Photodynamic Therapy and Immunological View in Gastrointestinal Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Gastric Cancers—Morbidity
1.2. Photodynamic Therapy—One of the Treatment Methods
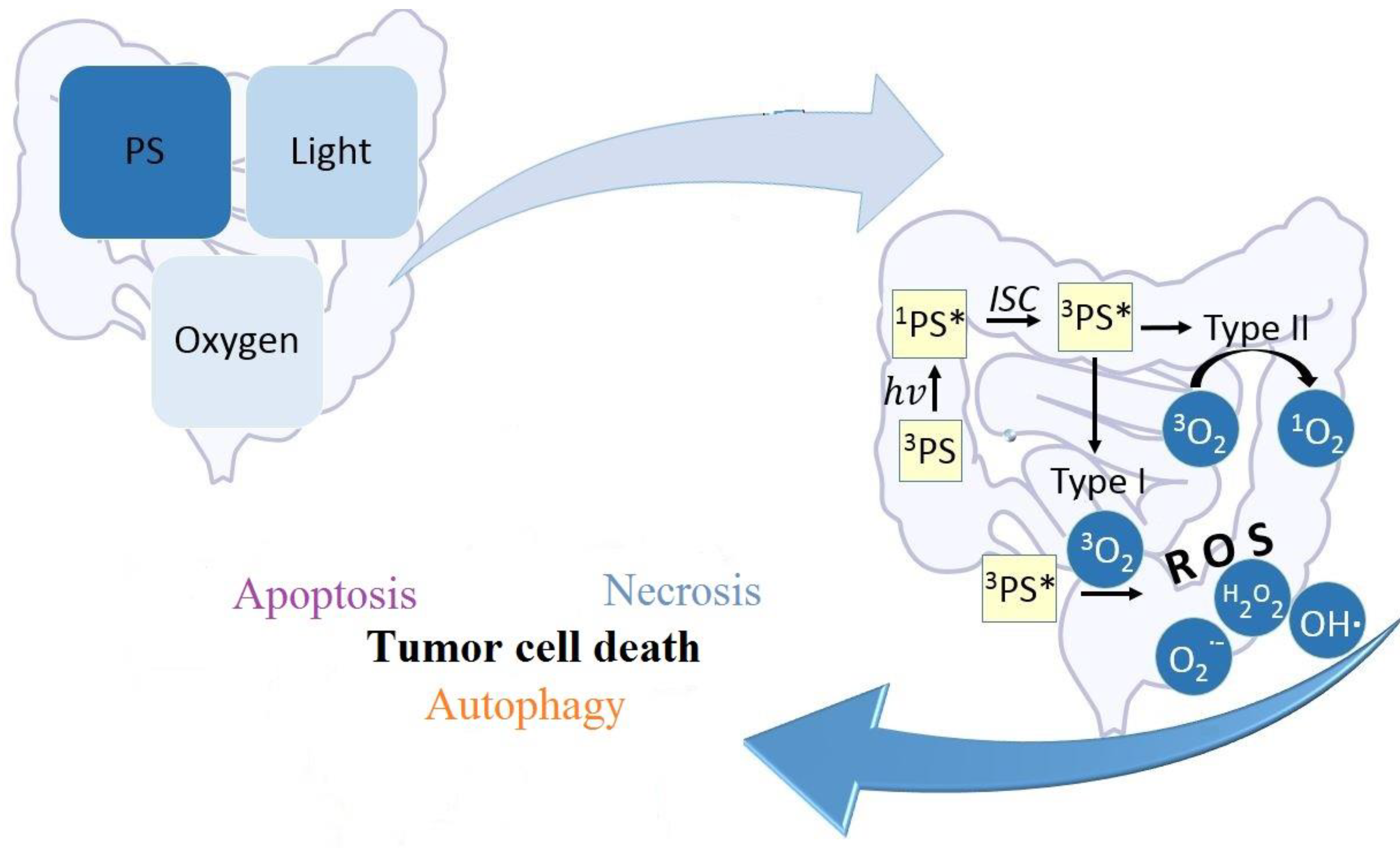
1.3. Overall Cellular Response to PDT
1.4. Merits and Defects of PDT
1.4.1. Merits
1.4.2. Defects
2. Materials and Methods
3. Literature Review
3.1. Esophageal Cancer
3.2. Stomach Cancer
3.3. Colon Cancer
3.4. Interaction of PDT with Gastrointestinal Tumor Cells
3.5. Clinical Challenges
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Krasna, M. Overview of esophageal cancer. Ann. Cardiothorac. Surg. 2017, 6, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Short, M.W.; Burgers, K.G.; Fry, V.T. Esophageal Cancer. Am. Fam. Physician 2017, 95, 22–28. [Google Scholar] [PubMed]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Labianca, R.; Beretta, G.D.; Gatta, G.; de Braud, F.; Van Cutsem, E. Gastric cancer. Crit. Rev. Oncol. Hematol. 2009, 71, 127–164. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Haraldsdottir, S.; Einarsdottir, H.M.; Smaradottir, A.; Gunnlaugsson, A.; Halfdanarson, T.R. Colorectal cancer—Review. Laeknabladid 2014, 100, 75–82. [Google Scholar]
- Cappell, M.S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. N. Am. 2008, 37, 1–24. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Serafin, I.; Dynarowicz, K.; Aebisher, D. Photodynamic therapy and associated targeting methods for treatment of brain cancer. Front. Pharmacol. 2023, 14, 1250699. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Aihara, Y.; Oda, Y.; Fukui, A.; Tsuzuk, S.; Saito, T.; Nitta, M.; Muragaki, Y.; Kawamata, T. Photodynamic therapy for malignant brain tumors in children and young adolescents. Front. Oncol. 2022, 12, 957267. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, L.I.N.; Hassan, S.A.B.; Alshadidi, A.A.F.; Rangaiah, G.C.; Divakar, D.D. Short-term influence of antimicrobial photodynamic therapy as an adjuvant to mechanical debridement in reducing soft-tissue inflammation and subgingival yeasts colonization in patients with peri-implant mucositis. Photodiagn. Photodyn. Ther. 2023, 42, 103320. [Google Scholar] [CrossRef] [PubMed]
- Gil-Pallares, P.; Navarro-Bielsa, A.; Almenara-Blasco, M.; Gracia-Cazaña, T.; Gilaberte, Y. Photodynamic Therapy, a successful treatment for granular parakeratosis. Photodiagn. Photodyn. Ther. 2023, 42, 103562. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M. Laser-mediated photodynamic therapy. Clin. Dermatol. 2006, 24, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Kübler, A.C. Photodynamic therapy. Med. Laser Appl. 2005, 20, 37–45. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Herman, M.; Kessel, D.; Fromm, D. Photodynamic treatment of neoplastic lesions of the gastrointestinal tract. Recent advances in techniques and results. Langenbecks Arch. Surg. 2000, 385, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cai, W.; Huang, J.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. Pyroptosis in development, inflammation and disease. Front. Immunol. 2022, 13, 991044. [Google Scholar] [CrossRef]
- Slastnikova, T.A.; Rosenkranz, A.A.; Lupanova, T.N.; Gulak, P.V.; Gnuchev, N.V.; Sobolev, A.S. Study of efficiency of the modular nanotransporter for targeted delivery of photosensitizers to melanoma cell nuclei in vivo. Dokl. Biochem. Biophys. 2012, 446, 235–237. [Google Scholar] [CrossRef]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, S.; Li, Y.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. Regulated cell death: Discovery, features and implications for neurodegenerative diseases. Cell Commun Signal. 2021, 19, 120. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Osuchowski, M.; Adamczyk, M.; Stopa, J.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Advancements in photodynamic therapy of esophageal cancer. Front. Oncol. 2022, 12, 1024576. [Google Scholar] [CrossRef]
