Enhanced Biomimetics of Three-Dimensional Osteosarcoma Models: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (a)
- Studies that included in vitro 3D cell culture models with explicit use of osteosarcoma cell lines (including humans, rodent, and canine species) and/or primary human cells;
- (b)
- Peer-reviewed journal articles reporting original research (as stated in (a)), published in English.
- (a)
- Use of in vitro 3D models that did not use osteosarcoma cell lines;
- (b)
- In vivo, in silico, xenografts, and two-dimensional (2D) experiments;
- (c)
- Review articles, commentaries, editorials, conference abstracts, non-full text, and non-English publications.
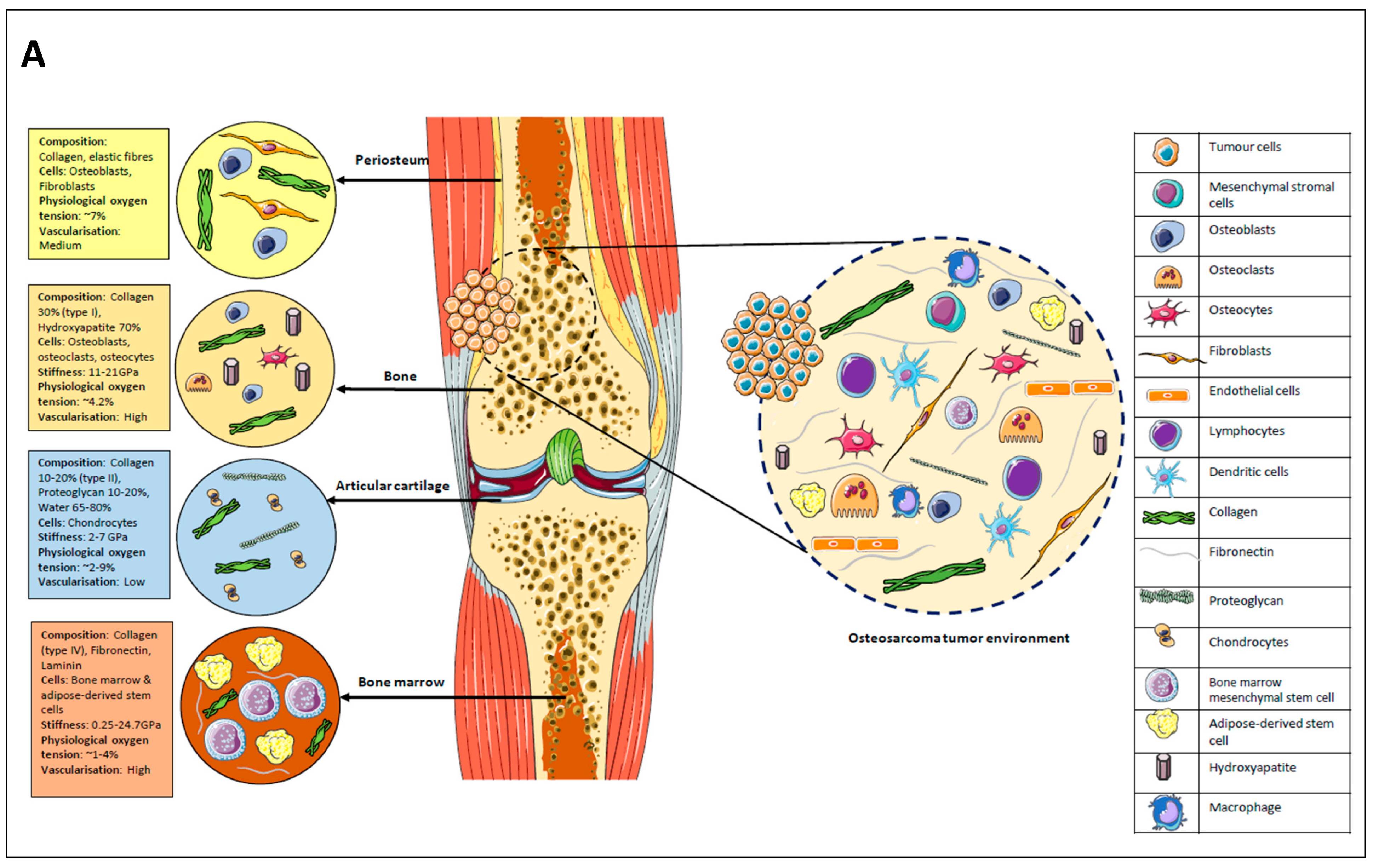
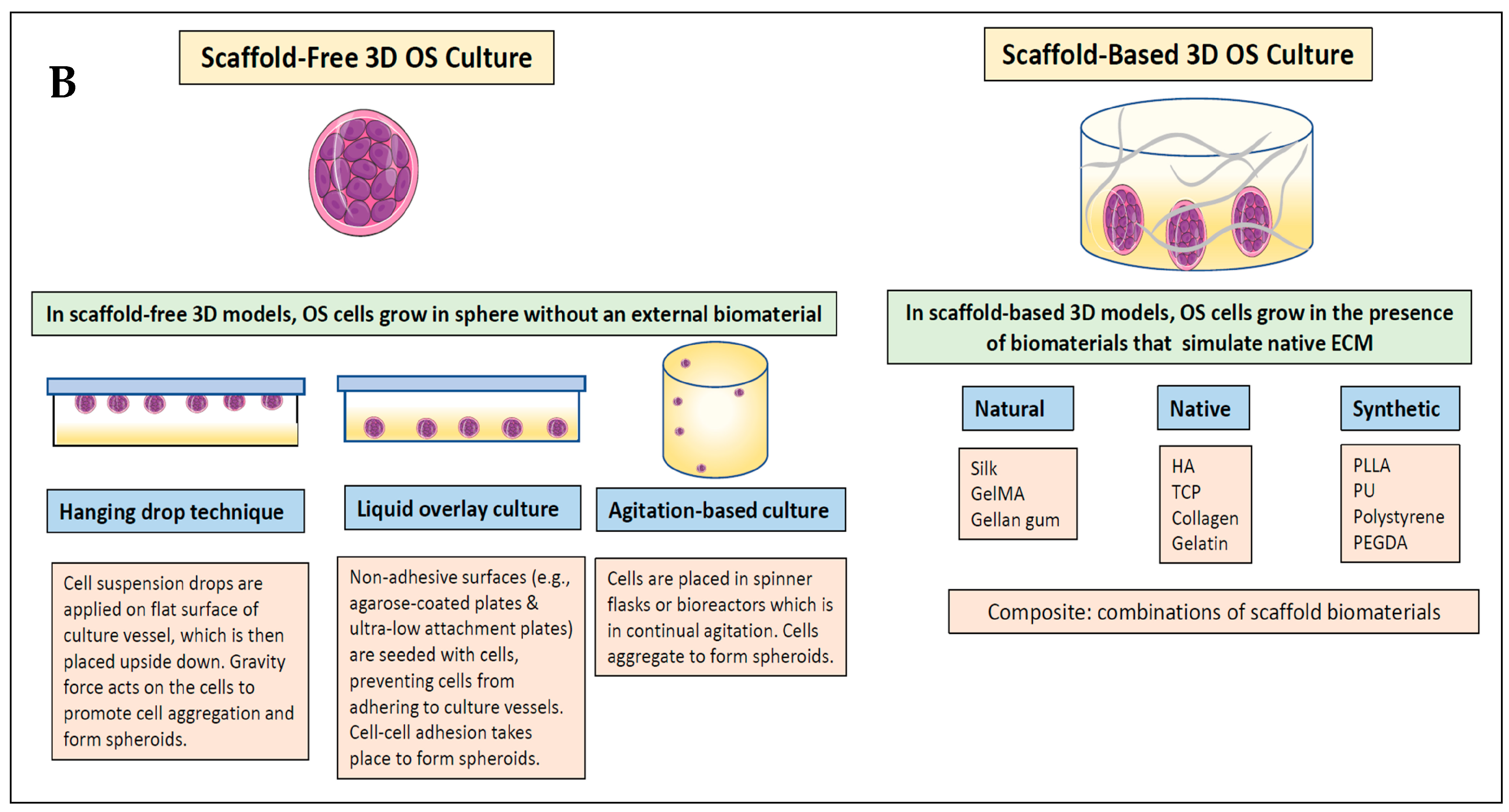
2.3. Definitions
2.4. Synthesising, Analysing, and Reporting Results
- (1)
- Biomimetic scaffolds used for 3D OS models.
- (2)
- Biomimetics of 3D OS models related to anticancer drug/therapy testing. This included comparing the differences in drug sensitivities between the 3D and 2D OS models and identifying any other biomimetic factors potentially influencing drug resistance.
- (3)
- Biomimetic response of stromal cells in 3D OS models.
3. Results
3.1. Flowchart for Study Selection
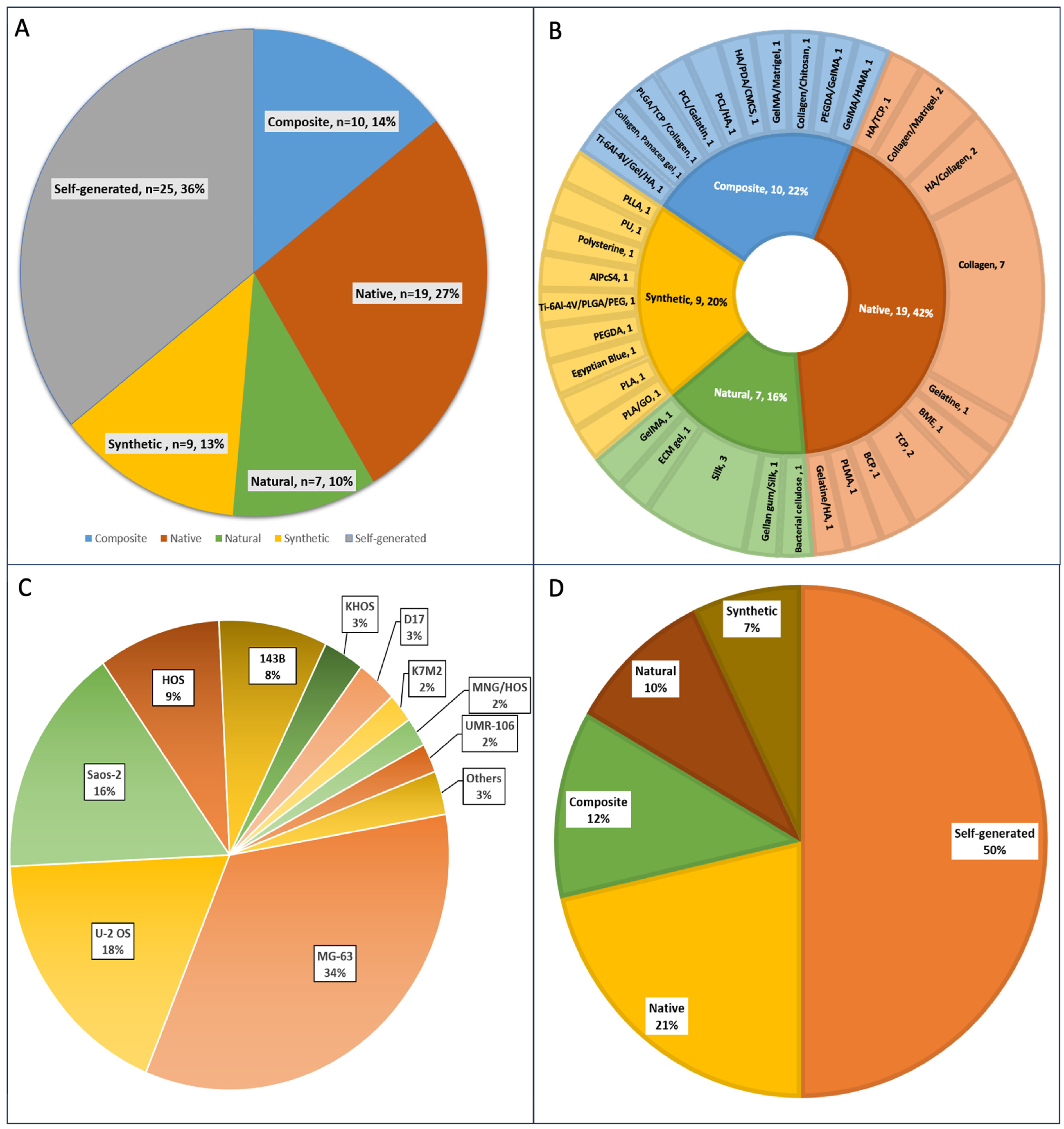
3.2. 3D Scaffolds Are Most Commonly Used to Engineer Osteosarcoma Models Compared to Self-Generated Spheroids
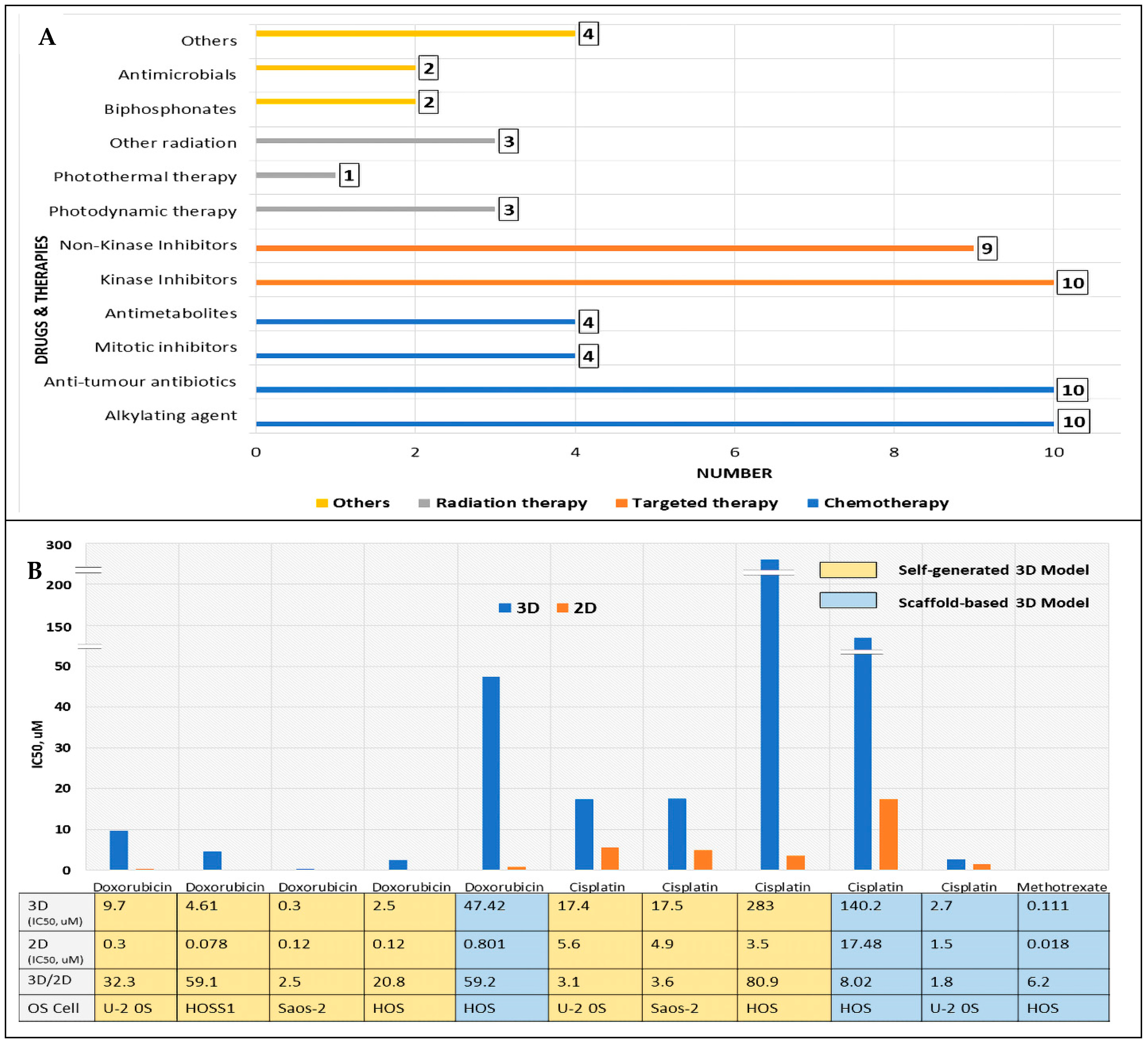
3.3. Three-Dimensional OS Models Are Utilised for Anticancer Drug/Therapy Screening
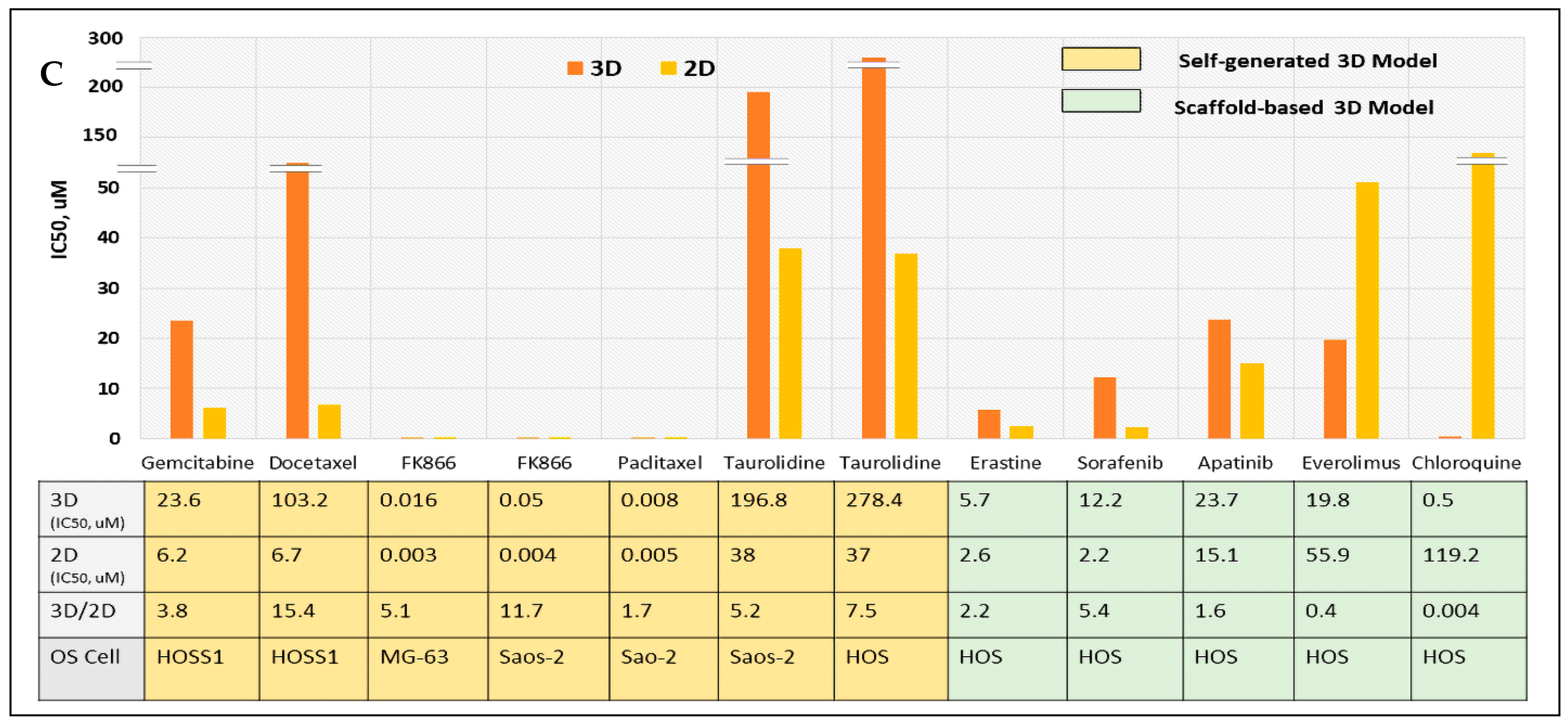
3.4. Increased Drug Resistance in Biomimetic 3D OS Models: 3D vs. 2D Comparison of Drug Sensitivity Based on IC50
3.5. Introducing Stromal Cells Can Induce Selective Anticancer Drug/Therapy Toxicity and Impact Other Biomimetic Responses
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gill, J.; Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021, 18, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Smith, H.; Beers, S.A.; Gray, J.; Kanczler, J.M. The role of pre-clinical 3-dimensional models of osteosarcoma. Int. J. Mol. Sci. 2020, 21, 5499. [Google Scholar] [CrossRef] [PubMed]
- Sitarski, A.M.; Fairfield, H.; Falank, C.; Reagan, M.R. 3D Tissue Engineered In Vitro Models of Cancer in Bone. ACS Biomater. Sci. Eng. 2018, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Pietrovito, L.; Leo, A.; Gori, V.; Lulli, M.; Parri, M.; Becherucci, V.; Piccini, L.; Bambi, F.; Taddei, M.L.; Chiarugi, P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018, 12, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-García, J.; Jubelin, C.; Loussouarn, A.; Goumard, M.; Griscom, L.; Renodon-Cornière, A.; Heymann, M.-F.; Heymann, D. In vitro three-dimensional cell cultures for bone sarcomas. J. Bone Oncol. 2021, 30, 100379. [Google Scholar] [CrossRef]
- Gao, S.; Shen, J.; Hornicek, F.; Duan, Z. Three-dimensional (3D) culture in sarcoma research and the clinical significance. Biofabrication 2017, 9, 32003. [Google Scholar] [CrossRef]
- Cortini, M.; Baldini, N.; Avnet, S. New Advances in the Study of Bone Tumors: A Lesson from the 3D Environment. Front. Physiol. 2019, 10, 814. [Google Scholar] [CrossRef]
