Simple Summary
Ferritin is a ferroxidase, which protects cellular components from the potentially toxic effects of free iron. The expression and localization of ferritin-heavy chain (FTH1), the catalytic subunit of ferritin, was shown to predict survival for triple-negative breast cancer (BC) patients and be related to T-cell response. Here, we studied the association between FTH1 and time to survival in primary BCs from 222 BRCA1/2 mutation carriers. We found that nuclear, but not cytoplasmic, localization of FTH1 expression was associated with a shorter time to recurrence. In a subset of 51 BRCA1/2 mutation carriers, we evaluated the relation between localization and expression of FTH1 and T-cell response. However, we did not detect any association between FTH1 and the amount or composition of CD8+ cytotoxic, CD4+ helper, or FOXP3+ regulatory T cells. Further research is necessary to unravel the mechanism by which nuclear FTH1 influences the clinical outcome of BRCA1/2-associated BC patients.
Abstract
The ferritin-heavy chain (FTH1) is the catalytic subunit of the ferroxidase ferritin, which prevents oxidative DNA damage via intracellular iron storage. FTH1 was shown to be a prognostic marker for triple-negative breast cancer (BC) patients and associated with an enrichment of CD8+ effector T cells. However, whether the expression and localization of FTH1 are also associated with clinical outcome in other BC subtypes is unknown. Here, we investigated the association of FTH1 with time to survival in BCs from 222 BRCA1/2 mutation carriers by immunohistochemistry on tissue microarrays. In addition, for 51 of these patients, the association between FTH1 and specific subsets of T cells was evaluated on whole slides using automatic scoring algorithms. We revealed that nuclear FTH1 (nFTH1) expression, in multivariable analyses, was associated with a shorter disease-free (HR = 2.71, 95% CI = 1.49–4.92, p = 0.001) and metastasis-free survival (HR = 3.54, 95% CI = 1.45–8.66, p = 0.006) in patients carrying a BRCA1/2 mutation. However, we found no relation between cytoplasmic FTH1 expression and survival of BRCA1/2 mutation carriers. Moreover, we did not detect an association between FTH1 expression and the amount of CD45+ (p = 0.13), CD8+ (p = 0.18), CD4+ (p = 0.20) or FOXP3+ cells (p = 0.17). Consequently, the mechanism underlying the worse recurrence-free survival of nFTH1 expression in BRCA1/2 mutation carriers needs further investigation.
1. Introduction
Ferritin is an iron-binding protein that is present in both the intra- and extracellular compartments. It mainly functions as a ferroxidase in iron sequestration, capturing and converting ferrous iron (Fe2+) into ferric iron (Fe3+). Ferritin is essential for iron homeostasis by making iron available for critical cellular processes, such as oxygen transport or conversion of oxygen into usable cellular energy. On the other hand, ferritin protects cellular components such as DNA and proteins from the potentially toxic effects of free iron, which can directly generate reactive oxygen species (ROS) via the Fenton reaction. The latter induces ferroptosis, a lipid peroxidation-driven and iron-dependent form of regulated cell death. Non-canonical induction of ferroptosis may occur through decreased ferritin expression, for example, through NCOA4-mediated ferritin autophagy. In addition, an excess of iron has also been linked to an altered distribution of T-cell subsets by increasing and decreasing the number and activity of CD8+ cytotoxic T cells and CD4+ helper T cells, respectively. Moreover, excess iron also alters the anti-tumor action of monocytes and macrophages [1,2,3,4].
The ferritin protein is made up of 24 subunits of two different subtypes: ferritin-heavy chains (FTH1; 21 kD) and ferritin-light chains (FTL; 18.5 kD) [5]. The ratio of FTH1, which possesses the ferroxidase catalytic activity, to FTL in the ferritin protein varies widely among tissue types and can be modulated under inflammatory or infectious circumstances [1]. Interestingly, tissue ferritin levels are significantly increased in breast cancer (BC) compared to normal or benign breast tumor tissue [6,7]. Moreover, both FTH1 and FTL expression have been associated with clinical outcomes of BC patients. FTL was shown to be associated with shorter metastasis-free survival (MFS) in node-negative BC patients [8]. However, in a study by Liu et al. FTL was not associated with MFS, while FTH1 was associated with longer MFS in triple-negative BC (TNBC) patients [9].
Previous research has also indicated that the subcellular localization of ferritin is relevant. Particularly in aggressive spindle-like TNBC cell lines, which displayed elevated levels of FTH1 and FTL compared with epithelial BC cell lines, increased ferritin was localized in the nucleus [10]. In line with this, Liu et al. showed that TNBC patients with high (>1%) nuclear FTH1 (nFTH1) expression had a higher risk of metastasis, while high (>75%) cytoplasmic FTH1 (cFTH1) was associated with lower risk of metastasis in these patients [11]. Intriguingly, high expression of cFTH1 was linked to an increase in CD8+ effector T cells but not CD4+ helper T cells. Finally, activation of the CXCL12-CXCR4 signaling pathway was shown to induce time-dependent cFTH1 to nFTH1 switching [12]. Concordantly, CXCL12-CXCR4 signaling promotes migration, invasion, and metastasis of malignant BC cells [13,14].