- Yano, T.; Hatogai, K.; Morimoto, H.; Yoda, Y.; Kaneko, K. Photodynamic therapy for esophageal cancer. Ann. Transl. Med. 2014, 2, 29. [Google Scholar]
- Qumseya, B.J.; David, W.; Wolfsen, H.C. Photodynamic Therapy for Barrett’s Esophagus and Esophageal Carcinoma. Clin. Endosc. 2013, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Barr, H. Photodynamic therapy in gastrointestinal cancer: A realistic option? Drugs Aging 2000, 16, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef]
- Xu, J.; Gao, J.; Wei, Q. Combination of photodynamic therapy with radiotherapy for cancer treatment. J. Nanomater. 2016, 2016, 8507924. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mukhopadhyay, M.; Shivam, K.; Tripathy, S.; Patra, R.; Pramanik, A. Recent developments in photodynamic therapy and its application against multidrug resistant cancers. Biomed. Mater. 2023, 18, 062005. [Google Scholar] [CrossRef]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers—A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef] [PubMed]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagn. Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Liao, W.; Xiao, S.; Yang, J.; Shi, X.; Zheng, Y. Multifunctional nanogel based on carboxymethyl cellulose interfering with cellular redox homeostasis enhances phycocyanobilin photodynamic therapy. Carbohydr. Polym. 2024, 323, 121416. [Google Scholar] [CrossRef]
- Kim, E.J.; Mangold, A.R.; DeSimone, J.A.; Wong, H.K.; Seminario-Vidal, L.; Guitart, J.; Appel, J.; Geskin, L.; Lain, E.; Korman, N.J.; et al. Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis Fungoides): The FLASH Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 1031–1039. [Google Scholar] [CrossRef]
- Niu, J.; Cheng, M.; Hong, Z.; Ling, J.; Di, W.; Gu, L.; Qiu, L. The effect of 5-Aminolaevulinic Acid Photodynamic Therapy versus CO2 laser in the Treatment of Cervical Low-grade Squamous Intraepithelial Lesions with High-Risk HPV Infection: A non-randomized, controlled pilot study. Photodiagn. Photodyn. Ther. 2021, 36, 102548. [Google Scholar] [CrossRef]
- Qiao, S.; Tang, H.; Xia, J.; Ding, M.; Qiao, S.; Niu, Y.; Jiang, G. Efficacy and safety of microneedling, fractional CO2 laser, and cryotherapy combined with 5-aminolevulinic acid photodynamic therapy in the treatment of actinic keratosis: A multicenter prospective randomized controlled study. Photodiagn. Photodyn. Ther. 2023, 43, 103700. [Google Scholar] [CrossRef]
- Filip, A.G.; Clichici, S.; Daicoviciu, D.; Olteanu, D.; Mureşan, A.; Dreve, S. Photodynamic therapy--indications and limits in malignant tumors treatment. Rom. J. Intern. Med. 2008, 46, 285–293. [Google Scholar]
- Gao, S.; Liang, S.; Ding, K.; Qu, Z.; Wang, Y.; Feng, X. Specific cellular accumulation of photofrin-II in EC cells promotes photodynamic treatment efficacy in esophageal cancer. Photodiagn. Photodyn. Ther. 2016, 14, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.G.; Wang, L.D.; Feng, X.S.; Qu, Z.F.; Shan, T.Y.; Xie, X.H. Absorption and elimination of photofrin-II in human immortalization esophageal epithelial cell line SHEE and its malignant transformation cell line SHEEC. Chin. J. Cancer 2009, 28, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, P.; de Rooij, F.W.; van Velthuysen, M.L.; Edixhoven, A.; van Hillegersberg, R.; Tilanus, H.W.; Wilson, J.H.; Siersema, P.D. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: A study in patients with (pre)malignant lesions of the oesophagus. Br. J. Cancer. 1998, 78, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B.; Feng, X.; Qu, F.; Wang, S.; Wu, L.; Wang, X.; Liu, Q.; Wang, P.; Zhang, K. Apoptosis and autophagy induced by DVDMs-PDT on human esophageal cancer Eca-109 cells. Photodiagn. Photodyn. Ther. 2018, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, P.; Chen, F.; Li, L.; Luo, R. Effect and mechanism of 5-aminolevulinic acid-mediated photodynamic therapy in esophageal cancer. Lasers Med. Sci. 2011, 26, 69–78. [Google Scholar] [CrossRef] [PubMed]