- Wadman, M. FDA no longer has to require animal testing for new drugs. Science 2023, 379, 127–128. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Raimondi, L.; Salamanna, F.; Carina, V.; Costa, V.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Relevance of 3d culture systems to study osteosarcoma environment. J. Exp. Clin. Cancer Res. 2018, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Yang, M.; Fan, Z.; Li, S.; Gao, T.; Fang, Z. Associations of chemo- and radio-resistant phenotypes with the gap junction, adhesion and extracellular matrix in a three-dimensional culture model of soft sarcoma. J. Exp. Clin. Cancer Res. 2015, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Seo, O.W.; Lee, J.; Hulme, J.; An, S.S.A. Real-time monitoring of cisplatin cytotoxicity on three-dimensional spheroid tumor cells. Drug Des. Devel. Ther. 2016, 10, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Koons, N.; Amato, N.; Sauer, S.; Warshawsky, D.; Barkan, D.; Khanna, C. Assessing a Novel 3D Assay System for Drug Screening against OS Metastasis. Pharmaceuticals 2021, 14, 971. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Tommaso, F.; et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020, 39, 40. [Google Scholar] [CrossRef] [PubMed]
- León, I.E.; Cadavid-Vargas, J.F.; Resasco, A.; Maschi, F.; Ayala, M.A.; Carbone, C.; Etcheverry, S.B. In vitro and in vivo antitumor effects of the VO-chrysin complex on a new three-dimensional osteosarcoma spheroids model and a xenograft tumor in mice. J. Biol. Inorg. Chem. 2016, 21, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, M.; Wang, W.; Wan, P.; Chu, X.; Zheng, Y.; Yang, K.; Zhang, Y. Nitrogen-containing bisphosphonate-loaded micro-arc oxidation coating for biodegradable magnesium alloy pellets inhibits osteosarcoma through targeting of the mevalonate pathway. Acta Biomater. 2021, 121, 682–694. [Google Scholar] [CrossRef]
- Ma, H.; Dean, D.C.; Wei, R.; Hornicek, F.J.; Duan, Z. Cyclin-dependent kinase 7 (CDK7) is an emerging prognostic biomarker and therapeutic target in osteosarcoma. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X21995069. [Google Scholar] [CrossRef]
- Ma, H.; Seebacher, N.A.; Hornicek, F.J.; Duan, Z. Cyclin-dependent kinase 9 (CDK9) is a novel prognostic marker and therapeutic target in osteosarcoma. EBioMedicine 2019, 39, 182–193. [Google Scholar] [CrossRef]
- Martella, E.; Ferroni, C.; Guerrini, A.; Ballestri, M.; Columbaro, M.; Santi, S.; Sotgiu, G.; Serra, M.; Donati, D.M.; Lucarelli, E.; et al. Functionalized Keratin as Nanotechnology-Based Drug Delivery System for the Pharmacological Treatment of Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 3670. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Yuan, K.; Yang, S.; Zhang, S.; Li, H.; Tang, T. Multi-omics analysis based on 3D-bioprinted models innovates therapeutic target discovery of osteosarcoma. Bioact. Mater. 2022, 18, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; De Maria, R.; Malatesta, D.; Romanucci, M.; D’Anselmo, A.; Della Salda, L. Establishment of three-dimensional canine osteosarcoma cell lines showing vasculogenic mimicry and evaluation of biological properties after treatment with 17-AAG. Vet. Comp. Oncol. 2019, 17, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.V.; Gaspar, V.M.; Ferreira, L.P.; Mano, J.F. Hydrogel 3D In vitro tumor models for screening cell aggregation mediated drug response. Biomater. Sci. 2020, 8, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Seo, O.W.; Kim, M.; Hulme, J.; An, S.S.A. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. Onco Targets Ther. 2016, 9, 7207–7218. [Google Scholar] [CrossRef] [PubMed]
