Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts
Abstract
Simple Summary
Abstract
1. Introduction
- 90Y-containing resin microspheres (SirSpheres®, Sirtex Medical, Woburn, MA, USA);
- 90Y-containing glass microspheres (TheraSphere®, Boston Scientific, Marlborough, MA, USA);
- 166Ho-containing poly-l-lactic acid (PLLA) microspheres (QuiremSpheres®, Terumo, Leuven, Belgium).
2. Materials and Methods
2.1. Inclusion Criteria and Patient Characteristics
2.2. TARE Procedures
2.3. Follow-Up
2.4. Outcome Evaluation and Statistics
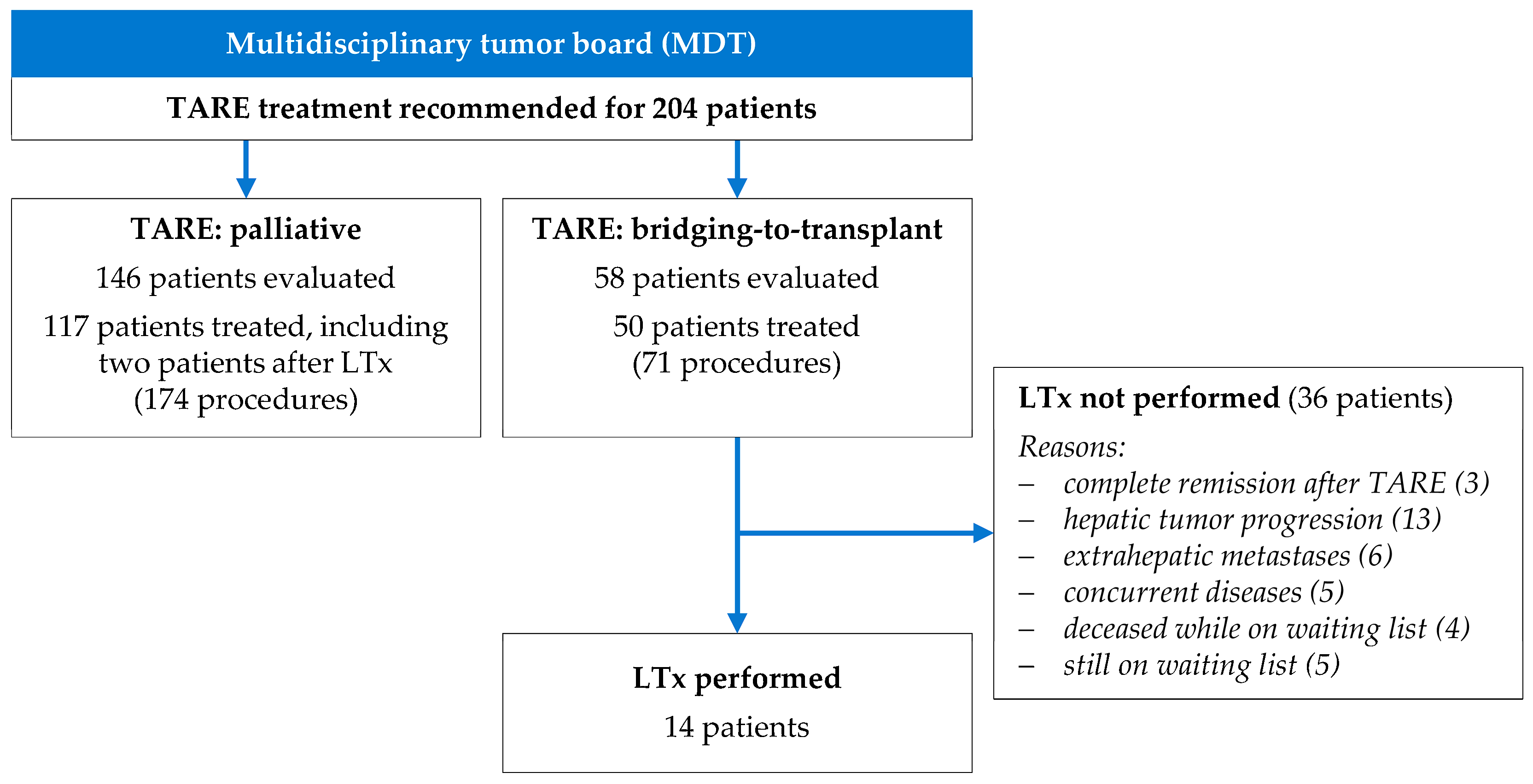
3. Results
3.1. Clinical Characteristics and Indications for TARE Treatment
3.2. TARE Interventional Procedures
3.3. Liver Transplantation and TARE
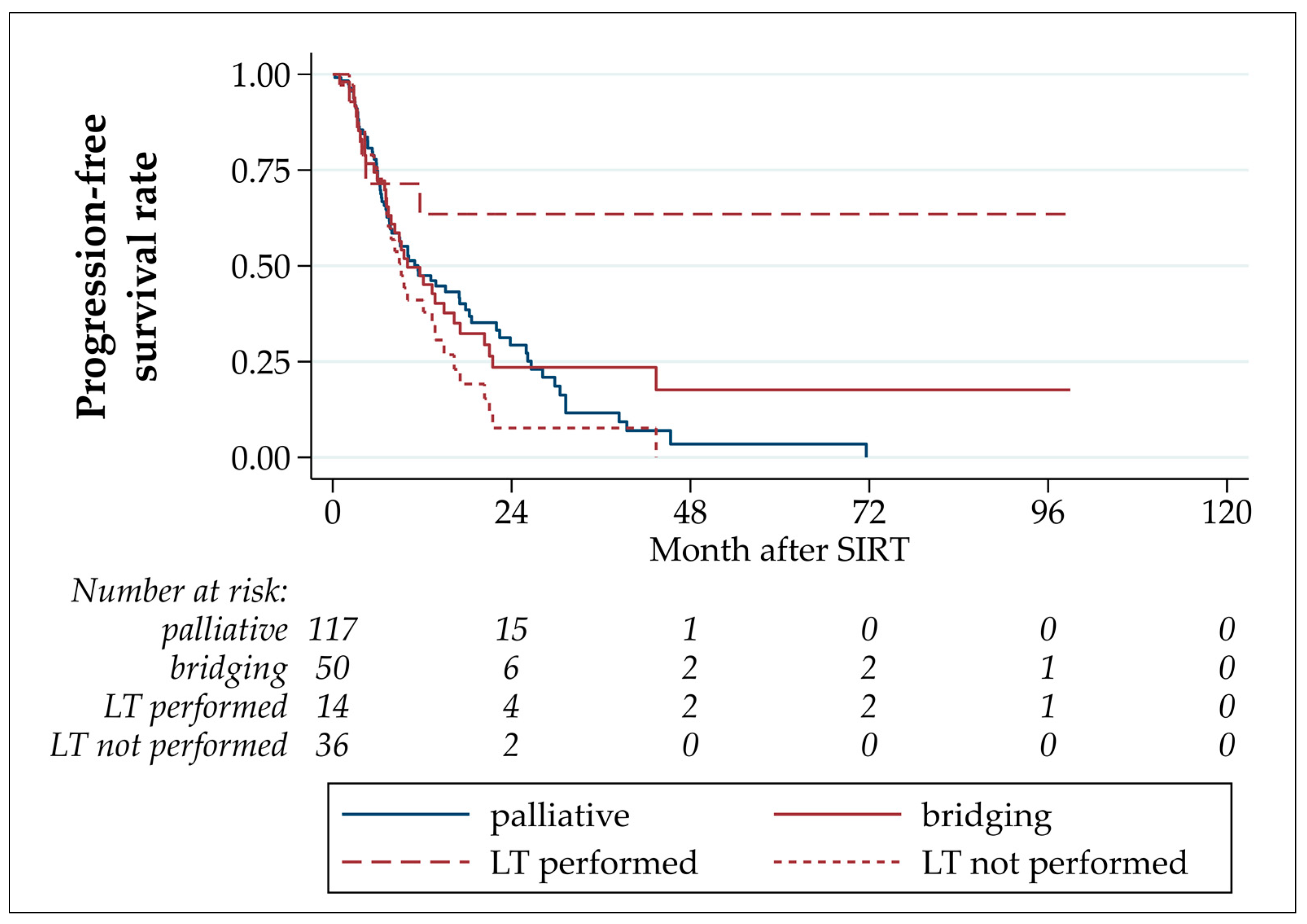
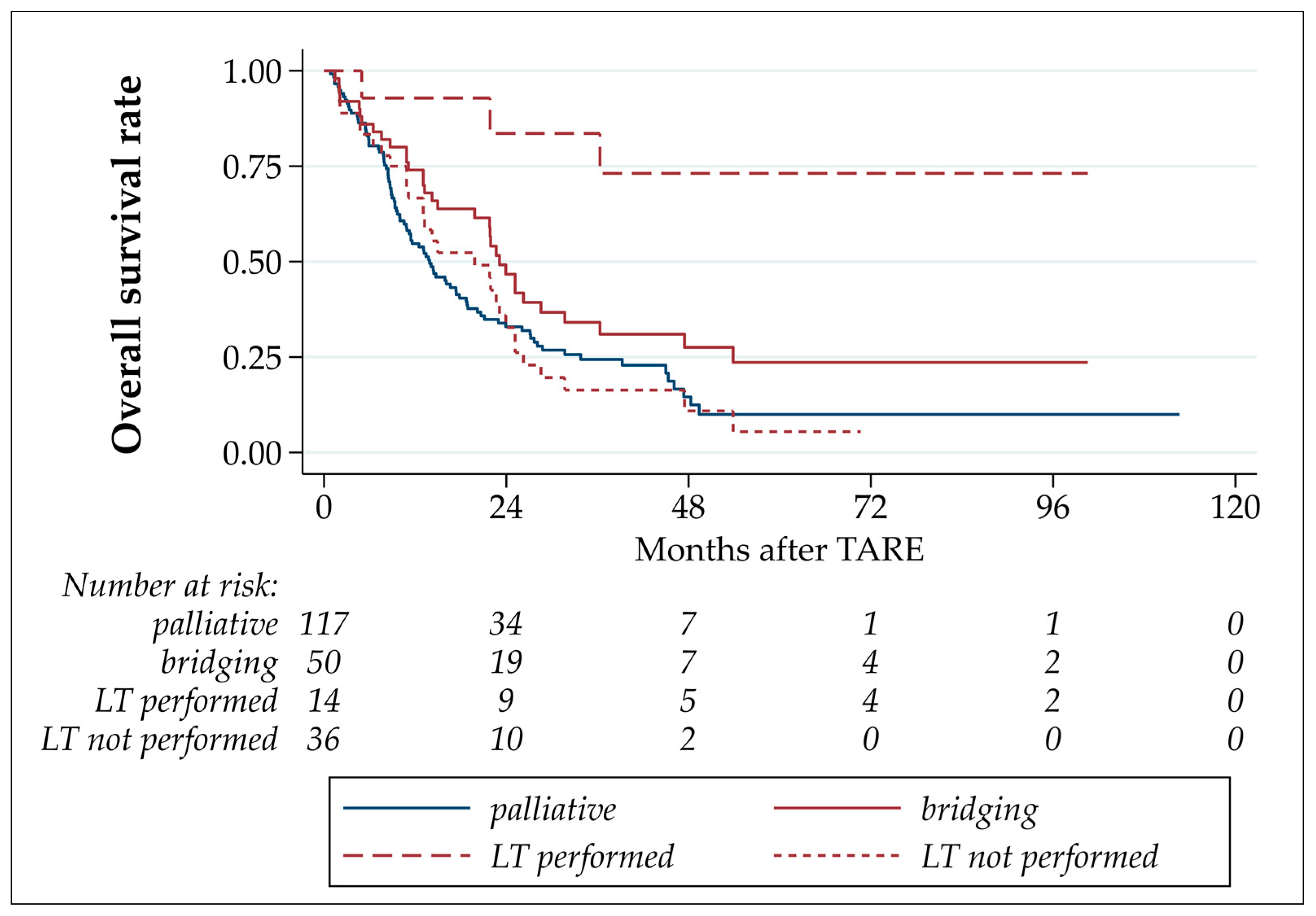
3.4. Progression-Free and Overall Survival
4. Discussion
4.1. Clinical Outcome
4.2. Comparison of Bridging to Palliative Treatments
4.3. Outcome of Patients Undergoing Liver Transplantation
4.4. Future Perspectives
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Coilly, A. Management of patients with liver diseases on the waiting list for transplantation: A major impact to the success of liver transplantation. BMC Med. 2018, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Rand, A.; Koch, T.; Ragaller, M. Organ donation-Not only a responsibility of intensive care medicine. Anaesthesist 2022, 71, 311–317. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, D.; Selzner, N.; Selzner, M. Bridging to liver transplantation in HCC patients. Langenbecks Arch. Surg. 2017, 402, 863–871. [Google Scholar] [CrossRef]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Vouche, M.; Atassi, R.; Memon, K.; Asadi, A.A.; Baker, T.; Caicedo, J.C.; Desai, K.; et al. Comparative study of post-transplant outcomes in hepatocellular carcinoma patients treated with chemoembolization or radioembolization. Eur. J. Radiol. 2017, 93, 100–106. [Google Scholar] [CrossRef]
- Oligane, H.C.; Xing, M.; Kim, H.S. Effect of Bridging Local-Regional Therapy on Recurrence of Hepatocellular Carcinoma and Survival after Orthotopic Liver Transplantation. Radiology 2017, 282, 869–879. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Vikoler, A.; Portenkirchner, M.; Mulleder, K.; Maglione, M.; Margreiter, C.; Moser, P.; Vogel, W.; Bale, R.; Freund, M.; et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int. 2016, 36, 688–695. [Google Scholar] [CrossRef]