Around 10% of BC patients have a family history of the disease. In this respect, BRCA1 and BRCA2 are the two most prevalently mutated and penetrant BC genes. In a cancer genetic clinic-based setting, BRCA1 and BRCA2 mutation carriers have a cumulative BC risk of 71% and 64% at 70 years old, while this is 65% and 45% at 70 years in a cohort unselected for family history [15,16]. Importantly, patients with a BRCA1 mutation mostly develop TNBC, while BCs of BRCA2 mutation carriers are generally ER+ [17]. Moreover, BRCA1 and BRCA2-deficient BCs are unable to adequately repair DNA double-strand breaks through the homologous recombination pathway, rendering these tumors highly sensitive to DNA-damaging agents, such as interstrand crosslinking agents, topo-isomerase II inhibitors or PARP inhibitors [18,19,20]. So far, a few studies have indicated either a direct or indirect link between BRCA1, PARP inhibition, and ferroptosis [21,22,23].
By using immunohistochemistry on tissue microarrays, we evaluated whether subcellular localization and expression of FTH1 in 222 BCs of BRCA1/2 mutation carriers were associated with clinical outcome after BC. In addition, we also analyzed whether cFTH1 and nFTH1 expression were correlated with different subsets of T cells in these BCs using an automated scoring algorithm.
2. Materials and Methods
2.1. Study Population
We retrieved primary formalin-fixed paraffin-embedded (FFPE) breast tumor blocks from 241 BRCA1/2 mutation carriers who were counseled at our Clinical Genetic Center and from whom we had previously collected clinical data for survival analyses, as well as treatment data and clinicopathological variables. Tumor blocks were retrieved from the pathology lab at the Erasmus University Medical Center as well as pathology labs from 12 other Dutch hospitals. Patients were diagnosed with BC between 1982 and 2008 and received genetic testing for BRCA1/2 genes between 1996 and 2008. In 191 patients (79.3%), a pathogenic BRCA1 germline mutation was detected, whereas 50 (20.7%) patients carried a pathogenic BRCA2 germline mutation. This study was approved by the Medical Ethics Committee of the Erasmus University Medical Center (MEC 02-953).
2.2. Tissue Microarray Generation
Primary FFPE tumor blocks were sectioned, and H&E stains were prepared. The invasive tumor area was marked on these H&E sections, and tumor grade and histology were reevaluated by a breast pathologist (CvD). Tissue microarray blocks were constructed by punching three 0.6 mm cores from the invasive tumor area from each patient’s primary tumor block and placing these in three different donor blocks using a TMA Grand Master (3DHisTech, Budapest, Hungary). This way, three donor TMA blocks were generated, all including one core from each patient, but organized in a different order.
2.3. Immunohistochemical Staining
Tissue microarray blocks were sectioned and placed on SuperFrost Plus slides (Fisher Scientific, Waltham, MA, USA). After deparaffination and dehydration of sections, antigen retrieval was performed by boiling sections for 40 min in DAKO Target Retrieval Solution (TRS) pH = 6 using a water bath (Glostrup, Denmark). Next, sections were blocked for endogenous peroxidase with 0.3% H2O2 in PBS and additionally blocked using 5% BSA in PBS. The primary antibody against FTH1 (rabbit anti-human FTH1, clone EPR3005Y; Genetex, Irvine, CA, USA) was diluted 1:100 in DAKO Antibody Diluent and incubated for 1 h at room temperature. FTH1 expression was visualized using the rabbit Envision+ system (DAKO), and nuclei were counterstained with hematoxylin.
FFPE blocks from 51 of the 241 BRCA1/2 (42 BRCA1 and 9 BRCA2) mutation carriers that were retrieved from the Erasmus University Medical Center were additionally stained for leukocyte marker CD45, cytotoxic T-cell marker CD8, helper T-cell marker CD4, and regulatory T-cell marker FOXP3 on whole sections, as described above. Antibodies were mouse anti-human CD45 clone 2B11 and PD7/26 (Cell Marque (Rocklin, CA, USA); TRS pH = 8, ready-to-use), mouse anti-human CD8 clone C8/144B (DAKO; TRS pH = 9, 1:100), mouse anti-human CD4 clone 4B12 (DAKO; TRS pH = 9, 1:80), and mouse anti-human FOXP3 clone 236A/E7 (Abcam (Cambridge, UK); TRS pH = 6, 1:50).