- Palubeckaitė, I.; Crooks, L.; Smith, D.P.; Cole, L.M.; Bram, H.; Le Maitre, C.; Clench, M.R.; Cross, N.A. Mass spectrometry imaging of endogenous metabolites in response to doxorubicin in a novel 3D osteosarcoma cell culture model. J. Mass Spectrom. 2019, 55, e4461. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.; Shah, M.; Gikas, P.; Briggs, T.; Roberts, S.J.; Cheema, U. Osteomimetic matrix components alter cell migration and drug response in a 3D tumour-engineered osteosarcoma model. Acta Biomater. 2019, 96, 247–257. [Google Scholar] [CrossRef]
- Pellegrini, E.; Desando, G.; Petretta, M.; Cellamare, A.; Cristalli, C.; Pasello, M.; Manara, M.C.; Grigolo, B.; Scotlandi, K. A 3D Collagen-Based Bioprinted Model to Study Osteosarcoma Invasiveness and Drug Response. Polymers 2022, 14, 4070. [Google Scholar] [CrossRef]
- Rimann, M.; Laternser, S.; Gvozdenovic, A.; Muff, R.; Fuchs, B.; Kelm, J.M.; Graf-Hausner, U. An in vitro osteosarcoma 3D microtissue model for drug development. J. Biotechnol. 2014, 189, 129–135. [Google Scholar] [CrossRef]
- Tan, P.H.S.; Chia, S.S.; Toh, S.L.; Goh, J.C.H.; Nathan, S.S. Three-dimensional spatial configuration of tumour cells confers resistance to chemotherapy independent of drug delivery. J. Tissue Eng. Regen. Med. 2013, 10, 637–646. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Li, X.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Establishment and Characterization of a Recurrent Osteosarcoma Cell Line: OSA 1777. J. Orthop. Res. 2019, 38, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Tornín, J.; Villasante, A.; Solé-Martí, X.; Ginebra, M.-P.; Canal, C. Osteosarcoma tissue-engineered model challenges oxidative stress therapy revealing promoted cancer stem cell properties. Free Radic. Biol. Med. 2021, 164, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.M.; Mickens, J.; Uskoković, V. Bisphosphonate-Functionalized Hydroxyapatite Nanoparticles for the Delivery of the Bromodomain Inhibitor JQ1 in the Treatment of Osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 25887–25904. [Google Scholar] [CrossRef] [PubMed]
- Dobos, A.; Steiger, W.; Theiner, D.; Gruber, P.; Lunzer, M.; Van Hoorick, J.; Van Vlierberghe, S.; Ovsianikov, A. Screening of two-photon activated photodynamic therapy sensitizers using a 3D osteosarcoma model. Analyst 2019, 144, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhao, J.; Yu, S.; Mao, Z.; Gao, C.; Zhu, Y.; Mao, C.; Zheng, L. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials 2019, 188, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Elie, J.; Feizbakhsh, O.; Desban, N.; Josselin, B.; Baratte, B.; Bescond, A.; Duez, J.; Fant, X.; Bach, S.; Marie, D.; et al. Design of new disubstituted imidazo[1,2-b]pyridazine derivatives as selective Haspin inhibitors. Synthesis, binding mode and anticancer biological evaluation. J. Enzym. Inhib. Med. Chem. 2020, 35, 1840–1853. [Google Scholar] [CrossRef] [PubMed]
- Komez, A.; Buyuksungur, A.; Antmen, E.; Swieszkowski, W.; Hasirci, N.; Hasirci, V. A two-compartment bone tumor model to investigate interactions between healthy and tumor cells. Biomed. Mater. 2020, 15, 35007. [Google Scholar] [CrossRef] [PubMed]
