Impact of the COVID-19 Pandemic on Incidence and Observed Survival of Malignant Brain Tumors in Belgium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
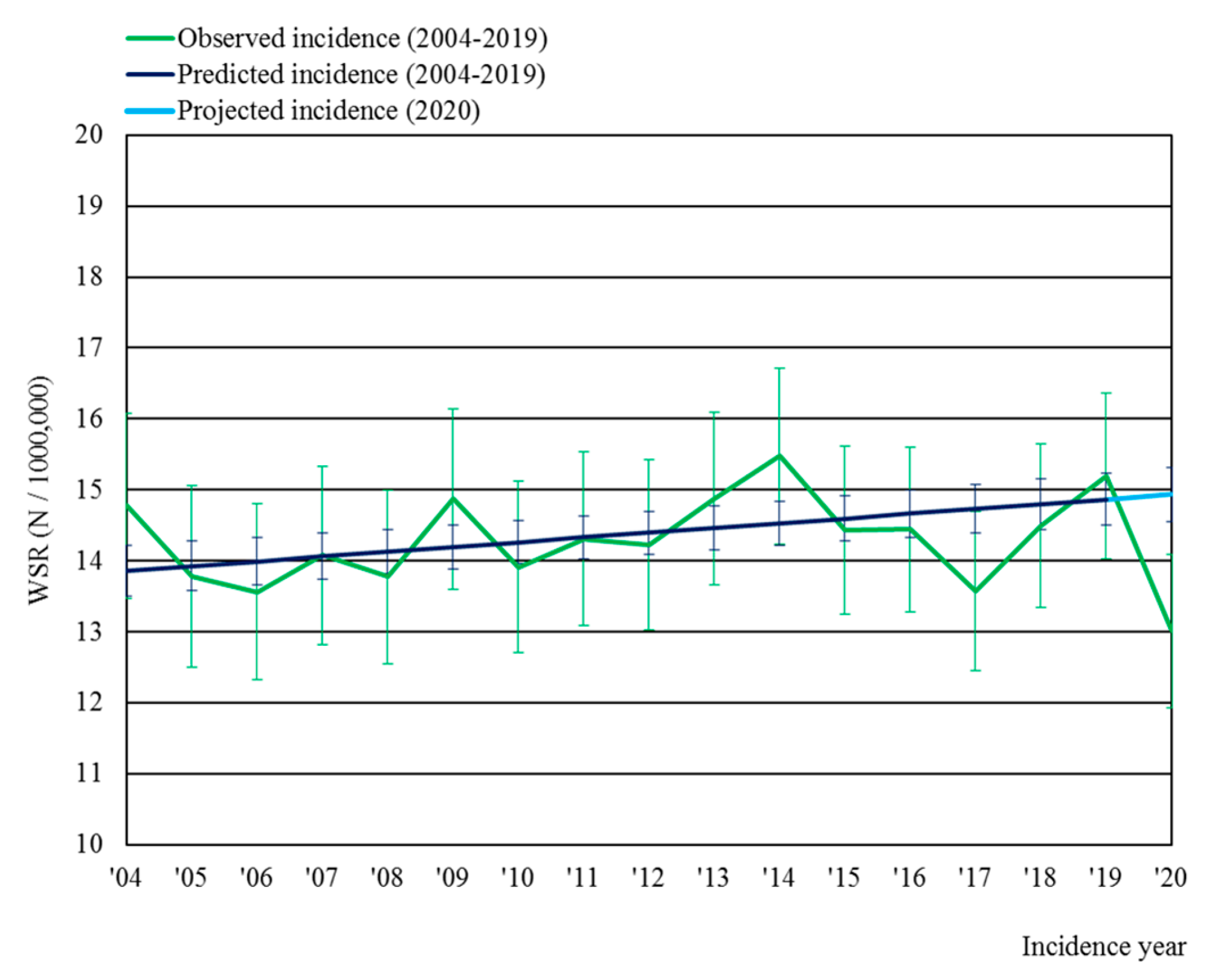
3.1. Incidence of Malignant Brain Tumors
3.2. Survival of Patients with Malignant Brain Tumors and Subtypes
3.3. Treatment for Glioblastoma and Hematolymphoid Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, H.M.; Tambuyzer, T.; Verdoodt, F.; Calay, F.; Poirel, H.A.; De Schutter, H.; Francart, J.; Van Damme, N.; Van Eycken, L. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: A year-long, population-level analysis. ESMO Open 2021, 6, 100197. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, C.W.; Friis, S.; Christensen, J.; Nilbert, M.C.; Morch, L.S. Drop in cancer diagnosis during the COVID-19 pandemic in Denmark: Assessment of impact during 2020. Acta Oncol. 2022, 61, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Walbaum, M.; Walbaum, B.; Guzman, M.J.; Jimenez de la Jara, J.; Nervi, B.; Atun, R. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: A simulation-based analysis. Lancet Oncol. 2021, 22, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- De Vincentiis, L.; Carr, R.A.; Mariani, M.P.; Ferrara, G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: An audit study from cellular pathology. J. Clin. Pathol. 2021, 74, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Paciotti, M.; Schmidt, A.L.; Lipsitz, S.R.; Choueiri, T.K.; Trinh, Q.D. Cancer Screening Tests and Cancer Diagnoses during the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Tam, S.; Bader, T.; Sorkin, A.; Benov, A. Pausing cancer screening during the severe acute respiratory syndrome coronavirus 2pandemic: Should we revisit the recommendations? Eur. J Cancer. 2020, 134, 86–89. [Google Scholar] [CrossRef]
- ElGhamry, A.N.; Jayakumar, N.; Youssef, M.; Shumon, S.; Mitchell, P. COVID-19 and Changes in Neurosurgical Workload in the United Kingdom. World Neurosurg. 2021, 148, e689–e694. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, Q.; Chiang, J.; Tan, S.H.; Chua, G.W.Y.; Xie, C.; Chua, M.L.K.; Soon, Y.Y.; Yang, V.S. Impact of cancer diagnoses on the outcomes of patients with COVID-19: A systematic review and meta-analysis. BMJ Open 2022, 12, e044661. [Google Scholar] [CrossRef]
- Pessina, F.; Navarria, P.; Bellu, L.; Clerici, E.; Politi, L.S.; Tropeano, M.P.; Simonelli, M.; Fornari, M.; Scorsetti, M. Treatment of patients with glioma during the COVID-19 pandemic: What we learned and what we take home for the future. Neurosurg. Focus 2020, 49, E10. [Google Scholar] [CrossRef]
- Azab, M.A.; Azzam, A.Y. Impact of COVID-19 pandemic on the management of glioma patients around the world. An evidence-based review. Brain Disord. 2021, 2, 100012. [Google Scholar] [CrossRef] [PubMed]
- Henau, K.; Van Eycken, E.; Silversmit, G.; Pukkala, E. Regional variation in incidence for smoking and alcohol related cancers in Belgium. Cancer Epidemiol. 2015, 39, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wet Houdende Diverse Bepalingen Betreffende Gezondheid van 13 December 2006, Artikel 39./Loi Portant Dispositions Diverses en Matière de Santé du 13 Décembre 2006, Article 39, (2006). Available online: https://etaamb.openjustice.be/nl/wet-van-13-december-2006_n2006023386.html (accessed on 2 August 2023).