2.4. Manual and Automated Scoring
Tissue microarray and whole slides were digitized using the Nanozoomer 2 digital slide scanner (Hamamatsu, Bridgewater, NJ, USA). Tissue microarray scans were uploaded to the Distiller TMA software v2.2 (Leica Microsystems, Wetzlar, Germany) and manually scored for cytoplasmic as well as nuclear FTH1 staining by quantifying the percentage of positively stained invasive tumor cells (Figure 1). Cytoplasmic FTH1 scores were dichotomized into low (≤75%) and high (>75%), whereas nuclear FTH1 scores were dichotomized into negative (≤1%) and positive (>1%), according to Liu et al. [11]. For 19 out of the 241 BRCA1/2 mutation carriers, we were unable to quantify the nFTH1 and cFTH1 scores from the breast tumor tissues due to lost or folded cores or cores not containing invasive tumor cells. Therefore, all analyses were performed with a maximum total of 222 BRCA1/2 mutation carriers, of whom 178 were BRCA1 and 44 were BRCA2 mutation carriers. The patient characteristics of these 222 BRCA1/2 mutation carriers can be found in Table 1.
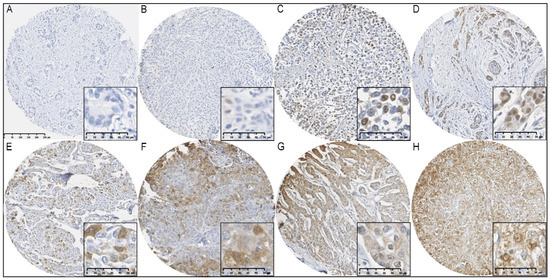
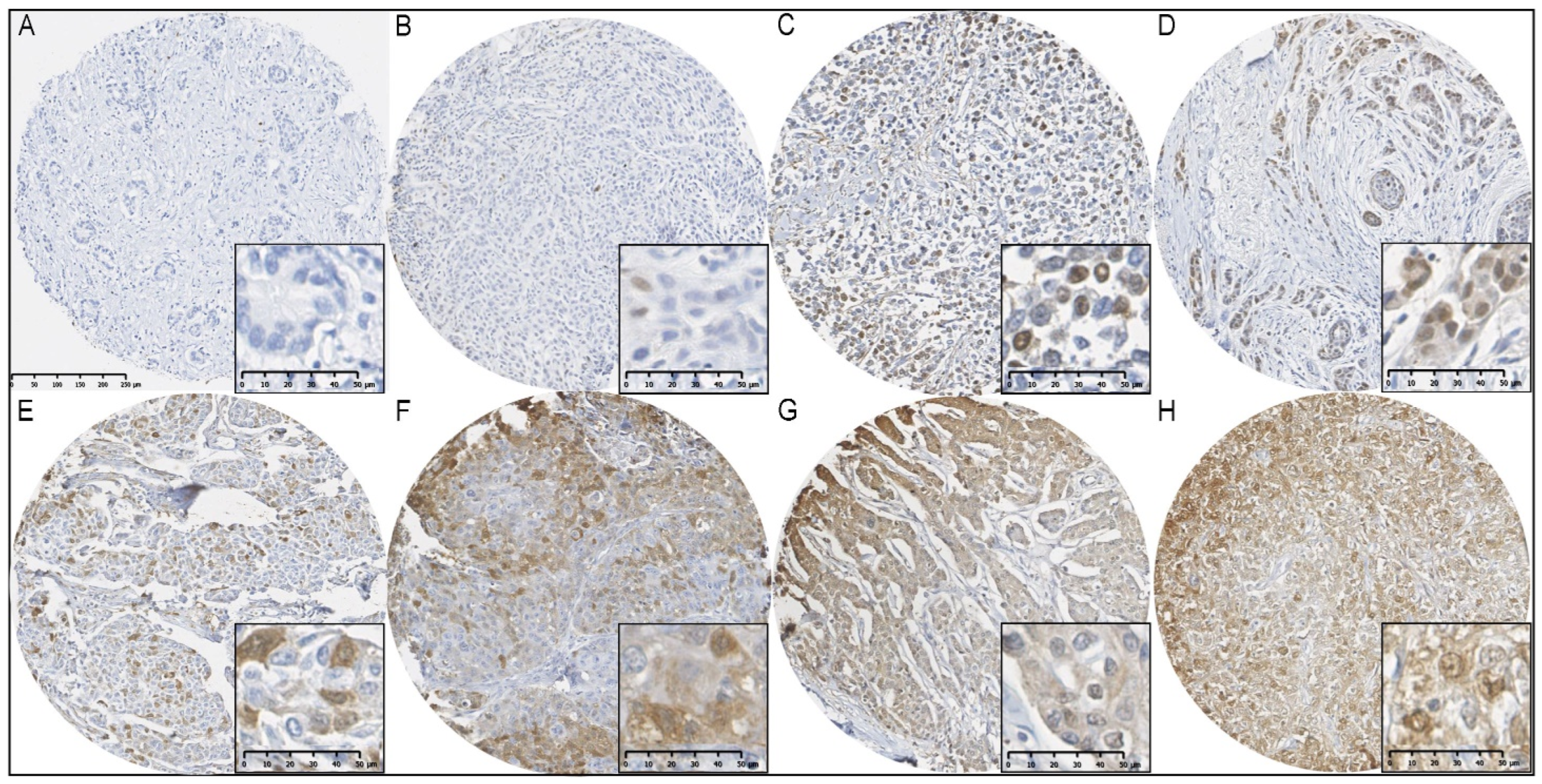
Figure 1.
