Technical and Clinical Outcomes of Laparoscopic–Laparotomic Hepatocellular Carcinoma Thermal Ablation with Microwave Technology: Case Series and Review of Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
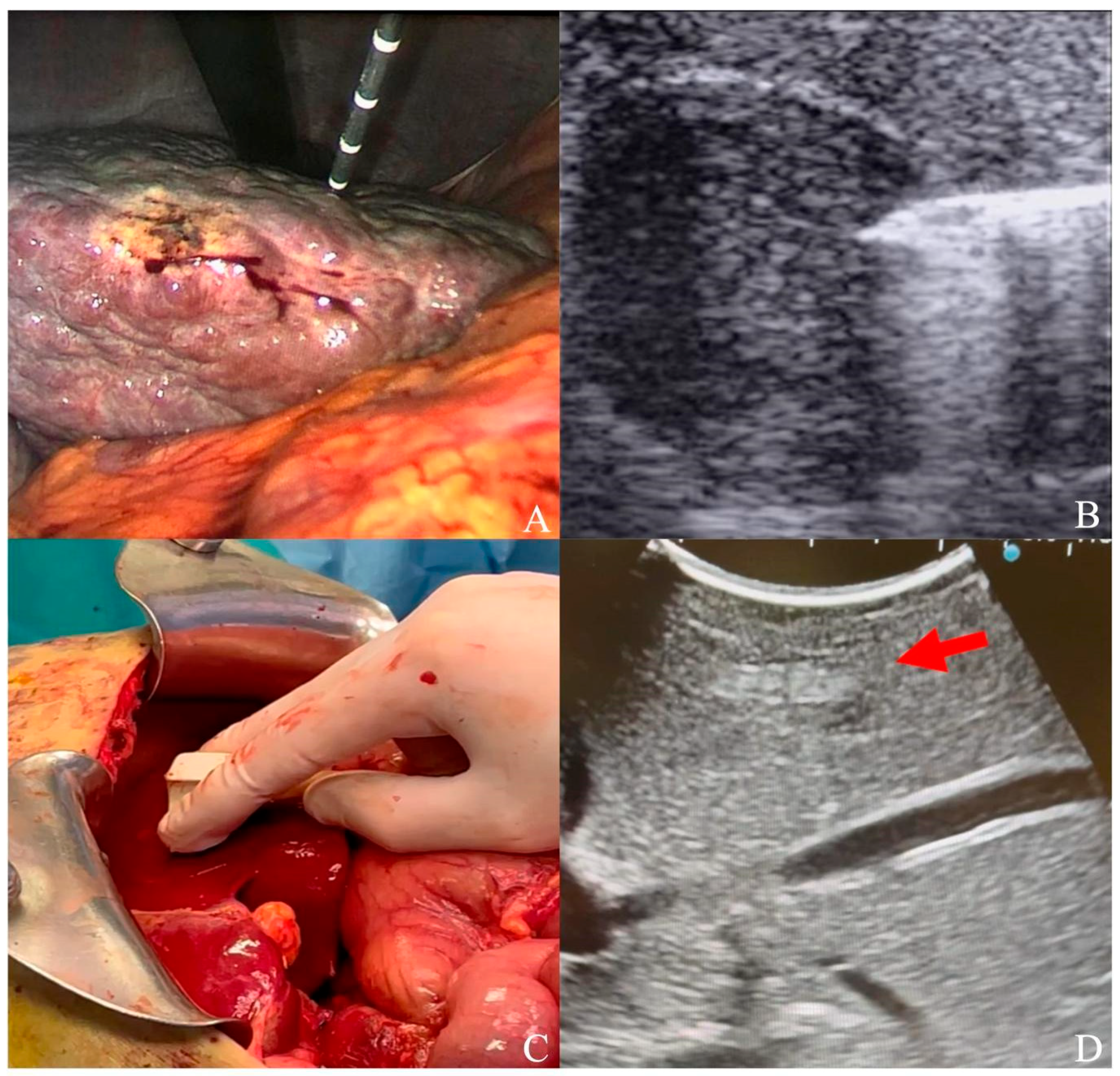
2.2. Laparoscopic and Laparotomic Ablation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Ganesan, P.; Kulik, L.M. Hepatocellular carcinoma: New developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Tümen, D.; Heumann, P.; Gülow, K.; Demirci, C.-N.; Cosma, L.-S.; Müller, M.; Kandulski, A. Pathogenesis and current treatment strategies of hepatocellular carcinoma. Biomedicines 2022, 10, 3202. [Google Scholar] [CrossRef]
- Pepple, P.T.; Gerber, D.A. Laparoscopic-assisted ablation of hepatic tumors: A review. Semin. Interv. Radiol. 2014, 31, 125–128. [Google Scholar] [CrossRef]
- Minami, Y.; Aoki, T.; Hagiwara, S.; Kudo, M. Tips for preparing and practicing thermal ablation therapy of hepatocellular carcinoma. Cancers 2023, 15, 4763. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, J.; Tu, B.; Liu, Y.; Yang, Y.; Hou, L.; Yang, X.; Liu, X.; Xie, H. Radiofrequency ablation combined with toripalimab for recurrent hepatocellular carcinoma: A prospective controlled trial. Cancer Med. 2023, 12, 20311–20320. [Google Scholar] [CrossRef]
- Taner, T.; Atwell, T.D.; Zhang, L.; Oberg, T.N.; Harmsen, W.S.; Slettedahl, S.W.; Kendrick, M.L.; Nagorney, D.M.; Que, F.G. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB 2013, 15, 190–195. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Carbonara, U.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Elbich, J.; Basile, G.; Hampton, L.J.; Cerrato, C.; et al. Microwave versus cryoablation and radiofrequency ablation for small renal mass: A multicenter comparative analysis. Minerva Urol. Nephrol. 2023, 75, 66–72. [Google Scholar] [CrossRef]
- Pedicini, V.; Gennaro, N.; Muglia, R.; Saita, A.; Casale, P.; Negro, A.; Cabassi, A. Renin-dependent hypertension cured with percutaneous radiofrequency ablation. J. Hypertens. 2019, 37, 653–656. [Google Scholar] [CrossRef]
- Shang, Y.; Li, G.; Zhang, B.; Wu, Y.; Chen, Y.; Li, C.; Zhao, W.; Liu, J. Image-guided percutaneous ablation for lung malignancies. Front. Oncol. 2022, 12, 1020296. [Google Scholar] [CrossRef]
- Matsui, Y.; Tomita, K.; Uka, M.; Umakoshi, N.; Kawabata, T.; Munetomo, K.; Nagata, S.; Iguchi, T.; Hiraki, T. Up-to-date evidence on image-guided thermal ablation for metastatic lung tumors: A review. Jpn. J. Radiol. 2022, 40, 1024–1034. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Bianchi, L.; Costa, G.; Carbonara, U.; Loizzo, D.; Lucarelli, G.; et al. Percutaneous Ablation vs Robot-Assisted Partial Nephrectomy for Completely Endophytic Renal Masses: A Multicenter Trifecta Analysis with a Minimum 3-Year Follow-Up. J. Endourol. 2023, 37, 279–285. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, C.-C.; Hung, C.-H.; Chen, C.-L.; Lu, S.-N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef]
