treatment durations on microbiome diversity and composition in the gut. PLoS ONE 2022, 17, e0275737. [Google Scholar] [CrossRef]
- Burdet, C.; Nguyen, T.T.; Duval, X.; Ferreira, S.; Andremont, A.; Guedj, J.; Mentré, F.; Group, D.-C.-S. Impact of antibiotic gut exposure on the temporal changes in microbiome diversity. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Zitvogel, L.; Kroemer, G.; Routy, B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 2019, 10, 424–428. [Google Scholar] [CrossRef]
| Variable | Total | No Antibiotics | Antibiotics | p Value |
|---|---|---|---|---|
| Age (years) | 72 (65, 79) | 70 (62, 78) | 73 (67, 81) | 0.23 |
| Male sex—n (%) | 51 (86.4) | 35 (89.7) | 16 (80) | 0.30 |
| Body mass index BMI (kg/m2) | 25.8 ± 5.3 | 26.5 ± 5.4 | 24.5 ± 4.9 | 0.32 |
| ECOG performance score—n (%) | ||||
| 0–1 | 42 (71.2) | 26 (66.7) | 16 (80) | 0.28 |
| 2–3 | 17 (28.8) | 13 (33.3) | 4 (20) | |
| Child-Pugh class—n (%) | ||||
| A | 38 (64.4) | 25 (64.1) | 13 (65) | 0.94 |
| B | 21 (35.6) | 14 (35.9) | 7 (35) | |
| BCLC stage—n (%) | ||||
| B | 17 (28.8) | 11 (28.2) | 6 (30) | 0.88 |
| C | 42 (71.2) | 28 (71.8) | 14 (70) | |
| α-feto protein level—n (%) | ||||
| Normal | 20 (33.9) | 16 (41) | 4 (20) | 0.10 |
| Abnormal | 39 (66.1) | 23 (59) | 16 (80) | |
| Etiology of cirrhosis—n (%) * | ||||
| Hepatitis B | 16 (27.1) | 12 (30) | 4 (20) | 0.25 |
| Hepatitis C | 12 (20.3) | 6 (15.4) | 6 (30) | |
| Non-viral | 30 (50) | 21 (53.8) | 9 (45) | |
| Comorbidities—n (%) | ||||
| Cardiac disease | 11 (18.6) | 6 (15.4) | 5 (25) | 0.13 |
| Diabetes mellitus | 32 (54.2) | 22 (56.4) | 10 (50) | 0.64 |
| Chronic kidney disease | 5 (8.5) | 3 (7.7) | 2 (10) | 0.76 |
| Hypertension | 26 (44.1) | 15 (38.5) | 11 (55) | 0.22 |
| Cirrhosis | 50 (84.7) | 34 (87.2) | 16 (80) | 0.46 |
| Antibiotic Types | Reason of Using | N (%) |
|---|---|---|
| Ceftriaxone | Spontaneous Bacterial Peritonitis (SBP) | 6 (30%) |
| Urinary Tract Infection (UTI) | 1 (5%) | |
| Ciprofloxacin | UTI | 5 (25%) |
| Piperacillin/tazobactam | Sepsis | 3 (15%) |
| Meropenem | 1 (5%) | |
| Augmentin | Upper Respiratory Tract Infections (URTIs) | 2 (10%) |
| Moxifloxacin | Pneumonia | 2 (10%) |
| Total | 20 (100%) | |
| Variable | Total | No Antibiotics (N = 39) | Antibiotics (N = 20) | p Value |
|---|---|---|---|---|
| Nivolumab Therapy—n (%) | ||||
| First line | 8 (13.6) | 6 (15.4) | 2 (10) | 0.12 |
| Second line | 49 (83.1) | 33 (84.6) | 16 (80) | |
| Third line | 2 (3.4) | 0 | 2 (10) | |
| Number of cycles | 7 | 7 | 6.5 | |
| Beta blockers—n (%) | 19 (32.2) | 11 (28.2) | 8 (40) | 0.39 |
| Oral hypoglycemics—n (%) | 11 (18.6) | 9 (23.1) | 2 (10) | 0.22 |
