Historical Perspective and Current Trends in Anticancer Drug Development
Abstract
Simple Summary
Abstract
1. Introduction
2. The Beginnings of Chemotherapy
3. Mustard Gases and Their Analogs
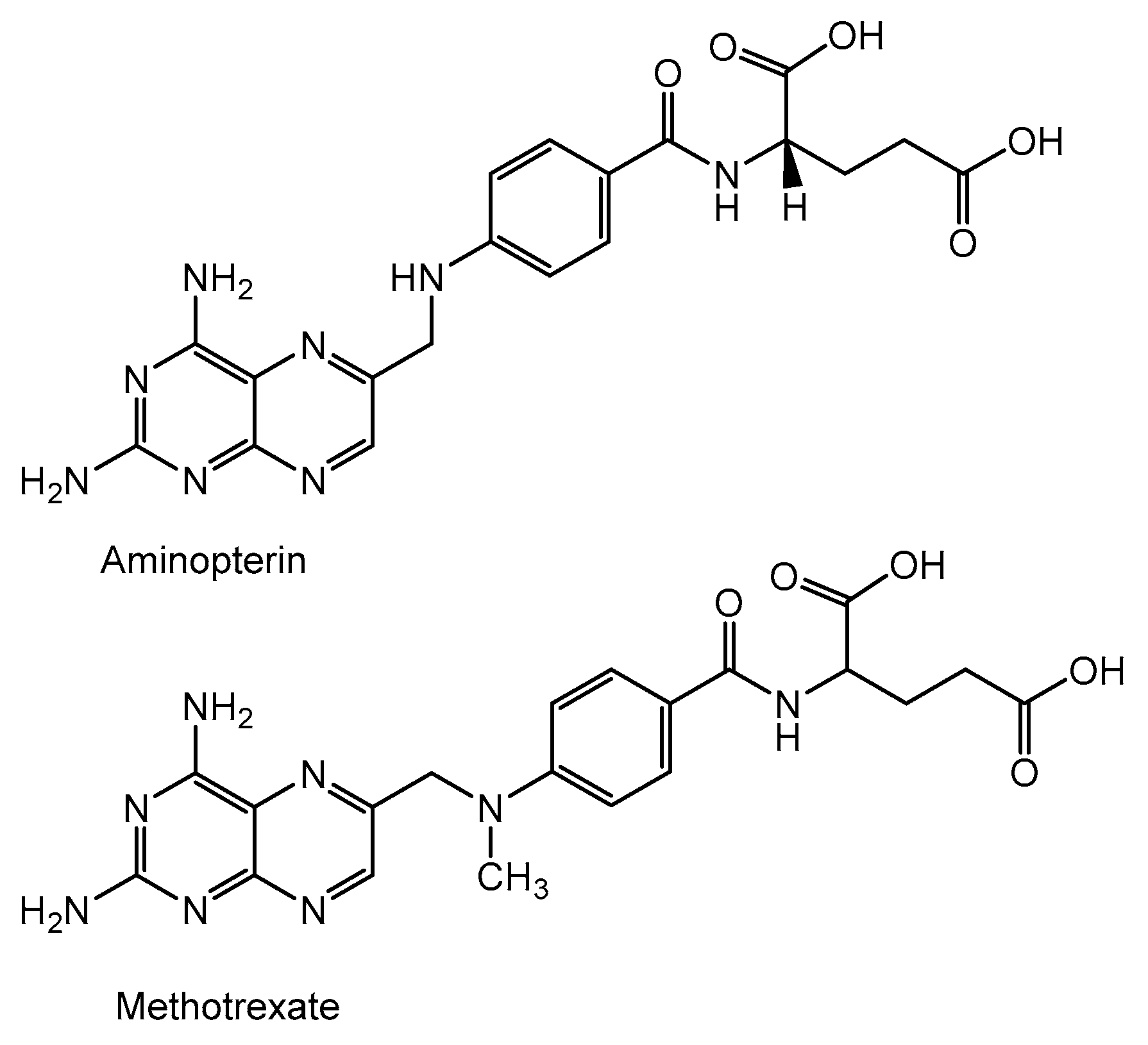
4. Folic Acid Antagonist
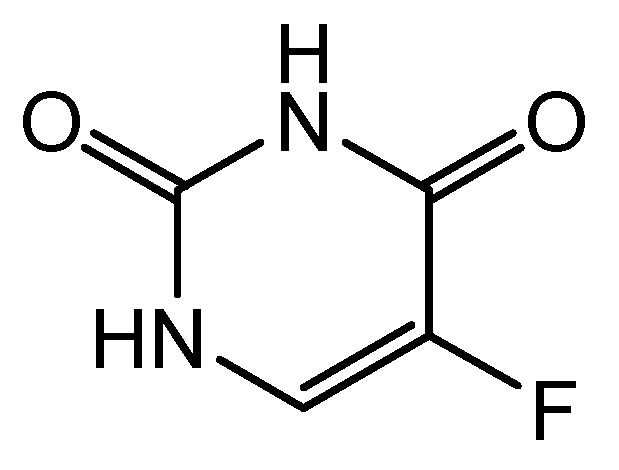
5. Antimetabolites
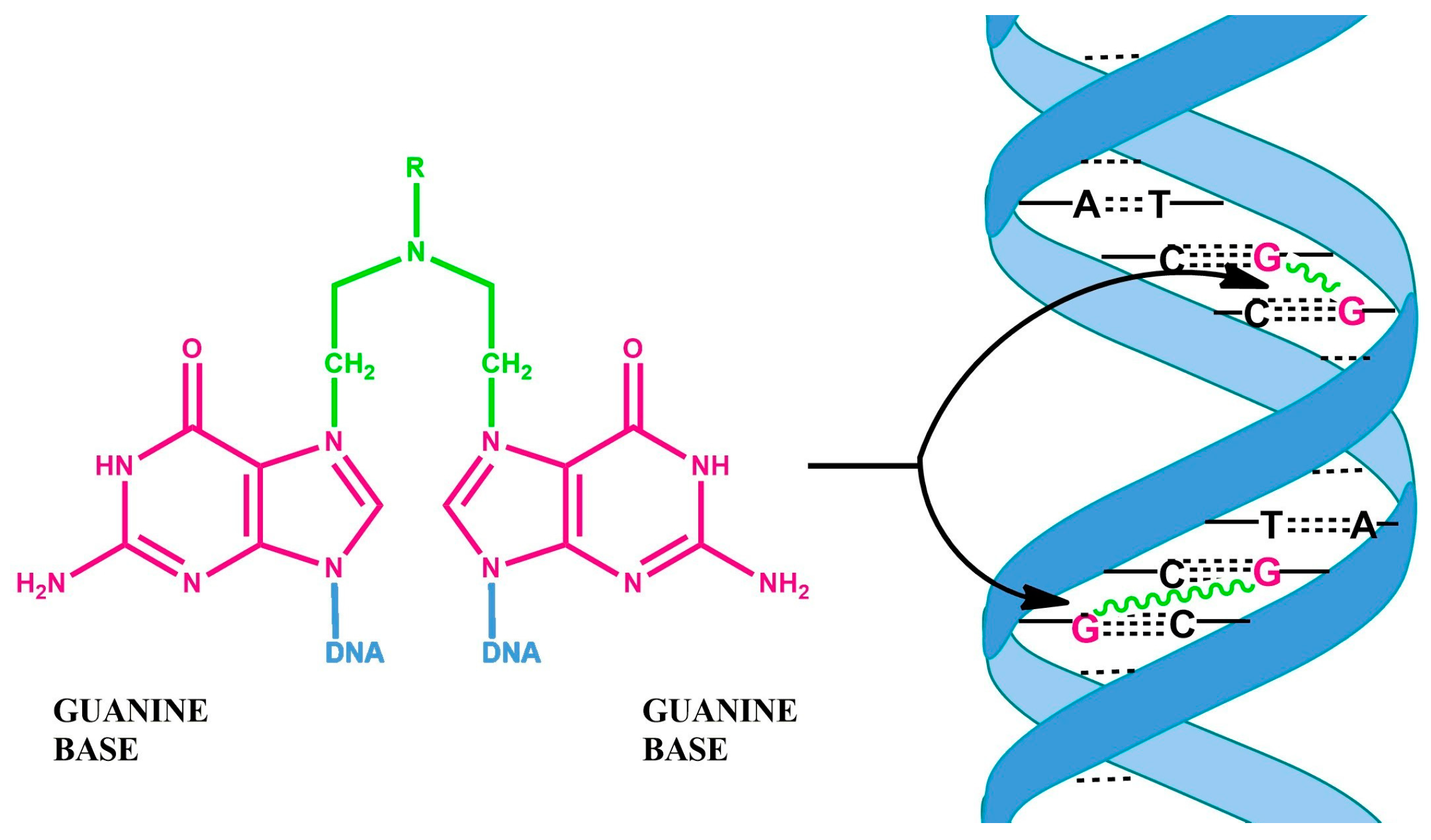
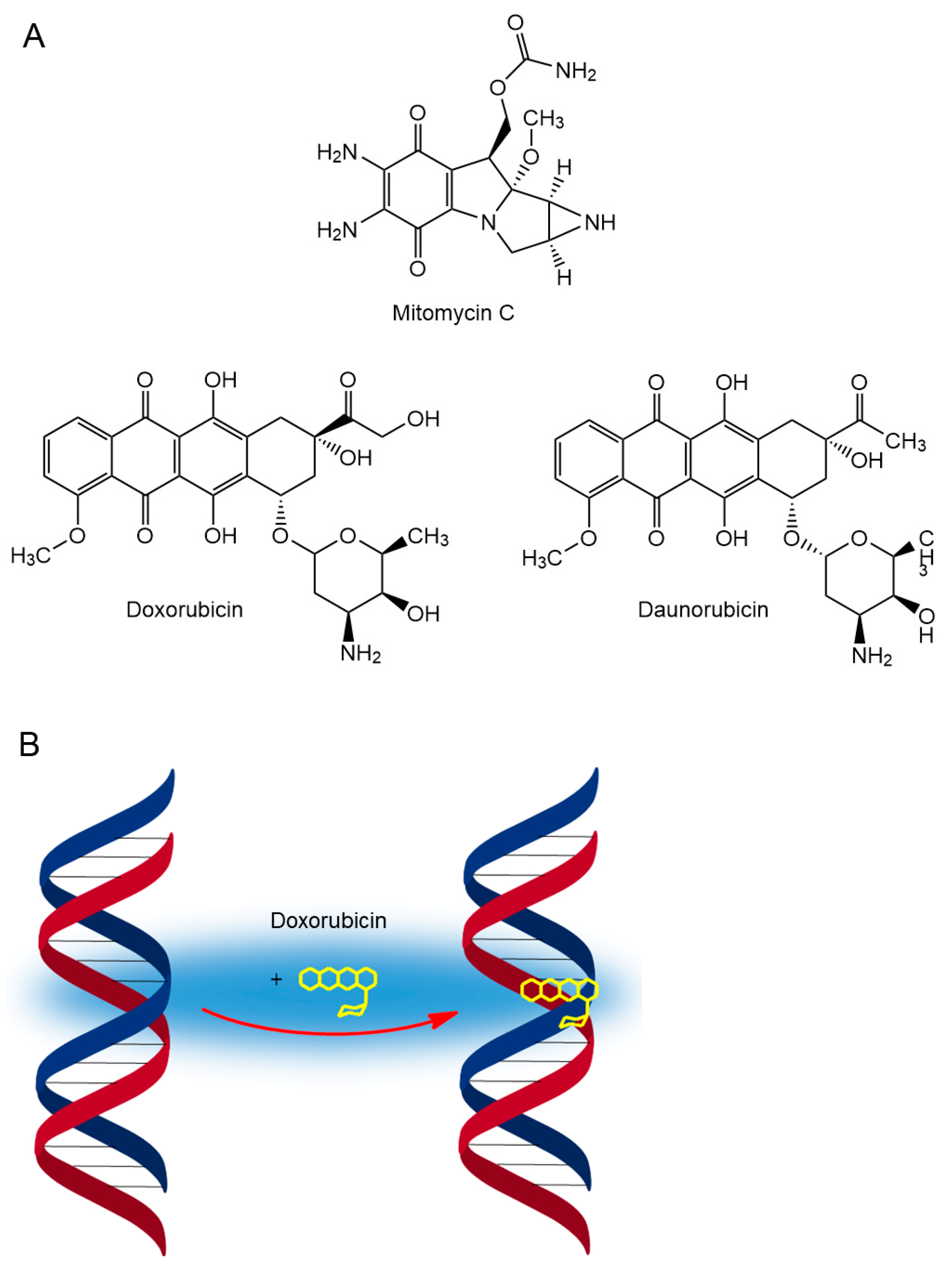
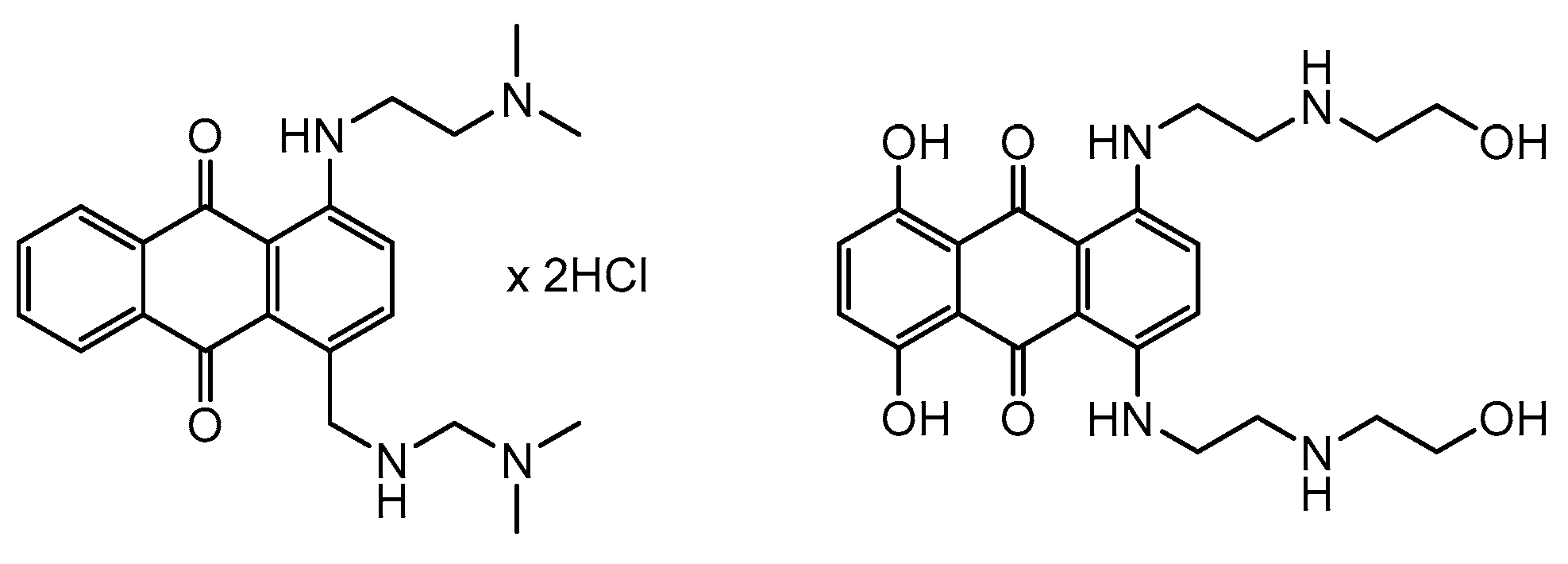
6. Anticancer Antibiotics
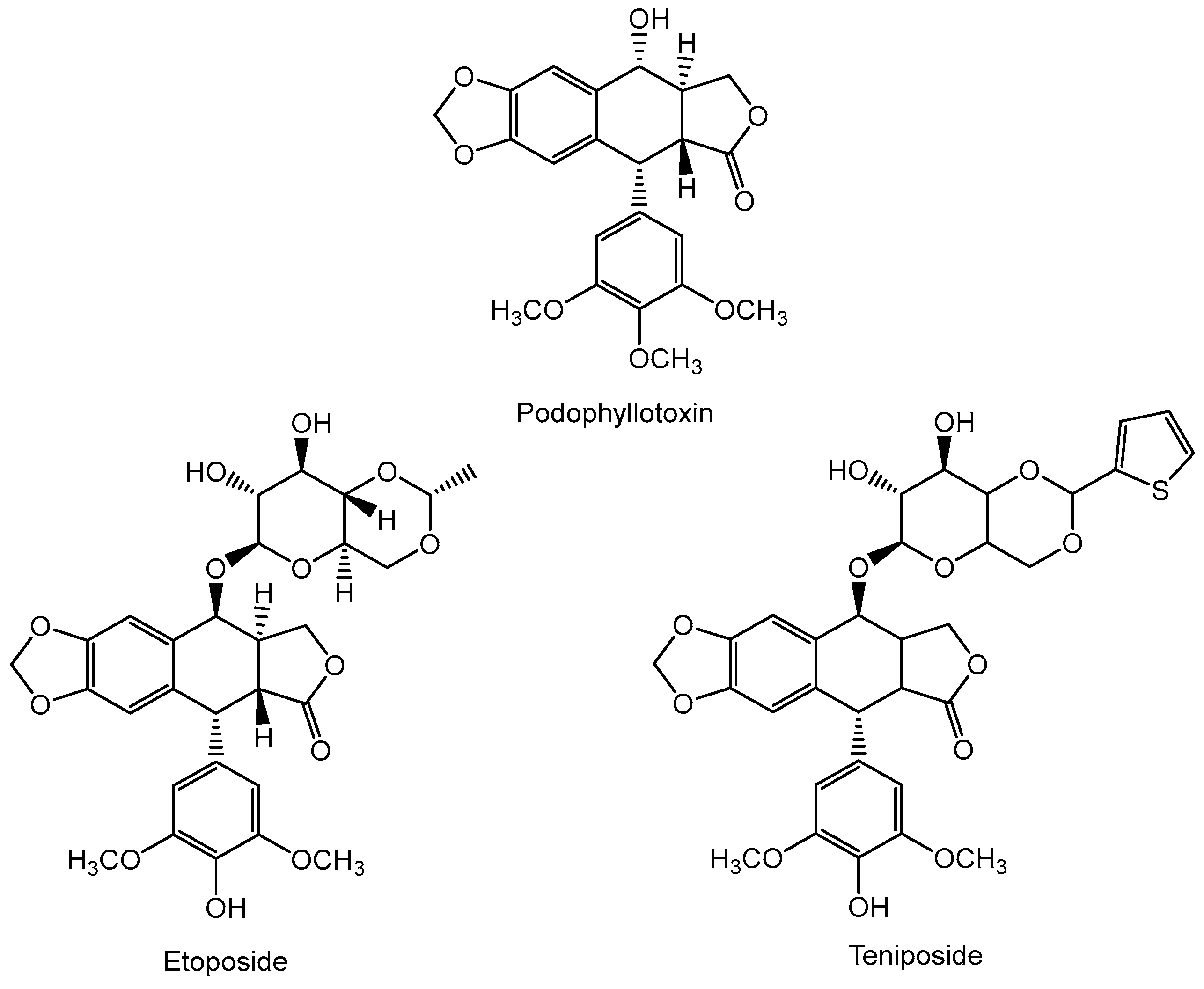
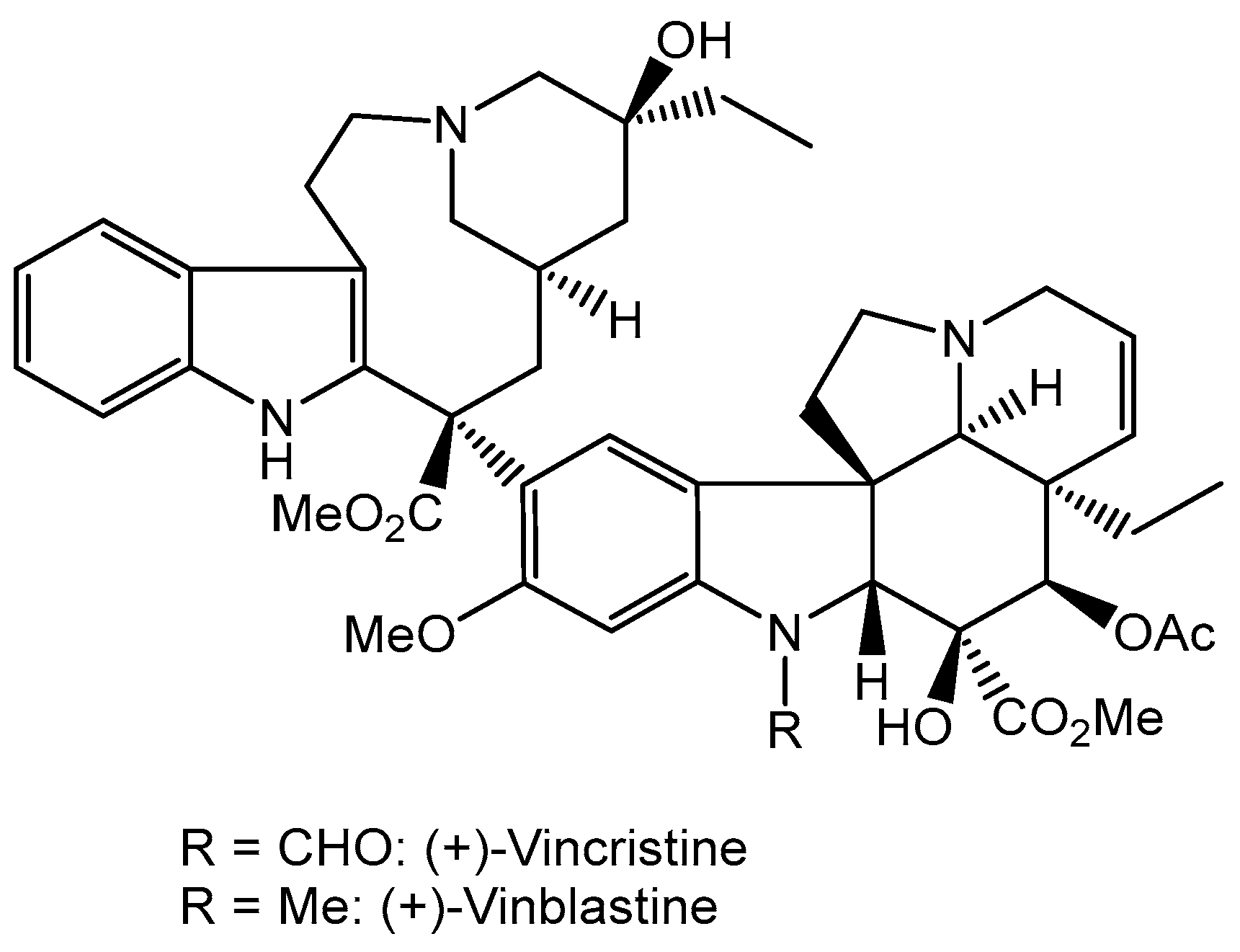
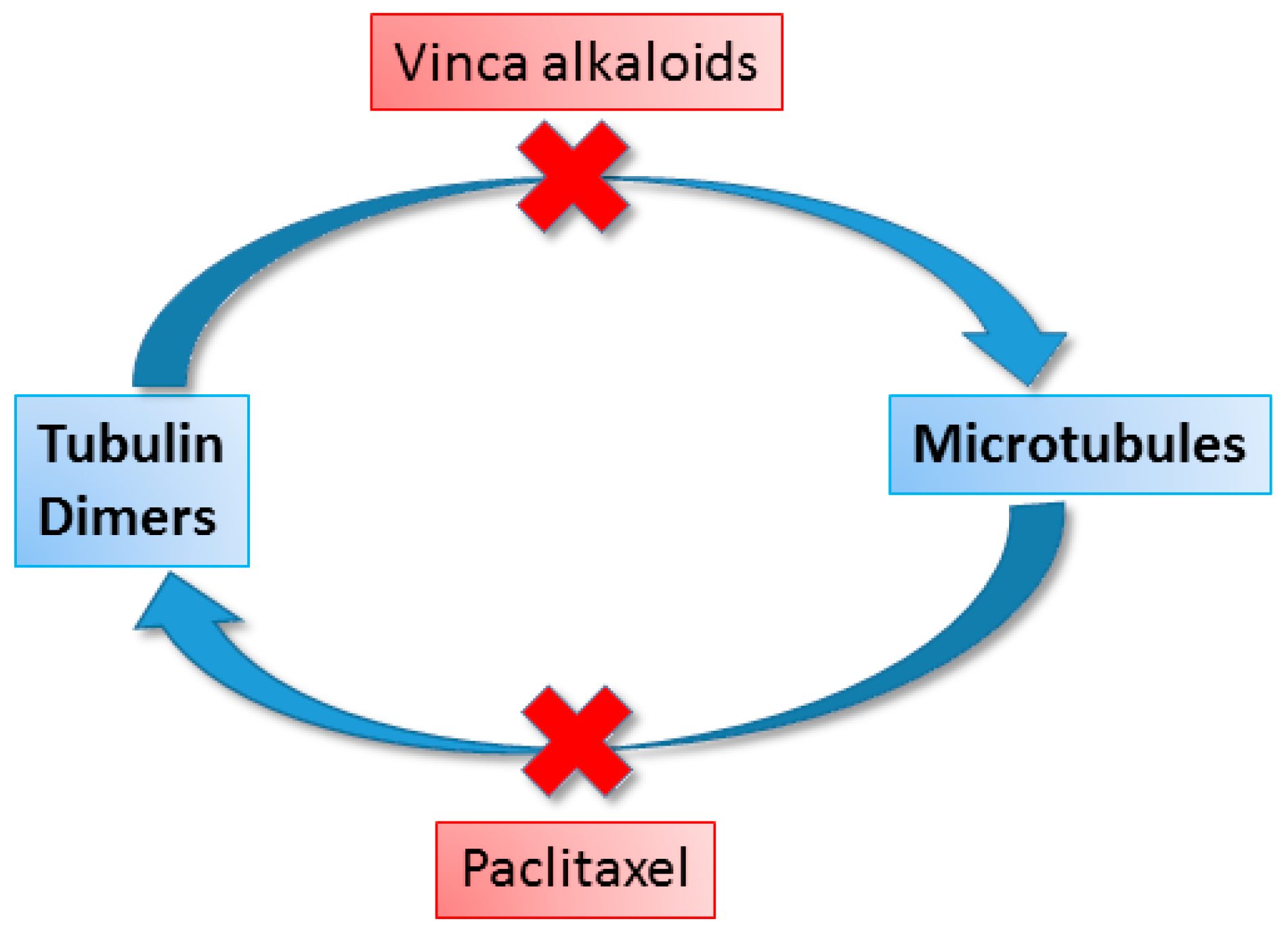
7. Anticancer Compounds of Plant Origin
8. Accidental Discoveries of Anticancer Drugs
9. Tyrosine Kinases Inhibitors
10. Drugs Targeting Cancer Stem Cells
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Davis, M.A.; Cho, E.; Teplensky, M.H. Harnessing biomaterial architecture to drive anticancer innate immunity. J. Mater. Chem. B. 2023, 11, 10982–11005. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Amarah, A.; Elsabagh, A.A.; Ouda, A.; Karen, O.; Ferih, K.; Elmakaty, I.; Malki, M.I. Emerging roles of activating transcription factor 2 in the development of breast cancer: A comprehensive review. Precis. Clin. Med. 2023, 6, pbad028. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Zhou, L.; Yang, H.; Lin, H.; Zhou, S.; Tan, Z.; Qian, J. Immunotherapy and targeted therapy as first-line treatment for advanced gastric cancer. Crit. Rev. Oncol. Hematol. 2023, 198, 104197. [Google Scholar] [CrossRef]