Immunohistochemical staining of FTH1 in BCs from BRCA1/2 mutation carriers. (A) FTH1 negative; (B) ≤1% nuclear FTH1 expression; (C,D) >1% nuclear FTH1 expression; (E) ≤75% cytoplasmic FTH1 expression; (F,G) >75% cytoplasmic FTH1 expression; (H) nuclear and cytoplasmic FTH1 expression.

Table 1.
Patient characteristics for 222 BRCA1/2 mutation carriers.
Whole slide scans were uploaded to QuPath v0.2.0, and the invasive tumor area was annotated. The percentages of CD45+, CD8+, CD4+, and FOXP3+ cells from the total number of cells in the annotated region were quantified using the positive cell detection algorithm. For all parameters except background radius (6 µm), sigma (1.65 µm), and cell expansion (4 µm), we used standard settings for this algorithm. In addition, we quantified the composition of the three different types of T cells by using the following ratios: CD8+/CD45+; CD4+/CD45+; CD4+/CD8+; and FOXP3+/CD4+.
2.5. Statistical Analysis
To evaluate the association between dichotomized FTH1 expression and clinicopathological factors, we performed a χ2 test or a Fisher’s Exact test when expected values in one of the groups were smaller than five. Because the majority of patients (87.1%) had their genetic DNA test after BC diagnosis, we accounted for potential survival bias by performing left-truncated survival analysis for disease-free, metastasis-free, overall, and BC-specific survival [24]. Consequently, time at risk started at the date of BC diagnosis or DNA test result, whichever came last, and ended either at the date of an event or censoring. This did not change the moment the event occurred but modified the time at risk until the event occurred. Events for DFS included contralateral BCs, local recurrences, lymph node metastases, or distant metastases, while for MFS, only distant metastases were considered. For OS, we considered death from all causes, whereas only death from BC was considered an event for BCSS. Censoring events were a secondary non-BC malignancy or end of follow-up time. Survival probabilities were plotted using the Kaplan–Meier method, and differences between survival curves were evaluated using the logrank test. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), and p-values were from the Wald test. Evaluation of proportional hazard assumptions was performed via plotting the Schoenfeld residuals. Multivariable Cox regression models included all clinicopathological values that were associated with survival time in the univariable model. Associations between dichotomized FTH1 expression and the amount and composition of T cells were evaluated using the Wilcoxon rank sum test with continuity correction. All reported p-values are two-sided.
3. Results
3.1. Clinicopathological Variables
The patient cohort consisted of 222 BC patients, of whom 178 were BRCA1 and 44 were BRCA2 mutation carriers. As expected, BCs from BRCA1 mutation carriers were more frequently of medullary histology and ER and PR negative. Moreover, BCs from these patients more often had a poor differentiation grade compared with BRCA2 mutation carriers (Table 1). For these 222 BC patients with a germline BRCA1 or BRCA2 mutation, we analyzed the association of cFTH1 (Table 2) and nFTH1 (Table 3) expression with the relevant clinicopathological variables. The data showed that cFTH1 expression levels were lower in tumors from patients diagnosed after 2000 in all mutation carriers (p = 0.033) and BRCA1 mutation carriers (p = 0.028) but not BRCA2 mutation carriers. In addition, BRCA2 carriers with ER- BC had a higher cFTH1 (p < 0.001; Table 2).

Table 2.
Association of cytoplasmatic FTH1 expression status with clinicopathological variables in primary breast cancers from 222 BRCA1 or BRCA2 mutation carriers.

Table 3.
Association of nuclear FTH1 expression status with clinicopathological variables in primary breast cancers from 222 BRCA1 or BRCA2 mutation carriers.
BRCA1/2 mutation carriers whose tumors displayed expression of nFTH1 more frequently had a favorable tumor grade (p = 0.015) as well as positive ER (p = 0.002) and PR status (p = 0.029). Moreover, for BRCA1 mutation carriers, nFTH1 expression in the tumor was associated with smaller tumor size (p = 0.045) and positive HER2 status (p = 0.049), while in BRCA2 mutation carriers, nFTH1 expression was associated with positive ER status (p = 0.004; Table 3).