- Bester, L.; Meteling, B.; Boshell, D.; Chua, T.C.; Morris, D.L. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: A review of the literature. J. Med. Imaging Radiat. Oncol. 2014, 58, 341–352. [Google Scholar] [CrossRef]
- Riemenschneider, T.; Ruf, C.; Kratzsch, H.C.; Ziegler, M.; Spath, G. Arterial, portal or combined arterio-portal regional chemotherapy in experimental liver tumours? J. Cancer Res. Clin. Oncol. 1992, 118, 597–600. [Google Scholar] [CrossRef]
- Aliseda, D.; Marti-Cruchaga, P.; Zozaya, G.; Rodriguez-Fraile, M.; Bilbao, J.I.; Benito-Boillos, A.; Martinez De La Cuesta, A.; Lopez-Olaondo, L.; Hidalgo, F.; Ponz-Sarvise, M.; et al. Liver Resection and Transplantation Following Yttrium-90 Radioembolization for Primary Malignant Liver Tumors: A 15-Year Single-Center Experience. Cancers 2023, 15, 733. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Miura, K.; Kuemmerli, C.; Capel, A.; Eshmuminov, D.; Ferreras, D.; Baroja-Mazo, A.; Cascales-Campos, P.; Jimenez-Mascunan, M.I.; Pons, J.A.; et al. Selecting the Appropriate Downstaging and Bridging Therapies for Hepatocellular Carcinoma: What Is the Role of Transarterial Radioembolization? A Pooled Analysis. Cancers 2023, 15, 2122. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- BTG International. TheraSphere Reference Manual Europe, version US-USTHSP-2013-0569(2)a(1); BTG International: London, UK, 2017. [Google Scholar]
- Quirem Medical B.V. Instructions for Use Ho-166-PLLA Microspheres, version LS-1101-10; Quirem Medical B.V.: Deventer, The Netherlands, 2017. [Google Scholar]
- Sirtex Medical Limited. SIR-Spheres Microspheres Training Manual, version TRN-RW-05; Sirtex Medical Limited: Woburn, MA, USA, 2014. [Google Scholar]