- Abraham, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2016, 44, 30–33. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef]
- Ibarra, A.M.C.; Aguiar, E.M.G.; Ferreira, C.B.R.; Siqueira, J.M.; Corrêa, L.; Nunes, F.D.; Franco, A.L.; Cecatto, R.B.; Hamblin, M.R.; Rodrigues, M.F.S.D. Photodynamic therapy in cancer stem cells—State of the art. Lasers Med. Sci. 2023, 38, 251. [Google Scholar] [CrossRef]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva, F.P., Jr. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef]
- Miranda-Vera, C.; Hernández, Á.P.; García-García, P.; Díez, D.; García, P.A.; Castro, M.Á. Podophyllotoxin: Recent Advances in the Development of Hybridization Strategies to Enhance Its Antitumoral Profile. Pharmaceutics 2023, 15, 2728. [Google Scholar] [CrossRef]
- Clark, P.I.; Slevin, M.L. The clinical pharmacology of etoposide and teniposide. Clin. Pharmacokinet. 1987, 12, 223–252. [Google Scholar] [CrossRef]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95–108. [Google Scholar]
- Baldwin, E.L.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Ann. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Penumallu, N.R.; Shafi, S.; Kamal, A. Prospects of Topoisomerase Inhibitors as Promising Anti-Cancer Agents. Pharmaceuticals 2023, 16, 1456. [Google Scholar] [CrossRef]