- Li Volsi, A.; Scialabba, C.; Vetri, V.; Cavallaro, G.; Licciardi, M.; Giammona, G. Near-Infrared Light Responsive Folate Targeted Gold Nanorods for Combined Photothermal-Chemotherapy of Osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 14453–14469. [Google Scholar] [CrossRef]
- Marshall, S.K.; Saelim, B.; Taweesap, M.; Pachana, V.; Panrak, Y.; Makchuchit, N.; Jaroenpakdee, P. Anti-EGFR Targeted Multifunctional I-131 Radio-Nanotherapeutic for Treating Osteosarcoma: In Vitro 3D Tumor Spheroid Model. Nanomaterials 2022, 12, 3517. [Google Scholar] [CrossRef]
- Monteiro, C.F.; Custódio, C.A.; Mano, J.F. Bioengineering a humanized 3D tri-culture osteosarcoma model to assess tumor invasiveness and therapy response. Acta Biomater. 2021, 134, 204–214. [Google Scholar] [CrossRef]
- Pierrevelcin, M.; Flacher, V.; Mueller, C.G.; Vauchelles, R.; Guerin, E.; Lhermitte, B.; Pencreach, E.; Reisch, A.; Muller, Q.; Doumard, L.; et al. Engineering Novel 3D Models to Recreate High-Grade Osteosarcoma and its Immune and Extracellular Matrix Microenvironment. Adv. Healthc. Mater. 2022, 11, e2200195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; León, I.E. In vitro and in vivo anticancer effects of two quinoline-platinum(II) complexes on human osteosarcoma models. Cancer Chemother. Pharmacol. 2019, 83, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Controlled release of soy isoflavones from multifunctional 3D printed bone tissue engineering scaffolds. Acta Biomater. 2020, 114, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Chia, S.S.; Toh, S.L.; Goh, J.C.; Nathan, S.S. The dominant role of IL-8 as an angiogenic driver in a three-dimensional physiological tumor construct for drug testing. Tissue Eng. Part A 2014, 20, 1758–1766. [Google Scholar] [CrossRef]
- Almela, T.; Brook, I.M.; Moharamzadeh, K. Development of three-dimensional tissue engineered bone-oral mucosal composite models. J. Mater. Sci. Mater. Med. 2016, 27, 65. [Google Scholar] [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F.; Lipreri, M.V.; Roncuzzi, L.; Columbaro, M.; Errani, C.; Longhi, A.; et al. The Release of Inflammatory Mediators from Acid-Stimulated Mesenchymal Stromal Cells Favours Tumour Invasiveness and Metastasis in Osteosarcoma. Cancers 2021, 13, 5855. [Google Scholar] [CrossRef]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Spentzos, D.; Hoffman, R.M.; Shi, H.; Duan, Z. Myc is a prognostic biomarker and potential therapeutic target in osteosarcoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922055. [Google Scholar] [CrossRef]
- Cai, B.; Huang, L.; Wang, J.; Sun, D.; Zhu, C.; Huang, Y.; Li, S.; Guo, Z.; Liu, L.; Feng, G.; et al. 3D Printed Multifunctional Ti6Al4V-Based Hybrid Scaffold for the Management of Osteosarcoma. Bioconjugate Chem. 2021, 32, 2184–2194. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Ricci, C.; Bongiorni, F.; Trombi, L.; D’Alessandro, D.; Danti, S.; Farè, S. An Osteosarcoma Model by 3D Printed Polyurethane Scaffold and In Vitro Generated Bone Extracellular Matrix. Cancers 2022, 14, 2003. [Google Scholar] [CrossRef]
- Kihara, T.; Umezu, C.; Sawada, K.; Furutani, Y. Osteogenic cells form mineralized particles, a few μm in size, in a 3D collagen gel culture. PeerJ 2019, 7, e7889. [Google Scholar] [CrossRef]