- Bauschke, A.; Altendorf-Hofmann, A.; Ardelt, M.; Kissler, H.; Tautenhahn, H.M.; Settmacher, U. Impact of successful local ablative bridging therapy prior to liver transplantation on long-term survival in patients with hepatocellular carcinoma in cirrhosis. J. Cancer Res. Clin. Oncol. 2020, 146, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Benko, T.; Konig, J.; Theysohn, J.M.; Schotten, C.; Saner, F.H.; Treckmann, J.; Radunz, S. Bridging treatment prior to liver transplantation for hepatocellular carcinoma: Radioembolization or transarterial chemoembolization? Eur. J. Med. Res. 2022, 27, 74. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.; Kulik, L.; Mouli, S.; Riaz, A.; Ali, R.; Desai, K.; Mora, R.A.; Ganger, D.; Maddur, H.; Flamm, S.; et al. Liver Transplantation Following Yttrium-90 Radioembolization: 15-Year Experience in 207-Patient Cohort. Hepatology 2021, 73, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Jang, H.; Choi, N.R.; Nam, J.Y.; Lee, Y.B.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, H.C.; Chung, J.W.; et al. Yttrium-90 Radioembolization Is Associated with Better Clinical Outcomes in Patients with Hepatocellular Carcinoma Compared with Conventional Chemoembolization: A Propensity Score-Matched Study. J. Hepatocell. Carcinoma 2021, 8, 1565–1577. [Google Scholar] [CrossRef]
- Crocetti, L.; Bozzi, E.; Scalise, P.; Bargellini, I.; Lorenzoni, G.; Ghinolfi, D.; Campani, D.; Balzano, E.; De Simone, P.; Cioni, R. Locoregional Treatments for Bridging and Downstaging HCC to Liver Transplantation. Cancers 2021, 13, 5558. [Google Scholar] [CrossRef]
- Goyal, P.; Salem, R.; Mouli, S.K. Role of interventional oncology in hepatocellular carcinoma: Future best practice beyond current guidelines. Br. J. Radiol. 2022, 95, 20220379. [Google Scholar] [CrossRef]
- Yim, S.Y.; Chun, H.S.; Lee, J.S.; Lim, J.H.; Kim, T.H.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Kim, G.M.; et al. Transarterial Radioembolization for Unresectable Hepatocellular Carcinoma: Real-Life Efficacy and Safety Analysis of Korean Patients. Cancers 2022, 14, 385. [Google Scholar] [CrossRef]
- Yu, C.Y.; Huang, P.H.; Tsang, L.L.; Hsu, H.W.; Lim, W.X.; Weng, C.C.; Huang, T.L.; Hsu, C.C.; Chen, C.L.; Ou, H.Y.; et al. Yttrium-90 Radioembolization as the Major Treatment of Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.W.; Mak, L.Y.; Cheung, T.T.; Lee, V.H.; Seto, W.K.; Yuen, M.F. Clinical practice guidelines and real-life practice on hepatocellular carcinoma: The Hong Kong perspective. Clin. Mol. Hepatol. 