3.2. Survival Analysis
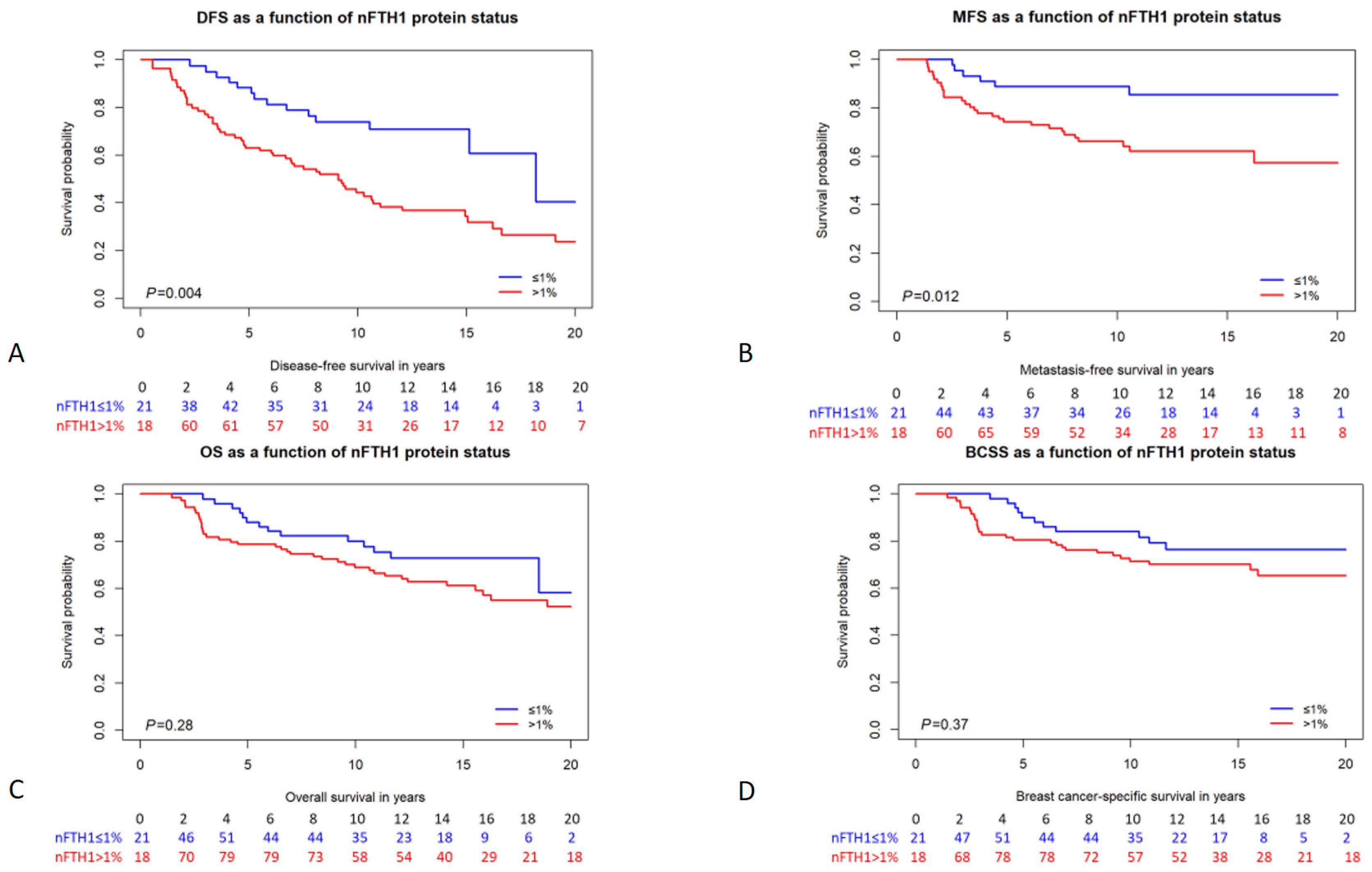
We analyzed the association of FTH1 localization and expression with different survival parameters, including disease-free survival (DFS), MFS, overall survival (OS), and BC-specific survival (BCSS). In patients carrying a germline BRCA1/2 mutation, cFTH1 expression was not associated with any of the survival parameters in Cox proportional hazards analysis (DFS HR = 1.17, 95% CI = 0.72–1.90, p = 0.53; MFS HR = 1.10, 95% CI = 0.55–2.21, p = 0.79; OS HR = 1.09, 95% CI = 0.62–1.91, p = 0.78; BCSS HR = 1.17, 95% CI = 0.61–2.25, p = 0.64; Table 4). Also, when analyzing BRCA1 and BRCA2 mutation carriers separately, cFTH1 expression was not associated with time to survival in either of the two subgroups (Table 4). We also analyzed the relationship between the expression of nFTH1 and the time to survival in patients carrying BRCA1/2 mutations. Interestingly, we found nFTH1 expression to be associated with a shorter DFS and MFS in both univariable (DFS HR = 2.32, 95% CI = 1.29–4.20, p = 0.005; MFS HR = 2.94, 95% CI = 1.21–7.12, p = 0.017; Figure 2 and Table 4) and multivariable analyses (DFS HR = 2.71, 95% CI = 1.49–4.92, p = 0.001; MFS HR = 3.54, 95% CI = 1.45–8.66, p = 0.006; Table 4). However, OS (HR = 1.40, 95% CI = 0.75–2.61, p = 0.28) and BCSS (HR = 1.38, 95% CI = 0.68–2.80, p = 0.37) were not associated with nFTH1 expression in BRCA1/2 mutation carriers (Figure 2 and Table 4). Because their clinicopathological characteristics differ considerably, we also performed subanalyses in BRCA1 and BRCA2 mutation carriers separately. Consistent with the results in all mutation carriers, nFTH1 expression was associated with a shorter DFS and MFS in both univariable (DFS HR = 2.29, 95% CI = 1.20–4.39, p = 0.012; MFS HR = 3.14, 95% CI = 1.18–8.39, p = 0.022) and multivariable (DFS HR = 3.02, 95% CI = 1.54–5.91, p = 0.001; MFS HR = 4.47, 95% CI = 1.62–12.3, p = 0.004) analyses in BRCA1 mutation carriers, but not with differences in OS and BCSS (Supplementary Figure S1 and Table 4). However, we found no association between nFTH1 and survival in BRCA2 mutation carriers (Supplementary Figure S1 and Table 4).