- Lencioni, R.; Cioni, D.; Crocetti, L.; Franchini, C.; Pina, C.D.; Lera, J.; Bartolozzi, C. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005, 234, 961–967. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Solbiati, L.; Hahn, P.F.; Cosman, E.; Conrad, J.E.; Fogle, R.; Gazelle, G.S. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: Laboratory and clinical experience in liver metastases. Radiology 1998, 209, 371–379. [Google Scholar] [CrossRef]
- Ikeda, K.; Osaki, Y.; Nakanishi, H.; Nasu, A.; Kawamura, Y.; Jyoko, K.; Sano, T.; Sunagozaka, H.; Uchino, K.; Minami, Y.; et al. Recent progress in radiofrequency ablation therapy for hepatocellular carcinoma. Oncology 2014, 87 (Suppl. S1), 73–77. [Google Scholar] [CrossRef]
- Solbiati, L.; Ierace, T.; Gennaro, N.; Muglia, R.; Cosman, E.R.; Goldberg, S.N. Percutaneous radiofrequency ablation of HCC: Reduced ablation duration and increased ablation size using single, internally cooled electrodes with an optimized pulsing algorithm. Int. J. Hyperth. 2020, 37, 861–867. [Google Scholar] [CrossRef]
- Cillo, U.; Noaro, G.; Vitale, A.; Neri, D.; D’Amico, F.; Gringeri, E.; Farinati, F.; Vincenzi, V.; Vigo, M.; Zanus, G. HePaTIC Study Group Laparoscopic microwave ablation in patients with hepatocellular carcinoma: A prospective cohort study. HPB 2014, 16, 979–986. [Google Scholar] [CrossRef]
- Kong, W.-T.; Zhang, W.-W.; Qiu, Y.-D.; Zhou, T.; Qiu, J.-L.; Zhang, W.; Ding, Y.-T. Major complications after radiofrequency ablation for liver tumors: Analysis of 255 patients. World J. Gastroenterol. 2009, 15, 2651–2656. [Google Scholar] [CrossRef]
- Eun, H.S.; Lee, B.S.; Kwon, I.S.; Yun, G.Y.; Lee, E.S.; Joo, J.S.; Sung, J.K.; Moon, H.S.; Kang, S.H.; Kim, J.S.; et al. Advantages of laparoscopic radiofrequency ablation over percutaneous radiofrequency ablation in hepatocellular carcinoma. Dig. Dis. Sci. 2017, 62, 2586–2600. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse quality assurance document and standards for classification of complications: The cirse classification system. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baère, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef]