- McGarrity, T.J.; Peiffer, L.P.; Granville, D.J.; Carthy, C.M.; Levy, J.G.; Khandelwal, M.; Hunt, D.W. Apoptosis associated with esophageal adenocarcinoma: Influence of photodynamic therapy. Cancer Lett. 2001, 163, 33–41. [Google Scholar] [CrossRef]
- Chen, X.H.; Luo, R.C.; Li, L.B.; Ding, X.M.; Lv, C.W.; Zhou, X.P.; Yan, X. Mechanism of photodynamic therapy against human esophageal carcinoma xenografts in nude mice. J. South. Med. Univ. 2009, 29, 2222–2224. [Google Scholar]
- Li, L.; Song, D.; Qi, L.; Jiang, M.; Wu, Y.; Gan, J.; Cao, K.; Li, Y.; Bai, Y.; Zheng, T. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 2021, 520, 143–159. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, P.; Wang, X.; Zhang, K.; Liu, Q. Targeted inhibition of p38MAPK-enhanced autophagy in SW620 cells resistant to photodynamic therapy-induced apoptosis. Lasers Med. Sci. 2015, 30, 1967–1975. [Google Scholar] [CrossRef]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr. Opin. Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Puente, Y.; Zapata-Benavides, P.; Tari, A.M.; López-Berestein, G. Bcl-2-related antisense therapy. Semin. Oncol. 2002, 29, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Li, Y.; Xu, D.; Shi, Y.; Tang, H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J. Biol. Chem. 2005, 280, 10491–11500. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Harrison, L. Tumor hypoxia: Causative factors, compensatory mechanisms, and cellular response. Oncologist 2004, 9, 4–9. [Google Scholar] [CrossRef]
- Gan, J.; Li, S.; Meng, Y.; Liao, Y.; Jiang, M.; Qi, L.; Li, Y.; Bai, Y. The influence of photodynamic therapy on the Warburg effect in esophageal cancer cells. Lasers Med. Sci. 2020, 35, 1741–1750. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; He, J.; Li, X.; Li, L.; Chen, X.; Lan, P. Influence and mechanism of 5-aminolevulinic acid-photodynamic therapy on the metastasis of esophageal carcinoma. Photodiagn. Photodyn. Ther. 2017, 20, 78–85. [Google Scholar] [CrossRef]
- Li, Y.; Sui, H.; Jiang, C.; Li, S.; Han, Y.; Huang, P.; Du, X.; Du, J.; Bai, Y. Dihydroartemisinin Increases the Sensitivity of Photodynamic Therapy Via NF-κB/HIF-1α/VEGF Pathway in Esophageal Cancer Cell in vitro and in vivo. Cell Physiol. Biochem. 2018, 48, 2035–2045. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Vlahopoulos, S.A. Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: Molecular mode. Cancer Biol. Med. 2017, 14, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Du, X.X.; Jia, D.X.; Wu, C.L.; Huang, P.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; Yuan, H.H.; et al. Dihydroartemisinin accentuates the anti-tumor effects of photodynamic therapy via inactivation of NF-κB in Eca109 and Ec9706 esophageal cancer cells. Cell Physiol. Biochem. 2014, 33, 1527–1536. [Google Scholar]
- Kurokawa, H.; Ito, H.; Terasaki, M.; Matano, D.; Taninaka, A.; Shigekawa, H.; Matsui, H. Nitric oxide regulates the expression of heme carrier protein-1 via hypoxia inducible factor-1α stabilization. PLoS ONE 2019, 14, e0222074. [Google Scholar] [CrossRef]
- Yoo, J.O.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. Differential cytotoxic responses to low- and high-dose photodynamic therapy in human gastric and bladder cancer cells. J. Cell Biochem. 2011, 112, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.J.; Huang, Z.H.; Yu, J.L.; Li, Z.; Ding, L.S. Effect of 5-aminolevulinic acid-mediated photodynamic therapy on human gastric cancer xenografts in nude mice in vivo. Chin. J. Gastrointest. Surg. 2008, 11, 580–583. [Google Scholar]