Table 4.
Uni- and multivariable Cox regression analysis of 222 BRCA1 or BRCA2 mutation carriers by FTH1 expression and localization.
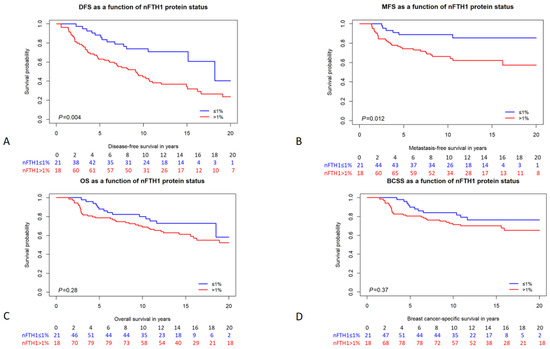
Figure 2.
Kaplan–Meier survival curves of BRCA1/2 mutation carriers stratified by nFTH1 expression. (A) disease-free survival; (B) metastasis-free survival; (C) overall survival; (D) breast cancer-specific survival. The number of persons at risk per time point is indicated below each graph. p-values are from the logrank test.
3.3. Immune Cells
In 2014, Liu et al. showed that high cFTH1 expression was associated with a favorable prognosis and a clearly decreased ratio between CD4+ and CD8+ T cells in TNBC. In addition, they hypothesized that the decreased CD4+ T-cell density in cFTH1 high TNBC tumors could be related to a lower density of FOXP3+ regulatory T cells [11]. Although we only found an association between nFTH1, not cFTH1, expression, and clinical outcome in BRCA1/2 mutation carriers, we still aimed to verify whether T-cell response played a role in this. Therefore, we determined the percentages of CD45+, CD8+, CD4+, and FOXP3+ cells in whole slides of BCs from 51 BRCA1/2 mutation carriers using an automated scoring algorithm. In this cohort, we did not find any association between cFTH1 expression and the amount (CD45+ p = 0.32, CD8+ p = 0.34, CD4+ p = 0.93, FOXP3+ p = 0.48) or composition (CD8+/CD45+ p = 0.33, CD4+/CD45+ p = 0.80, CD4+/CD8+ p = 0.41, FOXP3+/CD4+ p = 0.63) of the various immune cells. Similarly, we did not find any relation between the amount (CD45+ p = 0.13, CD8+ p = 0.18, CD4+ p = 0.20, FOXP3+ p = 0.17) or composition (CD8+/CD45+ p = 0.91, CD4+/CD45+ p = 0.98, CD4+/CD8+ p = 0.84, FOXP3+/CD4+ p = 1) of the various immune cells and nFTH1 expression.
4. Discussion
Here, we studied the expression and subcellular localization of FTH1 in BCs from 222 BRCA1/2 mutation carriers and investigated the association with the patients’ clinicopathological variables, survival, as well as their T-cell response in a subset of 51 BRCA1/2 mutation carriers.
We noticed that the expression of nFTH1 was associated with more favorable clinicopathological parameters in our study, including a smaller tumor size and a lower tumor grade. In addition, nFTH1-positive BCs were also more frequently ER- and PR-positive. This seems counterintuitive since we also established that nFTH1 is an independent predictor of shorter DFS and MFS. Positive associations between cFTH1 as well as nFTH1 and the clinicopathological variables, however, could have been false positive associations as a consequence of the multiple statistical tests we performed. Indeed, after conservative Bonferroni adjustment for multiple tests, only the association between high cFTH1 expression and negative ER status in BRCA2 mutation carriers remained significant.
Based on our results in BRCA1/2 mutation carriers, nuclear expression of FTH1 was only associated with patients’ DFS and MFS but not with OS or BCSS. It is, however, implausible that nFTH1 is associated with the recurrence of the disease but not death. Therefore, we considered that the number of events is likely higher for DFS and MFS compared with OS and BCSS, and the absence of an association with OS and BCSS might be a consequence of statistical power. In line with this, 39.0% of BRCA1/2 mutation carriers had a DFS event, while 23.5% and 17.9% of carriers had an OS or BCSS event. However, the rate of MFS events in these patients was similar to the rate of OS and BCSS events (i.e., 18.8%), suggesting that power was likely not an issue in our analyses unless the effect size for OS and BCSS was smaller. Considering the survival probability curves of the two nFTH1 groups were closer in Figure 2 for BCSS and OS than for DFS and MFS, this might be a plausible explanation. Since we had a relatively long median follow-up time of 111 months for our cohort, it is likely that conclusive evidence requires an expansion of the number of patients rather than the follow-up time. We do have to point out, though, that numbers at risk become very low after 15 years of follow-up time.