- Koski, C.; Sarkar, N.; Bose, S. Cytotoxic and osteogenic effects of crocin and bicarbonate from calcium phosphates for potential chemopreventative and anti-inflammatory applications in vitro and in vivo. J. Mater. Chem. B 2020, 8, 2048–2062. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Bastos, A.R.F.; Brancato, V.; Cerqueira, M.T.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Mechanical Property of Hydrogels and the Presence of Adipose Stem Cells in Tumor Stroma Affect Spheroid Formation in the 3D Osteosarcoma Model. ACS Appl. Mater. Interfaces 2019, 11, 14548–14559. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, W.; Tang, P.; Jiang, D.; Gu, C.; Huang, Y.; Gong, F.; Rong, Y.; Qian, D.; Chen, J.; et al. miR-624-5p promoted tumorigenesis and metastasis by suppressing hippo signaling through targeting PTPRB in osteosarcoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 488. [Google Scholar] [CrossRef]
- Piazzi, M.; Kojic, S.; Capanni, C.; Stamenkovic, N.; Bavelloni, A.; Marin, O.; Lattanzi, G.; Blalock, W.; Cenni, V. Ectopic Expression of Ankrd2 Affects Proliferation, Motility and Clonogenic Potential of Human Osteosarcoma Cells. Cancers 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.W.; Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Kajikuri, J.; Endo, K.; Kito, H.; Elboray, E.E.; Suzuki, T. Ca2+-activated K+channel KCa 1.1 as a therapeutic target to overcome chemoresistance in three-dimensional sarcoma spheroid models. Cancer Sci. 2021, 112, 3769–3783. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.M.; Huynh, E.; Tang, S.; Uskoković, V. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles). Acta Biomater. 2019, 88, 422–447. [Google Scholar] [CrossRef]
- Franceschini, N.; Oosting, J.; Tamsma, M.; Niessen, B.; Bruijn, I.B.-D.; van den Akker, B.; Kruisselbrink, A.B.; Palubeckaitė, I.; Bovée, J.V.M.G.; Cleton-Jansen, A.-M. Targeting the NAD Salvage Synthesis Pathway as a Novel Therapeutic Strategy for Osteosarcomas with Low NAPRT Expression. Int. J. Mol. Sci. 2021, 22, 6273. [Google Scholar] [CrossRef]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; Tampieri, A.; Montesi, M. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci. Rep. 2020, 10, 22294. [Google Scholar] [CrossRef]
- Charoen, K.M.; Fallica, B.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug-device combinations. Biomaterials 2014, 35, 2264–2271. [Google Scholar] [CrossRef]
- Gebhard, C.; Miller, I.; Hummel, K.; Ondrovics, M.; Schlosser, S.; Walter, I. Comparative proteome analysis of monolayer and spheroid culture of canine osteosarcoma cells. J. Proteom. 2018, 177, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Ozturk, S.; Gokalp, S.; Vatansever, S.; Gurhan, S.I.D.; Urkmez, A.S. Synergistic role of three dimensional niche and hypoxia on conservation of cancer stem cell phenotype. Int. J. Biol. Macromol. 2016, 90, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gorska, M.; Krzywiec, P.B.; Kuban-Jankowska, A.; Zmijewski, M.A.; Wozniak, M.; Wierzbicka, J.; Piotrowska, A.; Siwicka, K. Growth Inhibition of Osteosarcoma Cell Lines in 3D Cultures: Role of Nitrosative and Oxidative Stress. Anticancer Res. 2016, 36, 221–230. [Google Scholar] [PubMed]