- Krumbhaar, E.B.; Krumbhaar, H.D. The blood and bone marrow in yelloe cross gas (mustard gas) poisoning: Changes produced in the bone marrow of fatal cases. J. Med. Res. 1919, 40, 497–508. [Google Scholar]
- Pappenheimer, A.M.; Vance, M. The effects of intravenous injections of dichloroethylsulfide in rabbits, with special reference to its leucotoxic action. J. Exp. Med. 1920, 31, 71–94. [Google Scholar] [CrossRef][Green Version]
- Stenglova Netíkova, I.R.; Petruzelka, L.; Stastny, M.; Stengl, V. Safe decontamination of cytostatics from the nitrogen mustards family. Part one: Cyclophosphamide and ifosfamide. Int. J. Nanomed. 2018, 13, 7971–7985. [Google Scholar] [CrossRef]
- Farmer, P.B.; Jarman, M.; Facchinetti, T.; Pankiewicz, K.; Stec, W.J. The metabolism and antitumour activity of the enantiomers of cis- and trans-4-methylcyclophosphamide. Chem. Biol. Interact. 1977, 18, 47–57. [Google Scholar] [CrossRef]
- Voelcker, G. Oxazaphosphorine cytostatics: From serendipity to rational drug design. Anticancer Drugs 2019, 30, 435–440. [Google Scholar] [CrossRef]
- Voelcker, G. Causes and possibilities to circumvent cyclophosphamide toxicity. Anticancer Drugs 2020, 31, 617–622. [Google Scholar] [CrossRef]