- Gruttadauria, S.; Pagano, D.; Tropea, A.; Cintorino, D.; Castellana, L.; Bonsignore, P.; Ricotta, C.; Piccolo, G.; Vizzini, G.; Luca, A. Laparoscopic approach for thermoablation microwave in the treatment of hepatocellular carcinoma: A single center experience. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 808–811. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.-Y.; Lu, X.; Zhai, B. Laparoscopic Microwave Ablation of Hepatocellular Carcinoma at Liver Surface: Technique Effectiveness and Long-Term Outcomes. Technol. Cancer Res. Treat. 2019, 18, 1533033818824338. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, W.J.; Rhim, H.; Lim, H.K.; Choi, D.; Lee, J.Y. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am. J. Roentgenol. 2010, 195, 758–765. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Wakui, N.; Ikehara, T.; Iida, K.; Sumino, Y. Risk factors for the local recurrence of hepatocellular carcinoma after single-session percutaneous radiofrequency ablation with a single electrode insertion. Mol. Med. Rep. 2009, 2, 89–95. [Google Scholar] [CrossRef]
- Kim, P.N.; Choi, D.; Rhim, H.; Rha, S.E.; Hong, H.P.; Lee, J.; Choi, J.-I.; Kim, J.W.; Seo, J.W.; Lee, E.J.; et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (≤3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: A multicenter prospective study to assess factors affecting ultrasound visibility. J. Vasc. Interv. Radiol. 2012, 23, 627–634. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012, 262, 43–58. [Google Scholar] [CrossRef]
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1054–1063. [Google Scholar] [CrossRef]
- Yu, N.C.; Raman, S.S.; Kim, Y.J.; Lassman, C.; Chang, X.; Lu, D.S.K. Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model. J. Vasc. Interv. Radiol. 2008, 19, 1087–1092. [Google Scholar] [CrossRef]
- Glassberg, M.B.; Ghosh, S.; Clymer, J.W.; Qadeer, R.A.; Ferko, N.C.; Sadeghirad, B.; Wright, G.W.; Amaral, J.F. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. OncoTargets Ther. 2019, 12, 6407–6438. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef]
- Radosevic, A.; Quesada, R.; Serlavos, C.; Sánchez, J.; Zugazaga, A.; Sierra, A.; Coll, S.; Busto, M.; Aguilar, G.; Flores, D.; et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: A randomized controlled phase 2 trial. Sci. Rep. 2022, 12, 316. [Google Scholar] [CrossRef]
- Alonzo, M.; Bos, A.; Bennett, S.; Ferral, H. The EmprintTM Ablation System with ThermosphereTM Technology: One of the Newer Next-Generation Microwave Ablation Technologies. Semin. Interv. Radiol. 2015, 32, 335–338. [Google Scholar] [CrossRef]
- Tamai, H.; Okamura, J. New next-generation microwave thermosphere ablation for small hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 564–574. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Han, H.-S.; Cho, J.Y.; Yoon, C.J.; Kim, J.H. Laparoscopic approach for treatment of multiple hepatocellular carcinomas. Surg. Endosc. 2012, 26, 3133–3140. [Google Scholar] [CrossRef]