2023, 29, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Mantry, P.S.; Mehta, A.; Madani, B.; Mejia, A.; Shahin, I. Selective internal radiation therapy using yttrium-90 resin microspheres in patients with unresectable hepatocellular carcinoma: A retrospective study. J. Gastrointest. Oncol. 2017, 8, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Radunz, S.; Treckmann, J.; Baba, H.A.; Best, J.; Muller, S.; Theysohn, J.M.; Paul, A.; Benko, T. Long-Term Outcome after Liver Transplantation for Hepatocellular Carcinoma Following Yttrium-90 Radioembolization Bridging Treatment. Ann. Transplant. 2017, 22, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zori, A.G.; Ismael, M.N.; Limaye, A.R.; Firpi, R.; Morelli, G.; Soldevila-Pico, C.; Suman, A.; Vogel, J.D.; Lazarowicz, M.; Geller, B.S.; et al. Locoregional Therapy Protocols with and Without Radioembolization for Hepatocellular Carcinoma as Bridge to Liver Transplantation. Am. J. Clin. Oncol. 2020, 43, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Drescher, R.; Kohler, A.; Seifert, P.; Aschenbach, R.; Ernst, T.; Rauchfuss, F.; Freesmeyer, M. Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer. Biomedicines 2023, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Newell, P.H.; Wu, Y.; Hoen, H.; Uppal, R.; Thiesing, J.T.; Sasadeusz, K.; Cassera, M.A.; Wolf, R.F.; Hansen, P.; Hammill, C.W. Multimodal treatment of unresectable hepatocellular carcinoma to achieve complete response results in improved survival. HPB 2015, 17, 454–460. [Google Scholar] [CrossRef][Green Version]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.H.; Lee, S.J.; Lee, E.J.; Shin, E.C.; Seong, J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget 2017, 8, 41242–41255. [Google Scholar] [CrossRef]
- D’Avola, D.; Granito, A.; Torre-Alaez, M.; Piscaglia, F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1185–1198. [Google Scholar] [CrossRef]
- Fischer, J.; Wellhoner, S.; Ebel, S.; Lincke, T.; Bohlig, A.; Gerhardt, F.; Veelken, R.; Goessmann, H.; Steinhoff, K.G.; Denecke, T.; et al. The Liver Maximum Capacity Test (LiMAx) Is Associated with Short-Term Survival in Patients with Early Stage HCC Undergoing Transarterial Treatment. Cancers 2022, 14, 5323. [Google Scholar] [CrossRef] [PubMed]