- Lowenberg, D.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ifosfamide pathways, pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2014, 24, 133–138. [Google Scholar] [CrossRef]
- Voelcker, G. The mechanism of action of cyclophosphamide and its consequences for the development of a new generation of oxazaphosphorinecytostatics. Sci. Pharm. 2020, 88, 42. [Google Scholar] [CrossRef]
- Welch, A.D. Folic acid: Discovery and the exciting first decade. Perspect. Biol. Med. 1983, 27, 64–75. [Google Scholar] [CrossRef]
- Rosenberg, I.H. A history of the isolation and identification of folic acid (folate). Ann. Nutr. Metab. 2012, 61, 231–235. [Google Scholar] [CrossRef]
- Farber, S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood 1949, 4, 160–167. [Google Scholar] [CrossRef]
- Miller, D.R. A tribute to Sidney Farber—The father of modern chemotherapy. Br. J. Haematol. 2006, 134, 20–26. [Google Scholar] [CrossRef]
- Huennekens, F.M. The methotrexate story: A paradigm for development of cancer chemotherapeutic agents. Adv. Enzym. Reg. 1994, 34, 397–419. [Google Scholar] [CrossRef]
- Maksimovic, V.; Pavlovic-Popovic, Z.; Vukmirovic, S.; Cvejic, J.; Mooranian, A.; Al-Salami, H.; Golocorbin-Kon, S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020, 47, 4699–4708. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020, 21, 3483–3521. [Google Scholar] [CrossRef]