- Fritz, A.; Percy, C.; Jack, A.; Shanmugratnam, K.; Sobin, L.; Parkin, M.D.; Whelan, S. International Classification of Diseases for Oncology; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Kaplan, E.L.; Meier, P. Non parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Belgian Cancer Registry. Cancer Incidence Projections in Belgium 2015 to 2025; Belgian Cancer Registry: Brussels, Belgium, 2017. [Google Scholar]
- Jayakumar, N.; Kennion, O.; Villabona, A.R.; Paranathala, M.; Holliman, D. Neurosurgical Referral Patterns During the Coronavirus Disease 2019 Pandemic: A United Kingdom Experience. World Neurosurg. 2020, 144, e414–e420. [Google Scholar] [CrossRef] [PubMed]
- Castano-Leon, A.M.; Paredes, I.; Lagares, A.; Gomez, P.A.; Gonzalez-Leon, P.; Perez-Nunez, A.; Jiménez-Roldán, L.; Delgado-Fernández, J.; Fernández, C.E.; García-Pérez, D.; et al. Patients awaiting surgery for neurosurgical diseases during the first wave of the COVID-19 pandemic in Spain: A multicentre cohort study. BMJ Open 2022, 12, e061208. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, J.; Zhang, T.; Feng, Y.; Xiong, Z.; Xu, C.; Gong, P.; Si, J.; Chen, J. Characteristics and operation outcomes of neuro-oncology patients after COVID-19 pandemic—A case series. Interdiscip. Neurosurg. 2021, 25, 101172. [Google Scholar] [CrossRef] [PubMed]
- Mischkulnig, M.; Hopp, B.; Wadiura, L.I.; Khalaveh, F.; Kiesel, B.; Rossler, K.; Widhalm, G.; Dorfer, C. Treatment of high-grade glioma patients during the COVID-19 pandemic: Impact on overall survival, tumor size and delay of treatment. PLoS ONE 2023, 18, e0287993. [Google Scholar] [CrossRef]
- Chahal, M.; Aljawi, G.; Harrison, R.; Nichol, A.; Thiessen, B. Treatment Patterns and Outcomes of Patients with Grade 4 Glioma Treated with Radiation during the COVID-19 Pandemic. Curr. Oncol. 2023, 30, 3091–3101. [Google Scholar] [CrossRef]
- de Pelsemaeker, M.C.; Guiot, Y.; Vanderveken, J.; Galant, C.; Van Bockstal, M.R. The Impact of the COVID-19 Pandemic and the Associated Belgian Governmental Measures on Cancer Screening, Surgical Pathology and Cytopathology. Pathobiology 2021, 88, 46–55. [Google Scholar] [CrossRef]
- Hamilton, W. Cancer diagnostic delay in the COVID-19 era: What happens next? Lancet Oncol. 2020, 21, 1000–1002. [Google Scholar] [CrossRef]
- Luo, Q.; O’Connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.R.; Oliver, K.; Farrimond, S.; Chee, T.; Arzbaecher, J.; Kruchko, C.; Maher, M.E.; Tse, C.; Cashman, R.; Daniels, M.; et al. Brain tumors and COVID-19: The patient and caregiver experience. Neurooncol. Adv. 2020, 2, vdaa104. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Ramakrishna, S.; Long, A.H.; Phillips, C.A.; Montiel-Esparza, R.; Diorio, C.J.; Bailey, L.C.; Maude, S.L.; Aplenc, R.; Batra, V.; et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr. Blood Cancer 2020, 67, e28427. [Google Scholar] [CrossRef] [PubMed]