- Takahira, K.; Sano, M.; Arai, H.; Hanai, H. Apoptosis of gastric cancer cell line MKN45 by photodynamic treatment with photofrin. Lasers Med. Sci. 2004, 19, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ewetse, M.P.; Ma, C.; Pu, W.; Xu, B.; He, P.; Wang, Y.; Zhu, J.; Chen, H. The “Light Knife” for Gastric Cancer: Photodynamic Therapy. Pharmaceutics 2022, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, B.; Xiang, L.; Ding, T.; Wang, N.; Yu, R.; Gu, B.; Gao, L.; Maswikiti, E.P.; Wang, Y.; et al. Photodynamic therapy improves the outcome of immune checkpoint inhibitors via remodelling anti-tumour immunity in patients with gastric cancer. Gastric Cancer 2023, 26, 798–813. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Chen, Y.; Wu, L.; Hang, H.; Jiang, C.; Zhou, X. B2M gene expression shapes the immune landscape of lung adenocarcinoma and determines the response to immunotherapy. Immunology 2021, 164, 507–523. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, X.; Wang, P.; Zhang, K.; Liu, Q. Role of p38MAPK in apoptosis and autophagy responses to photodynamic therapy with Chlorin e6. Photodiagn. Photodyn. Ther. 2015, 12, 84–91. [Google Scholar] [CrossRef]
- Peng, C.L.; Lin, H.C.; Chiang, W.L.; Shih, Y.H.; Chiang, P.F.; Luo, T.Y.; Cheng, C.C.; Shieh, M.J. Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer. Photodiagn. Photodyn. Ther. 2018, 23, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Schlottmann, K.; Endlicher, E.; Seeger, S.; Schölmerich, J.; Knuechel, R. Intracellular localization is a cofactor for the phototoxicity of protoporphyrin IX in the gastrointestinal tract: In vitro study. Photochem. Photobiol. 2003, 78, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Siboni, G.; Weitman, H.; Freeman, D.; Mazur, Y.; Malik, Z.; Ehrenberg, B. The correlation between hydrophilicity of hypericins and helianthrone: Internalization mechanisms, subcellular distribution and photodynamic action in colon carcinoma cells. Photochem. Photobiol. Sci. 2002, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, A.; Kostenich, G.; Roitman, L.; Shechtman, Y.; Kopolovic, Y.; Ehrenberg, B.; Malik, Z. A comparative study of tissue distribution and photodynamic therapy selectivity of chlorin e6, Photofrin II and ALA-induced protoporphyrin IX in a colon carcinoma model. Br. J. Cancer 1996, 73, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Porcal, G.V.; Macor, L.P.; Ponzio, R.A.; Spada, R.M.; Lorente, C.; Chesta, C.A.; Rivarola, V.A.; Palacios, R.E. Metallated porphyrin-doped conjugated polymer nanoparticles for efficient photodynamic therapy of brain and colorectal tumor cells. Nanomedicine 2018, 13, 605–624. [Google Scholar] [CrossRef]
- Zheng, J.H.; Shi, D.; Zhao, Y.; Chen, Z.L. Role of calcium signal in apoptosis and protective mechanism of colon cancer cell line SW480 in response to 5-aminolevulinic acid-photodynamic therapy. Chin. J. Cancer 2006, 25, 683–688. [Google Scholar]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Šemeláková, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. The pro-apoptotic and anti-invasive effects of hypericin-mediated photodynamic therapy are enhanced by hyperforin or aristoforin in HT-29 colon adenocarcinoma cells. J. Photochem. Photobiol. B. 2012, 117, 115–125. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, Y.; Wang, L.; Chen, H.; Tan, N. Hyaluronic Acid Coated Liposomes Co-Delivery of Natural Cyclic Peptide RA-XII and Mitochondrial Targeted Photosensitizer for Highly Selective Precise Combined Treatment of Colon Cancer. Int. J. Nanomed. 2021, 16, 4929–4942. [Google Scholar] [CrossRef]