- Jabbari, E.; Sarvestani, S.K.; Daneshian, L.; Moeinzadeh, S. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. PLoS ONE 2015, 10, e0132377. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.R.; Chim, L.K.; Salazar, M.C.; Mehta, S.M.; Menegaz, B.A.; Lamhamedi-Cherradi, S.-E.; Satish, T.; Mohiuddin, S.; McCall, D.; Zaske, A.M.; et al. Mechanically tunable coaxial electrospun models of YAP/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta Biomater. 2019, 100, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Leitner, N.; Hlavatý, J.; Ertl, R.; Gabner, S.; Fuchs-Baumgartinger, A.; Walter, I. Lipid droplets and perilipins in canine osteosarcoma. Investigations on tumor tissue, 2D and 3D cell culture models. Vet. Res. Commun. 2022, 46, 1175–1193. [Google Scholar] [CrossRef] [PubMed]
- Solernó, L.M.; Sobol, N.T.; Gottardo, M.F.; Capobianco, C.S.; Ferrero, M.R.; Vásquez, L.; Alonso, D.F.; Garona, J. Propranolol blocks osteosarcoma cell cycle progression, inhibits angiogenesis and slows xenograft growth in combination with cisplatin-based chemotherapy. Sci. Rep. 2022, 12, 15058. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Gorgun, C.; Gokalp, S.; Vatansever, S.; Sendemir, A. Development and characterization of cancer stem cell-based tumoroids as an osteosarcoma model. Biotechnol. Bioeng. 2020, 117, 2527–2539. [Google Scholar] [CrossRef]
- González Diaz, E.C.; Lee, A.G.; Sayles, L.C.; Feria, C.; Sweet-Cordero, E.A.; Yang, F. A 3D Osteosarcoma Model with Bone-Mimicking Cues Reveals a Critical Role of Bone Mineral and Informs Drug Discovery. Adv. Healthc. Mater. 2022, 11, e2200768. [Google Scholar] [CrossRef]
- Picone, G.; Cappadone, C.; Pasini, A.; Lovecchio, J.; Cortesi, M.; Farruggia, G.; Lombardo, M.; Gianoncelli, A.; Mancini, L.; Ralf, H.M.; et al. Analysis of Intracellular Magnesium and Mineral Depositions during Osteogenic Commitment of 3D Cultured Saos2 Cells. Int. J. Mol. Sci. 2020, 21, 2368. [Google Scholar] [CrossRef]
- Porta, M.; Tonda-Turo, C.; Pierantozzi, D.; Ciardelli, G.; Mancuso, E. Towards 3D Multi-Layer Scaffolds for Periodontal Tissue Engineering Applications: Addressing Manufacturing and Architectural Challenges. Polymers 2020, 12, 2233. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Giordano, C.; Pietrabissa, R. Oxygen measurement in interstitially perfused cellularized constructs cultured in a miniaturized bioreactor. J. Appl. Biomater. Funct. Mater. 2015, 13, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Peng, C.-C.; Tung, Y.-C. Comparison of VEGF-A secretion from tumor cells under cellular stresses in conventional monolayer culture and microfluidic three-dimensional spheroid models. PLoS ONE 2020, 15, e0240833. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-L.; Xu, N.-Y.; Tang, R.-Z.; Liu, X.-Q. A 3D-printed scaffold-based osteosarcoma model allows to investigate tumor phenotypes and pathogenesis in an in vitro bone-mimicking niche. Mater. Today Bio 2022, 15, 100295. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zou, Q.; Zou, W.; Xie, Z.; Li, Z.; Zhao, X.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater. Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, C.; Ma, X.; Li, Y. Optimization of macroporous 3-D silk fibroin scaffolds by salt-leaching procedure in organic solvent-free conditions. J. Mater. Sci. Mater. Med. 2011, 23, 315–324. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef]
- He, C.; Dong, C.; Yu, L.; Chen, Y.; Hao, Y. Ultrathin 2D Inorganic Ancient Pigment Decorated 3D-Printing Scaffold Enables Photonic Hyperthermia of Osteosarcoma in NIR-II Biowindow and Concurrently Augments Bone Regeneration. Adv. Sci. 2021, 8, e2101739. [Google Scholar] [CrossRef]