- Wasim, U.; Tahir, M.J.; Siddiqi, A.R.; Jabbar, A.; Ullah, I. The impact of the COVID-19 pandemic on impending cancer deaths due to delays in diagnosis in the UK. J. Med. Virol. 2022, 94, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Joannides, A.; Plaha, P.; Afshari, F.T.; Albanese, E.; Barua, N.U.; Chan, H.W.; Critchley, G.; Flannery, T.; Fountain, D.M.; et al. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: A national survey (COVID-CNSMDT Study). BMJ Open 2020, 10, e040898. [Google Scholar] [CrossRef] [PubMed]
- Fountain, D.M.; Piper, R.J.; Poon, M.T.C.; Solomou, G.; Brennan, P.M.; Chowdhury, Y.A.; Colombo, F.; Elmoslemany, T.; Ewbank, F.G.; Grundy, P.L.; et al. CovidNeuroOnc: A UK multicenter, prospective cohort study of the impact of the COVID-19 pandemic on the neuro-oncology service. Neurooncol. Adv. 2021, 3, vdab014. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.; Ramos, A.; Giantini Larsen, A.M.; Bander, E.; Goldberg, J.; Parker, W.; Juthani, R.G. Impact of the COVID-19 pandemic on neuro-oncology outcomes. J. Neurooncol. 2021, 154, 375–381. [Google Scholar] [CrossRef]
- Bernhardt, D.; Wick, W.; Weiss, S.E.; Sahgal, A.; Lo, S.S.; Suh, J.H.; Chang, E.L.; Foote, M.; Perry, J.; Meyer, B.; et al. Neuro-oncology Management during the COVID-19 Pandemic with a Focus on WHO Grade III and IV Gliomas. Neuro Oncol. 2020, 22, 928–935. [Google Scholar] [CrossRef]
- Baba, M.A.; Adali, N. The Management of Glioblastoma during SARS-CoV-19 Pandemic: A Narrative Overview. Eur. J. Basic Med. Sci. 2021, 11, 19–22. [Google Scholar] [CrossRef]
- Silversmit, G.; Verdoodt, F.; Van Damme, N.; De Schutter, H.; Van Eycken, L. Excess mortality in a nationwide cohort of cancer patients during the Initial phase of the COVID-19 pandemic in Belgium. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1615–1619. [Google Scholar] [CrossRef]
- Directorate-General Statistics Belgium. Available online: http://www.statbel.fgov.be/ (accessed on 12 December 2023).
- Zhang, J.; Medeiros, L.J.; Young, K.H. Cancer Immunotherapy in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2018, 8, 351. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambuyzer, T.; Vanhauwaert, D.; Boterberg, T.; De Vleeschouwer, S.; Peacock, H.M.; Bouchat, J.; Silversmit, G.; Verdoodt, F.; De Gendt, C.; Van Eycken, L. Impact of the COVID-19 Pandemic on Incidence and Observed Survival of Malignant Brain Tumors in Belgium. Cancers 2024, 16, 63. https://doi.org/10.3390/cancers16010063
Tambuyzer T, Vanhauwaert D, Boterberg T, De Vleeschouwer S, Peacock HM, Bouchat J, Silversmit G, Verdoodt F, De Gendt C, Van Eycken L. Impact of the COVID-19 Pandemic on Incidence and Observed Survival of Malignant Brain Tumors in Belgium. Cancers. 2024; 16(1):63. https://doi.org/10.3390/cancers16010063
Chicago/Turabian StyleTambuyzer, Tim, Dimitri Vanhauwaert, Tom Boterberg, Steven De Vleeschouwer, Hanna M. Peacock, Joanna Bouchat, Geert Silversmit, Freija Verdoodt, Cindy De Gendt, and Liesbet Van Eycken. 2024. "Impact of the COVID-19 Pandemic on Incidence and Observed Survival of Malignant Brain Tumors in Belgium" Cancers 16, no. 1: 63. https://doi.org/10.3390/cancers16010063
APA StyleTambuyzer, T., Vanhauwaert, D., Boterberg, T., De Vleeschouwer, S., Peacock, H. M., Bouchat, J., Silversmit, G., Verdoodt, F., De Gendt, C., & Van Eycken, L. (2024). Impact of the COVID-19 Pandemic on Incidence and Observed Survival of Malignant Brain Tumors in Belgium. Cancers, 16(1), 63. https://doi.org/10.3390/cancers16010063