- Dinant, S.; de Graaf, W.; Verwer, B.J.; Bennink, R.J.; van Lienden, K.P.; Gouma, D.J.; van Vliet, A.K.; van Gulik, T.M. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J. Nucl. Med. 2007, 48, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Aquina, C.T.; Eskander, M.F.; Pawlik, T.M. Liver-Directed Treatment Options Following Liver Tumor Recurrence: A Review of the Literature. Front. Oncol. 2022, 12, 832405. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Makary, M.S. Recent Advances in Image-Guided Locoregional Therapies for Primary Liver Tumors. Biology 2023, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, B.; Ielasi, L.; Chen, R.; Abbati, C.; Tonnini, M.; Tovoli, F.; Granito, A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert. Rev. Anticancer. Ther. 2023, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Sangro, B.; Inarrairaegui, M.; Bilbao, J.I. Radioembolization for hepatocellular carcinoma. J. Hepatol. 2012, 56, 464–473. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Z.; Hu, R.; Dong, M.; Zhou, X.; Ren, S.; Zhang, Y.; Chen, C.; Huang, R.; Zhu, M.; et al. Metabolic Intervention Liposome Boosted Lung Cancer Radio-Immunotherapy via Hypoxia Amelioration and PD-L1 Restraint. Adv. Sci. 2023, 10, e2207608. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, H.; Li, J.; Jiang, X.; Li, Z.; Shen, J. Recent progress, perspectives, and issues of engineered PD-L1 regulation nano-system to better cure tumor: A review. Int. J. Biol. Macromol. 2024, 254, 127911. [Google Scholar] [CrossRef]
- Bastiaannet, R.; van Roekel, C.; Smits, M.L.J.; Elias, S.G.; van Amsterdam, W.A.C.; Doan, D.; Prince, J.F.; Bruijnen, R.C.G.; de Jong, H.; Lam, M. First Evidence for a Dose-Response Relationship in Patients Treated with 166Ho Radioembolization: A Prospective Study. J. Nucl. Med. 2020, 61, 608–612. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, P.; Rietbergen, D.D.D.; van Erkel, A.R.; Coenraad, M.J.; Arntz, M.J.; Bennink, R.J.; Braat, A.E.; Crobach, A.; van Delden, O.M.; van der Hulle, T.; et al. Study Protocol: Adjuvant Holmium-166 Radioembolization After Radiofrequency Ablation in Early-Stage Hepatocellular Carcinoma Patients-A Dose-Finding Study (HORA EST HCC Trial). Cardiovasc. Interv. Radiol. 2022, 45, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Stella, M.; Braat, A.; van Rooij, R.; de Jong, H.; Lam, M. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Granito, A.; Bolondi, L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017, 18, e101–e112. [Google Scholar] [CrossRef]