- He, J.; Chen, C.; Chen, L.; Cheng, R.; Sun, J.; Liu, X.; Wang, L.; Zhu, C.; Hu, S.; Xue, Y.; et al. Honeycomb-Like Hydrogel Microspheres for 3D Bulk Construction of Tumor Models. Research 2022, 2022, 9809763. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen-based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.B.; Li, Q.; Park, M.S.; Flanagan, W.M.; Semenza, G.L.; Dang, C.V. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 2001, 276, 7919–7926. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-Associated Mesenchymal Stroma Fosters the Stemness of Osteosarcoma Cells in Response to Intratumoral Acidosis via NF-ΚB Activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef]
- Rodrigues, J.; Sarmento, B.; Pereira, C.L. Osteosarcoma tumor microenvironment: The key for the successful development of biologically relevant 3D in vitro models. Vitr. Model. 2022, 1, 5–27. [Google Scholar] [CrossRef]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Fischetti, T.; Di Pompo, G.; Baldini, N.; Avnet, S.; Graziani, G. 3D Printing and Bioprinting to Model Bone Cancer: The Role of Materials and Nanoscale Cues in Directing Cell Behavior. Cancers 2021, 13, 4065. [Google Scholar] [CrossRef]
- Wang, X.; Tolba, E.; Schröder, H.C.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Müller, W.E.G. Effect of Bioglass on Growth and Biomineralization of SaOS-2 Cells in Hydrogel after 3D Cell Bioprinting. PLoS ONE 2014, 9, e112497. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Magdeldin, T.; Stamati, K.; Nyga, A.; Loizidou, M.; Emberton, M.; Cheema, U. Cancer-Associated Fibroblasts Mediate Cancer Progression and Remodel the Tumouroid Stroma. Br. J. Cancer 2020, 123, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Franz, E.M.; McAloney, C.A.; Vetter, T.A.; Cam, M.; Gross, A.C.; Taslim, C.; Wang, M.; Cannon, M.V.; Oles, A.; et al. Osteosarcoma Tumors Maintain Intra-Tumoral Transcriptional Heterogeneity during Bone and Lung Colonization. BMC Biol. 2023, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, X.; Miao, J.; Feng, W.; Xie, T.; Liao, S.; Qin, Z.; Tang, H.; Lin, C.; Li, B.; et al. A New Insight of Immunosuppressive Microenvironment in Osteosarcoma Lung Metastasis. Exp. Biol. Med. 2023, 248, 1056–1073. [Google Scholar] [CrossRef]
- Rothzerg, E.; Xu, J.; Wood, D. Different Subtypes of Osteosarcoma: Histopathological Patterns and Clinical Behaviour. J. Mol. Pathol. 2023, 4, 99–108. [Google Scholar] [CrossRef]

| Author [Ref.] | OS Cell | Drugs | 2D (Apoptosis) | 3D (Apoptosis) | 3D OS Model | Comments |
| Based on apoptosis of 3D OS models vs. 2D models (1 study) | ||||||
| He J, 2022 [81] | K7M2 MG-63 | Doxorubicin | 21.6% 43.1% | 3.14% 23.9% | Scaffold-based (GelMA) | Early apoptosis was lower for 3D compared to 2D culture. mRNA expression of BCL-2 (anti-apoptotic) in 3D culture was 3.22x higher than 2D culture. |
| Author | OS Cell | Drugs | Comparison Relative to | 3D OS Model | Comments | |
| Based on relative cell viability comparison (3 studies) | ||||||
| Ohya S, 2021 [56] | MG-63 | Paclitaxel Doxorubicin Cisplatin PAX (KCa1.1 inhibitor) | Relative to untreated OS cells | Self-generated | 3D culture showed greater resistance to paclitaxel, doxorubicin, and cisplatin compared to 2D culture. KCa1.1 activity (conferring resistance) was higher in 3D than in 2D. KCa1.1 inhibitor, PAX, improved sensitivity to drugs. | |