- Cillo, U.; Bertacco, A.; Fasolo, E.; Carandina, R.; Vitale, A.; Zanus, G.; Gringeri, E.; D’Amico, F.; Bassi, D.; Neri, D.; et al. Videolaparoscopic microwave ablation in patients with HCC at a European high-volume center: Results of 815 procedures. J. Surg. Oncol. 2019, 120, 956–965. [Google Scholar] [CrossRef]
- Torzilli, G. Sorafenib and surgery for hepatocellular carcinoma—A controversial relation: Lesson learned? GHM 2023, 5, 246–248. [Google Scholar] [CrossRef]
- Jin, M.; Yu, Q.; Liu, Y.; Xu, W.; Fu, X.; Ji, B. Safety and Efficacy of Physical Thermal Ablation Combined Sorafenib for Hepatocellular Carcinoma: A Meta-analysis. J. Clin. Transl. Hepatol. 2021, 9, 149–159. [Google Scholar] [CrossRef]
- Solomon, S.B.; Silverman, S.G. Imaging in interventional oncology. Radiology 2010, 257, 624–640. [Google Scholar] [CrossRef]
- Puijk, R.S.; Nieuwenhuizen, S.; van den Bemd, B.A.T.; Ruarus, A.H.; Geboers, B.; Vroomen, L.G.P.H.; Muglia, R.; de Jong, M.C.; de Vries, J.J.J.; Scheffer, H.J.; et al. Transcatheter CT Hepatic Arteriography Compared with Conventional CT Fluoroscopy Guidance in Percutaneous Thermal Ablation to Treat Colorectal Liver Metastases: A Single-Center Comparative Analysis of 2 Historical Cohorts. J. Vasc. Interv. Radiol. 2020, 31, 1772–1783. [Google Scholar] [CrossRef]
- Yao, X.-S.; Yan, D.; Jiang, X.-X.; Li, X.; Zeng, H.-Y.; Li, H. Short-term outcomes of radiofrequency ablation for hepatocellular carcinoma using cone-beam computed tomography for planning and image guidance. World J. Clin. Cases 2021, 9, 1580–1591. [Google Scholar] [CrossRef]
- Cha, D.I.; Lee, M.W.; Hyun, D.; Ahn, S.H.; Jeong, W.K.; Rhim, H. Combined Transarterial Chemoembolization and Radiofrequency Ablation for Hepatocellular Carcinoma Infeasible for Ultrasound-Guided Percutaneous Radiofrequency Ablation: A Comparative Study with General Ultrasound-Guided Radiofrequency Ablation Outcomes. Cancers 2023, 15, 5193. [Google Scholar] [CrossRef]
- Bargellini, I.; Sacco, R.; Bozzi, E.; Bertini, M.; Ginanni, B.; Romano, A.; Cicorelli, A.; Tumino, E.; Federici, G.; Cioni, R.; et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: A prospective cohort study. Eur. J. Radiol. 2012, 81, 1173–1178. [Google Scholar] [CrossRef]
- Hyun, D.; Cho, S.K.; Shin, S.W.; Park, K.B.; Park, H.S.; Choo, S.W.; Do, Y.S.; Choo, I.-W.; Lee, M.W.; Rhim, H.; et al. Early Stage Hepatocellular Carcinomas Not Feasible for Ultrasound-Guided Radiofrequency Ablation: Comparison of Transarterial Chemoembolization Alone and Combined Therapy with Transarterial Chemoembolization and Radiofrequency Ablation. Cardiovasc. Interv. Radiol. 2016, 39, 417–425. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Crocetti, L.; Rocco, B.; Bozzi, E.; Gasparrini, F.; Tanzilli, A.; Catalano, C.; Iezzi, R. Percutaneous thermal segmentectomy: Proof of concept. Cardiovasc. Interv. Radiol. 2022, 45, 665–676. [Google Scholar] [CrossRef]