- Chan, C.M.; Lo, P.C.; Yeung, S.L.; Ng, D.K.; Fong, W.P. Photodynamic activity of a glucoconjugated silicon(IV) phthalocyanine on human colon adenocarcinoma. Cancer Biol. Ther. 2010, 10, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Zhang, Y.; Lee, A.S. The ER Chaperone GRP78 and Cancer. In Protein Misfolding Disorders: A Trip into the ER; Hetz, C., Ed.; Bentham Science Publishers: Santiago, Chile, 2009; Volume 1, pp. 47–55. [Google Scholar]
- Gariboldi, M.B.; Ravizza, R.; Baranyai, P.; Caruso, E.; Banfi, S.; Meschini, S.; Monti, E. Photodynamic effects of novel 5,15-diaryl-tetrapyrrole derivatives on human colon carcinoma cells. Bioorg Med. Chem. 2009, 17, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Houreld, N.N. Genetic Aberrations Associated with Photodynamic Therapy in Colorectal Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3254. [Google Scholar] [CrossRef] [PubMed]
- Abdulrehman, G.; Xv, K.; Li, Y.; Kang, L. Effects of meta-tetrahydroxyphenylchlorin photodynamic therapy on isogenic colorectal cancer SW480 and SW620 cells with different metastatic potentials. Lasers Med. Sci. 2018, 33, 1581–1590. [Google Scholar] [CrossRef]
- Ouyang, G.; Liu, Z.; Xiong, L.; Chen, X.; Li, Q.; Huang, H.; Lin, L.; Miao, X.; Ma, L.; Chen, W.; et al. Role of PpIX-based photodynamic therapy in promoting the damage and apoptosis of colorectal cancer cell and its mechanisms. J. Cent. S. Univ. Med. Sci. 2017, 42, 874–881. [Google Scholar]
- Shahzidi, S.; Stokke, T.; Soltani, H.; Nesland, J.M.; Peng, Q. Induction of apoptosis by hexaminolevulinate-mediated photodynamic therapy in human colon carcinoma cell line 320DM. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 159–171. [Google Scholar] [CrossRef]
- Ali, S.M.; Olivo, M. Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int. J. Oncol. 2002, 21, 531–540. [Google Scholar] [CrossRef]
- Matroule, J.Y.; Carthy, C.M.; Granville, D.J.; Jolois, O.; Hunt, D.W.; Piette, J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene 2001, 20, 4070–4084. [Google Scholar] [CrossRef]
- Pérez-Arnaiz, C.; Acuña, M.I.; Busto, N.; Echevarría, I.; Martínez-Alonso, M.; Espino, G.; García, B.; Domínguez, F. Thiabendazole-based Rh(III) and Ir(III) biscyclometallated complexes with mitochondria-targeted anticancer activity and metal-sensitive photodynamic activity. Eur. J. Med. Chem. 2018, 157, 279–293. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Qiu, X.; Yang, K.; Zhou, H. The IL-12 family cytokines in fish: Molecular structure, expression profile and function. Dev. Comp. Immunol. 2023, 141, 104643. [Google Scholar] [CrossRef]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczynski, G.M.; Wilczek, E.; Chorazy-Massalska, M.; Radzikowska, A.; Maslinski, W.; Biały, L.; et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural photosensitizers in photodynamic therapy: In vitro activity against monolayers and spheroids of human colorectal adenocarcinoma SW480 cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Marcinkowska, E.; Ziółkowski, P.; Pacholska, E.; Latos-Grazyński, L.; Chmielewski, P.; Radzikowski, C. The new sensitizing agents for photodynamic therapy: 21-selenaporphyrin and 21-thiaporphyrin. Anticancer Res. 1997, 17, 3313–3319. [Google Scholar]
- Luo, M.; Ji, J.; Yang, K.; Li, H.; Kang, L. The role of autophagy in the treatment of colon cancer by chlorin e6 photodynamic therapy combined with oxaliplatin. Photodiagn. Photodyn. Ther. 2022, 40, 103082. [Google Scholar] [CrossRef]
- Marchal, S.; Fadloun, A.; Maugain, E.; D’Hallewin, M.A.; Guillemin, F.; Bezdetnaya, L. Necrotic and apoptotic features of cell death in response to Foscan photosensitization of HT29 monolayer and multicell spheroids. Biochem. Pharmacol. 2005, 69, 1167–1176. [Google Scholar] [CrossRef]
- Pansa, M.F.; Lamberti, M.J.; Cogno, I.S.; Correa, S.G.; Rumie Vittar, N.B.; Rivarola, V.A. Contribution of resident and recruited macrophages to the photodynamic intervention of colorectal tumor microenvironment. Tumour Biol. 2016, 37, 541–552. [Google Scholar] [CrossRef]