The expression of nFTH1 was significantly associated with shorter DFS and MFS in BRCA1/2 and BRCA1 mutation carriers but not in BRCA2 mutation carriers alone. This may not be entirely unexpected since nFTH1 expression was previously found to be related to worse prognosis in TNBC patients [11], and BRCA1 mutation is generally associated with TNBC [17]. Thus, the function of FTH1 may be associated with hormone receptor status. Alternatively, the loss of genome stability in both TNBC and BRCA1/2 mutation carriers, in combination with the deregulation of iron homeostasis, could also be the underlying mechanism. Since we were only able to include 42 BRCA2 mutation carriers in this study, we may not have had enough power to detect an association in this group. Therefore, conclusive evidence regarding the role of nFTH1 expression in the survival of BRCA2 mutation carriers requires a larger study population.
We did not detect any associations between cFTH1 expression and survival in BRCA1 mutation carriers. These results conflict with the study of Liu et al., in which they showed that high expression of cFTH1 was related to a favorable prognosis in TNBC [11]. In concordance with our results, though, Liu et al. showed that nFTH1 expression was associated with an adverse prognosis. Further research has to determine whether this discrepancy is a consequence of biological differences between TNBC and BCs of BRCA1/2 mutation carriers.
Liu et al. also suggested that high cFTH1 expression indicated a favorable prognosis via enrichment of CD8+ T cells in TNBCs, while CD4+ T cells were diminished [11]. They hypothesized that this lower density of CD4+ T cells could be related to a lower density of immune suppressive regulatory T cells. Moreover, in our data, we observed a lower percentage of medullary BCs among nFTH1-positive compared with nFTH1-negative BCs (16.9% vs. 30.1%), which suggests a lower amount of tumor-infiltrating lymphocytes among nFTH1 positive BCs. However, we do not find any relation between the expression and localization of FTH1 and the density of CD8+, CD4+, or FOXP3+ T cells, as well as the ratios between CD8+/CD45+, CD4+/CD45+, CD4+/CD8+, and FOXP3+/CD4+ cells. Compared with Liu et al.’s study, we used a more quantitative method involving automated scoring of the immune cell markers using QuPath v0.2.0 while they estimated the percentage of positively stained cells. However, this disconcordance may more likely be the result of true biological differences between TNBCs and BCs from BRCA1/2 mutation carriers. Therefore, future research avenues should focus on the biological effects of cellular FTH1 localization and its relation to BRCA1/2 mutation status, hormone receptor status, ferroptosis, other immune cells such as monocytes and macrophages and activation of the CXCL12-CXCR4 signaling pathway [1,2,3,4,12,21,22,23].
5. Conclusions
Nuclear localization of FTH1 was associated with a shorter DFS and MFS but not OS and BCSS in a cohort of 222 BRCA1/2 mutation carriers. This association was particularly pronounced in BRCA1 mutation carriers; however, conclusive evidence regarding this association in BRCA2 mutation carriers is pending. We found no evidence of an association between cytoplasmic localization of FTH1 and the time to survival. Moreover, we did not find a relation between the expression and localization of FTH1 and the density or composition of CD8+ cytotoxic T cells, CD4+ helper T cells, or FOXP3+ regulatory T cells in these BCs. Therefore, the mechanism by which nFTH1 influences the clinical outcome of BRCA1/2 mutation carriers is still unclear. Further research should focus on the biological effects of cellular FTH1 localization and its relation to BRCA1/2 mutation status, hormone receptor status, ferroptosis, and downstream immune response.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16010028/s1. Figure S1. Kaplan–Meier survival curves of BRCA1 and BRCA2 mutation carriers stratified by nFTH1 expression.
Author Contributions
Conceptualization, J.W.M.M., M.J.H. and A.H.; data curation, A.M.T., B.A.M.H.-G., C.H.M.v.D., M.J.H. and A.H.; formal analysis, S.Q., B.A.M.H.-G. and A.H.; methodology, A.M.T., B.A.M.H.-G., M.J.H. and A.H.; experimentation, A.M.T., A.M.A.C.T.-J., R.B.-F., W.J.C.P.-v.d.S., H.E.H. and T.v.T.; resources, C.K.B.-S., P.J.W. and C.H.M.v.D.; supervision, A.H.; funding acquisition, M.J.H.; writing—original draft, S.Q., A.M.T. and A.H.; writing—review and editing, S.Q., A.M.T., B.A.M.H.-G., P.J.W., C.H.M.v.D., J.W.M.M., M.J.H. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Dutch Cancer Society under DDHK 2004-3124.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethical Committee of the Erasmus MC under MEC 02.953.