| All | TARE as Bridging to LT | TARE as Palliative Treatment | p Value ** | |||
|---|---|---|---|---|---|---|
| All Bridging | LT Performed | LT not Performed | ||||
| No. of patients | 167 | 50 | 14 | 36 | 117 | |
| Gender | ||||||
| male female | 146 (87.4%) 21 (12.6%) | 45 (90.0%) 5 (10.0%) | 14 (100.0%) 0 | 31 (86.1%) 5 (13.9%) | 101 (86.3%) 16 (13.7%) | 0.616 |
| Age (years) | ||||||
| mean ± SD median, range | 67.5 ± 8.4 66.6, 45.7–85.5 | 62.6 ± 4.5 62.6, 50.6–70.7 | 63.4 ± 2.5 63.0, 59.4–69.0 | 62.3 ± 5.0 62.6, 50.6–70.7 | 69.6 ± 8.7 72.0, 45.7–85.5 | <0.001 |
| HCC diagnosis to TARE (months) | ||||||
| mean ± SD median, range | 12.9 ± 22.4 5.3, 1.3–177.5 | 10.8 ± 12.7 5.5, 1.3–54.3 | 9.9 ± 10.1 5.7, 1.3–40.3 | 11.1 ± 13.4 5.5, 1.5–54.3 | 13.8 ± 25.4 4.9, 1.3–177.5 | 0.509 |
| HCC treatments before TARE * | ||||||
| none surgery transarterial chemoembolization percutaneous radiation systemic therapy liver transplantation | 83 (49.7%) 33 (19.8%) 59 (35.3%) 4 (2.4%) 12 (7.2%) 2 (1.2%) | 23 (46.0%) 12 (24.0%) 17 (34.0%) 0 3 (6.0%) 0 | 6 (42.9%) 2 (14.3%) 6 (42.9%) 0 1 (7.1%) 0 | 17 (47.2%) 10 (27.8%) 11 (30.6%) 0 2 (5.6%) 0 | 60 (51.3%) 21 (17.9%) 42 (35.9%) 4 (3.4%) 9 (7.7%) 2 (1.7%) | 0.613 0.399 0.861 0.318 1.000 1.000 |
| HCC stage | ||||||
| IA IB II IIIA IIIB IVA | 1 (0.6%) 5 (3.0%) 94 (56.3%) 47 (28.1%) 13 (7.8%) 7 (4.2%) | 0 0 30 (60.0%) 13 (26.0%) 6 (12.0%) 1 (2.0%) | 0 0 8 (57.1%) 3 (21.4%) 3 (21.4%) 0 | 0 0 22 (61.1%) 10 (27.8%) 3 (8.3%) 1 (2.8%) | 1 (0.9%) 5 (4.3%) 64 (54.7%) 34 (29.1%) 7 (6.0%) 6 (5.1%) | 0.647 |
| Child–Pugh stage/score | ||||||
| A5 A6 B7 B8 | 47 (28.1%) 104 (62.3%) 14 (8.4%) 2 (1.2%) | 18 (36.0%) 29 (58.0%) 2 (4.0%) 1 (2.0%) | 5 (35.7%) 9 (64.3) 0 0 | 13 (36.1%) 20 (55.6%) 2 (5.6%) 1 (2.8%) | 29 (24.8%) 75 (64.1%) 12 (10.3%) 1 (0.9%) | 0.253 |
| All | TARE as Bridging to LT | TARE as Palliative Treatment | p Value ** | |||
|---|---|---|---|---|---|---|