- Mroz, P.; Szokalska, A.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PLoS ONE 2010, 5, e15194. [Google Scholar] [CrossRef]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Ma, H.; Yang, K.; Li, H.; Luo, M.; Wufuer, R.; Kang, L. Photodynamic effect of chlorin e6 on cytoskeleton protein of human colon cancer SW480 cells. Photodiagn. Photodyn. Ther. 2021, 33, 102201. [Google Scholar] [CrossRef]
- Wufuer, R.; Ma, H.X.; Luo, M.Y.; Xu, K.Y.; Kang, L. Downregulation of Rac1/PAK1/LIMK1/cofilin signaling pathway in colon cancer SW620 cells treated with Chlorin e6 photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102143. [Google Scholar] [CrossRef]
- Dobre, M.; Boscencu, R.; Neagoe, I.V.; Surcel, M.; Milanesi, E.; Manda, G. Insight into the Web of Stress Responses Triggered at Gene Expression Level by Porphyrin-PDT in HT29 Human Colon Carcinoma Cells. Pharmaceutics 2021, 13, 1032. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, C.; Sun, Y.; Li, Y.; Wu, H.; Ma, S.; Shang, J.; Zhan, Y.; Yin, P.; Gao, F. Bufalin exacerbates Photodynamic therapy of colorectal cancer by targeting SRC-3/HIF-1α pathway. Int. J. Pharm. 2022, 624, 122018. [Google Scholar] [CrossRef]
- West, C.M.; Moore, J.V. Mechanisms behind the resistance of spheroids to photodynamic treatment: A flow cytometry study. Photochem. Photobiol. 1992, 55, 425–430. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Catrinacio, C.; Ropolo, A.; Rivarola, V.A.; Vaccaro, M.I. A novel HIF-1α/VMP1-autophagic pathway induces resistance to photodynamic therapy in colon cancer cells. Photochem. Photobiol. Sci. 2017, 16, 1631–1642. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Fernández-Zapico, M.E.; Rumie Vittar, N.B.; Rivarola, V.A. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PLoS ONE 2017, 12, e0177801. [Google Scholar] [CrossRef]
- Wang, H.P.; Hanlon, J.G.; Rainbow, A.J.; Espiritu, M.; Singh, G. Up-regulation of Hsp27 plays a role in the resistance of human colon carcinoma HT29 cells to photooxidative stress. Photochem. Photobiol. 2002, 76, 98–104. [Google Scholar] [CrossRef]
- Gyurászová, K.; Mikeš, J.; Halaburková, A.; Jendželovský, R.; Fedoročko, P. YM155, a small molecule inhibitor of survivin expression, sensitizes cancer cells to hypericin-mediated photodynamic therapy. Photochem. Photobiol. Sci. 2016, 15, 812–821. [Google Scholar] [CrossRef]
- Jendzelovský, R.; Mikes, J.; Koval’, J.; Soucek, K.; Procházková, J.; Kello, M.; Sacková, V.; Hofmanová, J.; Kozubík, A.; Fedorocko, P. Drug efflux transporters, MRP1 and BCRP, affect the outcome of hypericin-mediated photodynamic therapy in HT-29 adenocarcinoma cells. Photochem. Photobiol. Sci. 2009, 8, 1716–1723. [Google Scholar] [CrossRef]
- Purkiss, S.F.; Grahn, M.F.; Williams, N.S. Haematoporphyrin derivative–photodynamic therapy of colorectal carcinoma, sensitized using verapamil and adriamycin. Surg. Oncol. 1996, 5, 169–175. [Google Scholar] [CrossRef]
- Halaburková, A.; Jendželovský, R.; Kovaľ, J.; Herceg, Z.; Fedoročko, P.; Ghantous, A. Histone deacetylase inhibitors potentiate photodynamic therapy in colon cancer cells marked by chromatin-mediated epigenetic regulation of CDKN1A. Clin. Epigenet. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, H.; Wen, Y.; Zhao, H.; Zhou, N.; Lei, S.; Xiong, L. Functional role of a long non-coding RNA LIFR-AS1/miR-29a/TNFAIP3 axis in colorectal cancer resistance to pohotodynamic therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, X.; Chen, H.N.; Nice, E.C.; Huang, C. Drug resistance in colorectal cancer: An epigenetic overview. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188623. [Google Scholar] [CrossRef] [PubMed]