Informed Consent Statement
This retrospective medical data and biospecimen study has been executed pursuant to Dutch legislation and international standards. Prior to 25 May 2018, national legislation on data protection applied, as well as the International Guideline on Good Clinical Practice. Since 25 May 2018 we have also adhered to the GDPR. Within this framework, patients are informed and have always had the opportunity to object or actively consent to the (continued) use of their personal data and biospecimens in research. Hence, the procedures comply both with (inter-)national legislative and ethical standards.
Data Availability Statement
Tissue microarray data are available upon request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the Erasmus University Medical Center’s department of Pathology for use of its facilities and services for TMA construction and immunohistochemical staining. The authors are also thankful to the pathologists and pathology lab members of Pathan, Maasstad ziekenhuis, PAL Dordrecht, Reinier de Graaf ziekenhuis, Groene Hart ziekenhuis, Lievensberg ziekenhuis, Amphia ziekenhuis, Elisabeth-Tweesteden ziekenhuis, Bronovo ziekenhuis, HMC Westeinde, Admiraal de Ruyter ziekenhuis, and Zorgzaam ziekenhuis for making formalin-fixed paraffin-embedded breast cancer tissues available for TMA construction.
Conflicts of Interest
Claudia K. Bes-Stobbe was employed by Pathan B.V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, X.B.; Ying, M.D.; He, Q.J.; Cao, J.; Yang, B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front. Pharmacol. 2017, 8, 992. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, Y.; Yu, X.; Li, X.; Fang, X. Induction and application of ferroptosis in cancer therapy. Cancer Cell Int. 2022, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Adelman, T.G.; Drysdale, J.W. On ferritin heterogeneity. Further evidence for heteropolymers. J. Biol. Chem. 1978, 253, 4451–4458. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.E.; Bond, B.H.; Silberberg, B.K. Tissue ferritin concentration in carcinoma of the breast. Cancer 1982, 50, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.L.; Elliott, M.C.; Wang, F.; Head, J.F. Breast carcinoma and the role of iron metabolism. A cytochemical, tissue culture, and ultrastructural study. Ann. N. Y. Acad. Sci. 1993, 698, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jezequel, P.; Campion, L.; Spyratos, F.; Loussouarn, D.; Campone, M.; Guerin-Charbonnel, C.; Joalland, M.-P.; André, J.; Descotes, F.; Grenot, C.; et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in node-negative breast cancer tumors: A multicentric 2004 national PHRC study. Int. J. Cancer 2012, 131, 426–437. [Google Scholar] [CrossRef]
- Liu, N.Q.; Stingl, C.; Look, M.P.; Smid, M.; Braakman, R.B.; De Marchi, T.; Sieuwerts, A.M.; Span, P.N.; Sweep, F.C.G.J.; Linderholm, B.K.; et al. Comparative proteome analysis revealing an 11-protein signature for aggressive triple-negative breast cancer. J. Natl. Cancer Inst. 2014, 106, djt376. [Google Scholar] [CrossRef]
- Shpyleva, S.I.; Tryndyak, V.P.; Kovalchuk, O.; Starlard-Davenport, A.; Chekhun, V.F.; Beland, F.A.; Pogribny, I.P. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res. Treat. 2011, 126, 63–71. [Google Scholar] [CrossRef]
- Liu, N.Q.; De Marchi, T.; Timmermans, A.M.; Beekhof, R.; Trapman-Jansen, A.M.; Foekens, R.; Look, M.P.; van Deurzen, C.H.M.; Span, P.N.; Sweep, F.C.G.J.; et al. Ferritin heavy chain in triple negative breast cancer: A favorable prognostic marker that relates to a cluster of differentiation 8 positive (CD8+) effector T-cell response. Mol. Cell. Proteom. 2014, 13, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, C.; Mines, M.; Zhang, J.; Fan, G.H. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J. Biol. Chem. 2006, 281, 37616–37627. [Google Scholar] [CrossRef] [PubMed]
- Luker, K.E.; Lewin, S.A.; Mihalko, L.A.; Schmidt, B.T.; Winkler, J.S.; Coggins, N.L.; Thomas, D.G.; Luker, G.D. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene 2012, 31, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.A.; Massagué, J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013, 154, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Con-tralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomarkers Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and syn-ergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Akatsuka, S.; Motooka, Y.; Zheng, H.; Cheng, Z.; Shiraki, Y.; Mashimo, T.; Imaoka, T.; Toyokuni, S. BRCA1 haploinsufficiency promotes chromosomal amplification under Fenton reaction-based carcinogenesis through ferroptosis-resistance. Redox Biol. 2022, 54, 102356. [Google Scholar] [CrossRef] [PubMed]
- Azzato, E.M.; Greenberg, D.; Shah, M.; Blows, F.; Driver, K.E.; Caporaso, N.E.; Pharoah, P.D.P. Prevalent cases in observational studies of cancer survival: Do they bias hazard ratio estimates? Br. J. Cancer 2009, 100, 1806–1811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).