| All Bridging | LT Performed | LT not Performed | ||||
| No. of TARE procedures | 245 | 71 | 16 | 55 | 174 | |
| Treated liver lobes | ||||||
| right left | 170 (69.4%) 75 (30.6%) | 51 (71.8%) 20 (28.2%) | 11 (68.8%) 5 (31.2%) | 40 (72.7%) 15 (27.3%) | 119 (68.4%) 55 (31.6%) | 0.649 |
| Treatment cycles | ||||||
| all monolobar bilobar sequential | 209 173 (82.8%) 36 (17.2%) | 61 51 (83.6%) 10 (16.4%) | 14 12 (86.7%) 2 (14.3%) | 47 39 (83.0%) 8 (17.0%) | 148 122 (82.4%) 26 (17.6%) | 0.999 |
| No. of procedures per patient | ||||||
| 1 2 3 4 | 101 (60.5%) 57 (34.1%) 6 (3.6%) 3 (1.8%) | 30 (60.0%) 19 (38.0%) 1 (2.0%) 0 | 12 (85.7%) 2 (14.3%) 0 0 | 18 (50.0%) 17 (47.2%) 1 (2.8%) 0 | 71 (60.7%) 38 (32.5%) 5 (4.3%) 3 (2.6%) | 0.763 |
| Tumor burden in target volume (%) | ||||||
| mean ± SD median, range | 18.0 ± 22.2 7.4, 1.7–100 | 12.7 ± 19.1 5.6, 1.7–100.0 | 10.4 ± 11.4 5.3, 1.7–37.6 | 13.5 ± 21.4 6.2, 1.9–100.0 | 20.2 ± 23.2 8.8, 1.7–100.0 | 0.013 |
| Microsphere type | ||||||
| 90Y glass 90Y resin 166Ho PLLA | 158 (64.5%) 84 (34.3%) 3 (1.2%) | 48 (66.2%) 26 (33.8%) 0 | 11 (68.8%) 5 (31.2%) 0 | 36 (65.5%) 19 (34.5%) 0 | 111 (63.8%) 60 (34.5%) 3 (1.7%) | 0.814 |
| Prescribed activity (GBq; median, range) | ||||||
| 90Y glass 90Y resin 166Ho PLLA | 2.1, 0.4–7.7 1.0, 0.3–3.1 1.8, 1.4–5.6 | 2.1, 0.6–7.7 1.0, 0.6–2.0 - | 1.9, 0.6–4.3 0.85, 0.6–1.4 - | 2.1, 0.6–7.7 1.0, 0.6–2.0 - | 2.2, 0.4–7.1 1.0, 0.3–3.1 1.8, 1.4–5.6 | 0.697 0.881 - |
| All | TARE as Bridging to LT | TARE as Palliative Treatment | p Value ** | |||
|---|---|---|---|---|---|---|
| All Bridging | LT Performed | LT not Performed | ||||
| No. of patients | 167 | 50 | 14 | 36 | 117 | |
| Follow-up (months) after TARE | ||||||
| mean ± SD median, range | 21.3 ± 19.4 14.5, 0.9–112.6 | 26.7 ± 23.5 21.8, 1.4–100.6 | 45.7 ± 30.2 38.1, 5.0–100.6 | 19.3 ± 14.8 14.7, 1.4–70.7 | 19.1 ± 16.8 13.8, 0.9–112.6 | 0.040 |
| HCC treatments after TARE * | ||||||
| none transarterial chemoembolization percutaneous radiation systemic therapy surgery (without LT) liver transplantation (LT) | 101 (60.5%) 31 (18.6%) 19 (11.4%) 34 (20.4%) 4 (2.4%) 14 (8.4%) | 20 (40.0%) 12 (24.0%) 9 (18.0%) 13 (26.0%) 1 (2.0%) 14 (28.0%) | 0 4 (28.6%) 3 (21.4%) 3 (21.4%) 0 14 (100%) | 20 (55.6%) 8 (22.2%) 6 (16.7%) 10 (27.8%) 1 (2.8%) 0 | 81 (69.2%) 19 (16.2%) 10 (8.5%) 21 (17.9%) 3 (2.6%) 0 | <0.001 0.279 0.108 0.294 0.999 |