- Sardoiwala, M.N.; Kushwaha, A.C.; Dev, A.; Shrimali, N.; Guchhait, P.; Karmakar, S.; Roy Choudhury, S. Hypericin-Loaded Transferrin Nanoparticles Induce PP2A-Regulated BMI1 Degradation in Colorectal Cancer-Specific Chemo-Photodynamic Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumour Biol. 2017, 39, 1010428317734691. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lei, K.; Du, W.; Yang, L.; Shi, H.; Gao, Y.; Yin, P.; Liang, X.; Liu, J. Enhancement of oxaliplatin sensitivity in human colorectal cancer by hypericin mediated photodynamic therapy via ROS-related mechanism. Int. J. Biochem. Cell Biol. 2016, 71, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Fan, G.; Wu, H.; Liu, C.; Zhan, Y.; Qiu, Y.; Shou, C.; Gao, F.; Zhang, J.; Yin, P.; et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol. Ther. 2021, 29, 2931–2948. [Google Scholar] [CrossRef]
- Kleban, J.; Mikes, J.; Horváth, V.; Sacková, V.; Hofmanová, J.; Kozubík, A.; Fedorocko, P. Mechanisms involved in the cell cycle and apoptosis of HT-29 cells pre-treated with MK-886 prior to photodynamic therapy with hypericin. J. Photochem. Photobiol. B. 2008, 93, 108–118. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.; Abrahamse, H. Molecular Effectors of Photodynamic Therapy-Mediated Resistance to Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13182. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent advances in strategies for overcoming hypoxia in photodynamic therapy of cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Wang, B.; Li, X.; Feng, Z.; Ma, C.; Gao, L.; Yu, Y.; Zhang, J.; Zheng, P.; Wang, Y.; et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front. Immunol. 2022, 13, 1050421. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Jouan-Hureaux, V.; Barberi-Heyob, M.; Frochot, C.; Svasti, J.; Niamsiri, N. Overcoming the diverse mechanisms of multidrug resistance in lung cancer cells by photodynamic therapy using pTHPP-loaded PLGA-lipid hybrid nanoparticles. Eur. J. Pharm. Biopharm. 2020, 149, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Deken, M.M.; Kijanka, M.M.; Beltrán Hernández, I.; Slooter, M.D.; de Bruijn, H.S.; van Diest, P.J.; van Bergen En Henegouwen, P.M.P.; Lowik, C.W.G.M.; Robinson, D.J.; Vahrmeijer, A.L.; et al. Nanobody-targeted photodynamic therapy induces significant tumor regression of trastuzumab-resistant HER2-positive breast cancer, after a single treatment session. J. Control Release 2020, 323, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, M.; Zhou, M.; Li, H.; Chen, Y.; Ren, X.; Dai, Y. O2-evolving and ROS-activable nanoparticles for treatment of multi-drug resistant Cancer by combination of photodynamic therapy and chemotherapy. Nanomedicine 2019, 19, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Yang, J.; Gong, Q.; Luo, K.; Gu, Z. Redox dual-responsive dendrimeric nanoparticles for mutually synergistic chemo-photodynamic therapy to overcome drug resistance. J. Control Release 2021, 329, 1210–1221. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv Mater. 2022, 34, e2206121. [Google Scholar] [CrossRef]
- Huis In‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Bhattarai, P.; Hameed, S.; Dai, Z. Perfluorocarbon@Porphyrin Nanoparticles for Tumor Hypoxia Relief to Enhance Photodynamic Therapy against Liver Metastasis of Colon Cancer. ACS Nano 2020, 14, 13569–13583. [Google Scholar] [CrossRef]
- Ding, D.; Zhong, H.; Liang, R.; Lan, T.; Zhu, X.; Huang, S.; Wang, Y.; Shao, J.; Shuai, X.; Wei, B. Multifunctional Nanodrug Mediates Synergistic Photodynamic Therapy and MDSCs-Targeting Immunotherapy of Colon Cancer. Adv. Sci. 2021, 8, e2100712. [Google Scholar] [CrossRef]