- Solbiati, M.; Ierace, T.; Muglia, R.; Pedicini, V.; Iezzi, R.; Passera, K.M.; Rotilio, A.C.; Goldberg, S.N.; Solbiati, L.A. Thermal ablation of liver tumors guided by augmented reality: An initial clinical experience. Cancers 2022, 14, 1312. [Google Scholar] [CrossRef]
- Muglia, R.; Solbiati, L. New technological advancements for interventional oncology. Chin. Clin. Oncol. 2019, 8, 65. [Google Scholar] [CrossRef]
- De Paolis, L.T.; De Luca, V. Augmented visualization with depth perception cues to improve the surgeon’s performance in minimally invasive surgery. Med. Biol. Eng. Comput. 2019, 57, 995–1013. [Google Scholar] [CrossRef]
- Luo, L.; He, X.; Li, K.; Long, Y.; Zeng, Q.; Tan, L.; Zheng, R.; Xu, E. Thermal ablation of medium-sized hepatocellular carcinomas using intraoperative ultrasound fusion imaging: A propensity score-matched analysis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101581. [Google Scholar] [CrossRef]
- Santambrogio, R.; Vertemati, M.; Barabino, M.; Zappa, M.A. Laparoscopic microwave ablation: Which technologies improve the results. Cancers 2023, 15, 1814. [Google Scholar] [CrossRef]
- Stern, S.M. Technology in radiology: Advances in diagnostic imaging & therapeutics. J. Clin. Eng. 1993, 18, 425–432. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muglia, R.; Marra, P.; Pinelli, D.; Dulcetta, L.; Carbone, F.S.; Barbaro, A.; Celestino, A.; Colledan, M.; Sironi, S. Technical and Clinical Outcomes of Laparoscopic–Laparotomic Hepatocellular Carcinoma Thermal Ablation with Microwave Technology: Case Series and Review of Literature. Cancers 2024, 16, 92. https://doi.org/10.3390/cancers16010092
Muglia R, Marra P, Pinelli D, Dulcetta L, Carbone FS, Barbaro A, Celestino A, Colledan M, Sironi S. Technical and Clinical Outcomes of Laparoscopic–Laparotomic Hepatocellular Carcinoma Thermal Ablation with Microwave Technology: Case Series and Review of Literature. Cancers. 2024; 16(1):92. https://doi.org/10.3390/cancers16010092
Chicago/Turabian StyleMuglia, Riccardo, Paolo Marra, Domenico Pinelli, Ludovico Dulcetta, Francesco Saverio Carbone, Alessandro Barbaro, Antonio Celestino, Michele Colledan, and Sandro Sironi. 2024. "Technical and Clinical Outcomes of Laparoscopic–Laparotomic Hepatocellular Carcinoma Thermal Ablation with Microwave Technology: Case Series and Review of Literature" Cancers 16, no. 1: 92. https://doi.org/10.3390/cancers16010092
APA StyleMuglia, R., Marra, P., Pinelli, D., Dulcetta, L., Carbone, F. S., Barbaro, A., Celestino, A., Colledan, M., & Sironi, S. (2024). Technical and Clinical Outcomes of Laparoscopic–Laparotomic Hepatocellular Carcinoma Thermal Ablation with Microwave Technology: Case Series and Review of Literature. Cancers, 16(1), 92. https://doi.org/10.3390/cancers16010092