| Occurrence of extrahepatic metastases | 35 (21.0%) | 9 (18.0%) | 3 (21.4%) | 6 (16.7%) | 26 (22.2%) | 0.679 |
| Progression-free survival | ||||||
| progression (events, %) median (months, 95% CI) est. PFS rate after 6 months (95% CI) est. PFS rate after 12 months (95% CI) | 109 (%) 11.0 (8.9–15.1) 74.0% (66.3–80.2%) 47.5% (38.9–55.5%) | 34 (68.0%) 10.0 (7.2–16.3) 72.2% (56.9–82.8%) 47.4% (32.4–61.0%) | 5 (35.7%) - (4.3-.) 71.4% (40.6–88.2%) 63.5% (33.1–83.0%) | 29 (80.6%) 9.2 (7.1–13.7) 72.7% (54.0–84.8%) 41.1% (24.2–57.2%) | 75 (64.1%) 11.4 (7.7–17.8) 74.8% (65.5–82.0%) 47.5% (37.1–57.2%) | 0.033 |
| Overall survival | ||||||
| death (events, %) median (months, 95% CI) est. survival rate after 6 months (95% CI) est. survival rate after 12 months (95% CI) | 125 (74.9%) 16.6 (13.2–21.8) 82.0% (75.3–87.1%) 60.5% (52.6–67.4%) | 33 (66.0%) 23.1 (15.0–31.7) 86.0% (72.9–93.1%) 74.0% (59.5–84.0%) | 3 (21.4%) - 92.9% (59.1–99.0%) 92.9% (59.1–99.0%) | 30 (83.3%) 19.8 (11.1–23.9) 83.3% (66.6–92.1%) 66.7% (48.8–79.5%) | 92 (78.6%) 13.9 (10.8–17.8) 80.3% (71.9–86.5%) 54.7% (45.3–63.2%) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönherr, J.; Seifert, P.; Gühne, F.; Winkens, T.; Rauchfuß, F.; Settmacher, U.; Freesmeyer, M.; Drescher, R. Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts. Cancers 2024, 16, 235. https://doi.org/10.3390/cancers16010235
Schönherr J, Seifert P, Gühne F, Winkens T, Rauchfuß F, Settmacher U, Freesmeyer M, Drescher R. Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts. Cancers. 2024; 16(1):235. https://doi.org/10.3390/cancers16010235
Chicago/Turabian StyleSchönherr, Jacqueline, Philipp Seifert, Falk Gühne, Thomas Winkens, Falk Rauchfuß, Utz Settmacher, Martin Freesmeyer, and Robert Drescher. 2024. "Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts" Cancers 16, no. 1: 235. https://doi.org/10.3390/cancers16010235
APA StyleSchönherr, J., Seifert, P., Gühne, F., Winkens, T., Rauchfuß, F., Settmacher, U., Freesmeyer, M., & Drescher, R. (2024). Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts. Cancers, 16(1), 235. https://doi.org/10.3390/cancers16010235