- Yan, S.; Tang, D.; Hong, Z.; Wang, J.; Yao, H.; Lu, L.; Yi, H.; Fu, S.; Zheng, C.; He, G.; et al. CD133 peptide-conjugated pyropheophorbide-a as a novel photosensitizer for targeted photodynamic therapy in colorectal cancer stem cells. Biomater. Sci. 2021, 9, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zou, H.; Liu, X.; He, J.; Zheng, Y.; Xiong, L.; Miao, X. miR-7112-3p targets PERK to regulate the endoplasmic reticulum stress pathway and apoptosis induced by photodynamic therapy in colorectal cancer CX-1 cells. Photodiagn. Photodyn Ther. 2020, 29, 101663. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kwon, S.; Jang, S.Y.; Park, E.; Lee, Y.; Koo, H. Overcoming the obstacles of current photodynamic therapy in tumors using nanoparticles. Bioact. Mater. 2021, 8, 20–34. [Google Scholar] [CrossRef] [PubMed]


| Mechanism of Interaction of PDT with Gastrointestinal Tumor Cells | ||
|---|---|---|
| Esophageal cancer | Accumulation of photosensitizer | Imbalance between the activity of porphobilinogen deaminase and ferrochelatase enzymes (5-ALA) |
| Mechanism of cell damage | Direct cell damage | |
| Destruction of tumor blood vessels | ||
| Activation of the immune response | ||
| Type of response and cell death | Apoptosis | |
| Necrosis | ||
| Pyroptosis | ||
| Autophagy | ||
| Gastric cancer | Accumulation of photosensitizer | Dependent on nitric oxide (NO) and heme carrier protein-1 (HCP-1) |
| Mechanism of cell damage | Direct cell damage | |
| Activation of the immune response | ||
| Type of response and cell death | Apoptosis | |
| Necrosis | ||
| Colorectal cancer | Accumulation of photosensitizer | Partitioning |
| Pinocytosis | ||
| Endocytosis | ||
| Difference in activity between porphobilinogen deaminase and ferrochelatase (PPIX) | ||
| Mechanism of cell damage | Direct cell damage | |
| Destruction of tumor blood vessels | ||
| Activation of the immune response | ||
| Type of response and cell death | Apoptosis | |
| Necrosis | ||
| Type of Disease | A Type of Third-generation Photosensitizer | Wavelength of Laser Light (nm) | Immunological Effect | References |
|---|---|---|---|---|
| Colon cancer | porphyrin grafted lipid (PGL) nanoparticles | 650 | The results confirmed that the designed nanoplatform effectively eliminates differences in oxygen content, which positively affects the process of generating singlet oxygen and the process of weakening COX-2 expression. | [140] |
| Colon cancer | liposome encapsulating phosphoinositide 3-kinase gamma (PI3Kγ) inhibitor IPI-549 and chlorin e6 | 660 | The proposed therapy significantly limited the development and growth of the tumor by positively affecting the physiology of dendritic cells and T lymphocytes. | [141] |
| Colorectal cancer | CD133-Pyro | 670 | The study showed that the designed composite increases ROS production and induces cell death. | [142] |
| Colorectal cancer | Sinoporphyrin sodium (DVDMS) | 635 | The therapy induced programmed cell death, among others, by generating the caspase pathway in CX-1 cells. | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Kawczyk-Krupka, A.; Cieślar, G.; Bartusik-Aebisher, D. Photodynamic Therapy and Immunological View in Gastrointestinal Tumors. Cancers 2024, 16, 66. https://doi.org/10.3390/cancers16010066
Aebisher D, Woźnicki P, Dynarowicz K, Kawczyk-Krupka A, Cieślar G, Bartusik-Aebisher D. Photodynamic Therapy and Immunological View in Gastrointestinal Tumors. Cancers. 2024; 16(1):66. https://doi.org/10.3390/cancers16010066
Chicago/Turabian StyleAebisher, David, Paweł Woźnicki, Klaudia Dynarowicz, Aleksandra Kawczyk-Krupka, Grzegorz Cieślar, and Dorota Bartusik-Aebisher. 2024. "Photodynamic Therapy and Immunological View in Gastrointestinal Tumors" Cancers 16, no. 1: 66. https://doi.org/10.3390/cancers16010066
APA StyleAebisher, D., Woźnicki, P., Dynarowicz, K., Kawczyk-Krupka, A., Cieślar, G., & Bartusik-Aebisher, D. (2024). Photodynamic Therapy and Immunological View in Gastrointestinal Tumors. Cancers, 16(1), 66. https://doi.org/10.3390/cancers16010066