| Proton pump inhibitors—n (%) | 11 (18.6) | 7 (17.9) | 4 (20) | 0.84 |
| Steroids—n (%) | 20 (33.9) | 14 (35.9) | 6 (30) | 0.65 |
| Variable | Total | No antibiotics | Antibiotics | p Value |
|---|---|---|---|---|
| Response Based on mRECIST, n (%) | ||||
| CR | 1 (2.4%) | 1 (3.7%) | 0 | 0.87 |
| PR | 17 (41.5%) | 11 (40.7%) | 6 (42.9%) | |
| SD | 16 (39%) | 10 (37%) | 6 (42.9%) | |
| PD | 7 (17.1%) | 5 (18.5%) | 2 (14.3%) | |
| Mean follow-up period | 7.03 months | |||
| Dependent Variable: Time to Death or Last Contact | RR | 95% CI | p Value | |
|---|---|---|---|---|
| Antibiotic use | 3.30 | 1.04 | 10.39 | 0.04 |
| Gender | 0.62 | 0.14 | 2.63 | 0.51 |
| Age | 1.01 | 0.94 | 1.09 | 0.61 |
| Diabetes mellitus | 1.02 | 0.34 | 3.06 | 0.96 |
| Hypertension | 0.93 | 0.24 | 3.57 | 0.91 |
| Cardiac disease | 0.60 | 0.12 | 3.04 | 0.54 |
| Chronic kidney disease | 0.87 | 0.08 | 9.04 | 0.91 |
| Presence of cirrhosis | 1.40 | 0.38 | 5.05 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, K.; Alotaibi, F.M.; Alsugheir, F.; Aldawoud, M.; Alolayan, A.; Algarni, M.A.; Sabatin, F.; Mohammad, M.F.; Alosaimi, A.; Sanai, F.M.; et al. Antibiotic Exposure Concurrently with Anti-PD1 Blockade Therapy Reduces Overall Survival in Patients with Child–Pugh Class A Advanced Hepatocellular Carcinoma. Cancers 2024, 16, 133. https://doi.org/10.3390/cancers16010133
Alshammari K, Alotaibi FM, Alsugheir F, Aldawoud M, Alolayan A, Algarni MA, Sabatin F, Mohammad MF, Alosaimi A, Sanai FM, et al. Antibiotic Exposure Concurrently with Anti-PD1 Blockade Therapy Reduces Overall Survival in Patients with Child–Pugh Class A Advanced Hepatocellular Carcinoma. Cancers. 2024; 16(1):133. https://doi.org/10.3390/cancers16010133
Chicago/Turabian StyleAlshammari, Kanan, Faizah M. Alotaibi, Futoon Alsugheir, Mohammad Aldawoud, Ashwaq Alolayan, Mohammed Ahmad Algarni, Fouad Sabatin, Mohammad F. Mohammad, Abdulaziz Alosaimi, Faisal M. Sanai, and et al. 2024. "Antibiotic Exposure Concurrently with Anti-PD1 Blockade Therapy Reduces Overall Survival in Patients with Child–Pugh Class A Advanced Hepatocellular Carcinoma" Cancers 16, no. 1: 133. https://doi.org/10.3390/cancers16010133
APA StyleAlshammari, K., Alotaibi, F. M., Alsugheir, F., Aldawoud, M., Alolayan, A., Algarni, M. A., Sabatin, F., Mohammad, M. F., Alosaimi, A., Sanai, F. M., Odah, H., Alshehri, A. S., Aldibasi, O. S., Alrehaily, S., & Al Saleh, A. S. (2024). Antibiotic Exposure Concurrently with Anti-PD1 Blockade Therapy Reduces Overall Survival in Patients with Child–Pugh Class A Advanced Hepatocellular Carcinoma. Cancers, 16(1), 133. https://doi.org/10.3390/cancers16010133