| Tan PH, 2013 [30] | 143.98.2 Saos-2 U-2 OS | Doxorubicin | Relative to untreated OS cells | Scaffold-based (Silk) | 3D cultures demonstrated decrease sensitivity to cisplatin (10-fold difference in IC50) and doxorubicin (100-fold difference) compared to 2D cells. | |
| 143.98.2 Saos-2 U-2 OS | Cisplatin | |||||
| Tornin J, 2021 [32] | MG-63 | PAR | Relative to untreated OS cells | Scaffold-based (Collagen/HA) | PAR (cold plasma-activated Ringer’s) solution reduced cell viability in a dose-response manner in 2D monolayer, while it enhanced proliferation in 3D cultures at the same increasing treatment times except at 240 s, which eliminated cells in both 2D and 3D cultures. | |
| Author | Stromal Cells | OS Cells | 3D Model | Drug | Comments |
|---|---|---|---|---|---|
| Dobos A, 2019 [34] | Adipose-derived stem cell (ASC) | MG-63 | Scaffold-based (Gelatine) | Two-photon excited Photodynamic Therapy (TPE-PDT) | 3D culture of ASC with OS spheroids showed no damage to healthy cells after TPE-PDT, showing precision of irradiation. |
| Komez A, 2020 [37] | Human foetal osteoblast cells (hFOB) and human umbilical vein endothelial cells (HUVECs). | Saos-2 | Scaffold-based (PLGA/TCP/Collagen) | Doxorubicin | Doxorubicin impact was explicitly evident on OS cells and not on healthy bone cells, human foetal osteoblast cells (hFOB), and human umbilical vein endothelial cells (HUVECs). |
| Koski C, 2020 [51] | Human foetal osteoblast cells (hFOB) | MG-63 | Scaffold-based (TCP) | Bicarbonate and Crocin | Crocin and bicarbonate showed selective cytotoxicity towards OS cells without affecting osteoblasts. |
| Li Volsi A, 2017 [38] | Human dermal fibroblast | U-2 OS | Scaffold-based (ECM gel) | Nutlin-3 loaded in nanorod | Nutlin-3 loaded in a nanorod system was more effective against OS cells compared to fibroblasts, while free nutlin-3 did not distinguish cancer from normal cells. |
| Sarkar N, 2020 [43] | Human foetal osteoblast cell (hFOB) | MG-63 | Scaffold-based (TCP) | Soy isoflavones (genistein, daidzein, and glycitein) | Isoflavones showed no toxicity (with improved proliferation, viability, and differentiation) in osteoblast cells while reducing OS cell viability and proliferation. |
| Wu VM, 2017 [33] | Fibroblasts | K7M2 | Self-generated | NP loaded with bisphosphonate and JQ1 | The NP reduced the viability of OS cells more than fibroblast cells. |
| Marshall SK, 2022 [39] | Fibroblast (EGFR-) | MG-63 (EGFR+) | Self-generated | Doxorubicin and Na131I nanoparticle (DIE- NP) | The anti-EGFR NP achieved 80-fold higher efficacy in targeting the OS cells compared to fibroblast cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, V.; Bakkalci, D.; Wei, S.; Cheema, U. Enhanced Biomimetics of Three-Dimensional Osteosarcoma Models: A Scoping Review. Cancers 2024, 16, 164. https://doi.org/10.3390/cancers16010164
Sandhu V, Bakkalci D, Wei S, Cheema U. Enhanced Biomimetics of Three-Dimensional Osteosarcoma Models: A Scoping Review. Cancers. 2024; 16(1):164. https://doi.org/10.3390/cancers16010164
Chicago/Turabian StyleSandhu, Vinesh, Deniz Bakkalci, Siyi Wei, and Umber Cheema. 2024. "Enhanced Biomimetics of Three-Dimensional Osteosarcoma Models: A Scoping Review" Cancers 16, no. 1: 164. https://doi.org/10.3390/cancers16010164