- Malaviya, A.N.; Sharma, A.; Agarwal, D.; Kapoor, S.; Garg, S.; Sawhney, S. Low-dose and high-dose methotrexate are two different drugs in practical terms. Int. J. Reum. Dis. 2010, 13, 288–293. [Google Scholar] [CrossRef]
- Gonen, N.; Assaraf, Y.G. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist. Updat. 2012, 15, 183–210. [Google Scholar] [CrossRef]
- Walling, J. From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Investig. New Drugs 2006, 24, 37–77. [Google Scholar] [CrossRef]
- Adjei, A.A. Pemetrexed: A multitargeted antifolate agent with promising activity in solid tumors. Ann. Oncol. 2000, 11, 1335–1341. [Google Scholar] [CrossRef]
- Joerger, M.; Omlin, A.; Cerny, T.; Fruh, M. The role of pemetrexed in advanced non small-cell lung cancer: Special focus on pharmacology and mechanism of action. Curr. Drug Targets 2010, 11, 37–47. [Google Scholar] [CrossRef]
- Takemura, Y.; Kobayashi, H.; Miyachi, H. Raltitrexed, a novel folate-based thymidylate synthase inhibitor, for the treatment of acute leukemia: Is this drug active against acute myelogenous leukemia? Int. J. Hematol. 2000, 72, 112–114. [Google Scholar]
- Lin, J.T.; Bertino, J.R. Clinical science review: Update on trimetrexate, a folate antagonist with antineoplastic and antiprotozoal properties. Cancer Investig. 1991, 9, 159–172. [Google Scholar] [CrossRef]
- Adjei, A.A. Pemetrexed (Alimta®): A novel multitargeted antifolate agent. Expert Rev. Anticancer Ther. 2003, 3, 145–156. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Moran, R.G.; Goldman, I.D. Pemetrexed: Biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol. Cancer Ther. 2007, 6, 404–417. [Google Scholar] [CrossRef]
- Wilson, K.S.; Malfair Taylor, S.C. Raltitrexed: Optimism and reality. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1447–1454. [Google Scholar] [CrossRef]
- Haller, D.G. Trimetrexate: Experience with solid tumors. Semin. Oncol. 1997, 24, 18–76. [Google Scholar]
- Tanaka, F.; Fukuse, T.; Wada, H.; Fukushima, M. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr. Pharmaceut. Biotech. 2000, 1, 137–164. [Google Scholar] [CrossRef]
- Chalabi-Dchar, M.; Fenouil, T.; Machon, C.; Vincent, A.; Catez, F.; Marcel, V.; Mertani, H.C.; Saurin, J.C.; Bouvet, P.; Guitton, J.; et al. A novel view on an old drug, 5-fluorouracil: An unexpected RNA modifier with intriguing impact on cancer cell fate. NAR Cancer 2021, 3, zcab032. [Google Scholar] [CrossRef]
- WHO. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee (Including the 20th WHO Model List of Essential Medicines and the 6th WHO Model List of Essential Medicines for Children); WHO Technical Report Series; 30 January 2017 Meeting Report, No. 1006; WHO: Geneva, Switzerland, 2017; ISBN 978-92-4-121015-7. [Google Scholar]
- Hussain, S. On a new proposed mechanism of 5-fluorouracil-mediated cytotoxicity. Trends Cancer 2020, 6, 365–368. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nature Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Bradner, W.T. Mitomycin C: A clinical update. Cancer Treat. Rev. 2001, 27, 35–50. [Google Scholar] [CrossRef]
- Bargonetti, J.; Champeil, E.; Tomasz, M. Differential toxicity of DNA adducts of mitomycin C. J. Nucleic Acids 2010, 2010, 698960. [Google Scholar] [CrossRef]
- Paz, M.M.; Zhang, X.; Lu, J.; Holmgren, A. A new mechanism of action for the anticancer drug mitomycin C: Mechanism-based inhibition of thioredoxin reductase. Chem. Res. Toxicol. 2012, 25, 1502–1511. [Google Scholar] [CrossRef]
- Lown, J. Discovery and development of anthracycline antitumour antibiotics. Chem. Soc. Rev. 1993, 22, 165–176. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Jawad, B.; Poudel, L.; Podgornik, R.; Steinmetz, N.F.; Ching, W.Y. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys. Chem. Chem. Phys. 2019, 21, 3877–3893. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Moiseeva, A.A. Anthracycline derivatives and their anticancer activity. Apoptosis 2019, 25, 26. [Google Scholar] [CrossRef]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shaik, B.B.; Katari, N.K.; Jonnalagadda, S.B. Role of natural products in developing novel anticancer agents: A perspective. Chem. Biodivers. 2022, 19, e202200535. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Khan, A.W.; Farooq, M.; Haseeb, M.; Choi, S. Role of plant-derived active constituents in cancer treatment and their mechanisms of action. Cells 2022, 11, 1326. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Cho, W.C. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Liu, L.F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988, 48, 1722–1726. [Google Scholar] [PubMed]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Lee, W.L.; Shiau, J.Y.; Shyur, L.F. Taxol, camptothecin and beyond for cancer therapy. Rec. Trends Med. Plants Res. 2012, 133–178. [Google Scholar]
- Mathijssen, R.H.; Loos, W.J.; Verweij, J.; Sparreboom, A. Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Curr. Cancer Drug Targets 2002, 2, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Causes and consequences of DNA single-strand breaks. Trends Biochem. Sci. 2024, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.P. Camptothecin and podophyllotoxin derivatives: Inhibitors of topoisomerase I and II—Mechanisms of action, pharmacokinetics and toxicity profile. Drug Safety 2006, 29, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M. Paclitaxel (Taxol®): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about taxol: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Sati, P.; Sharma, E.; Dhyani, P.; Attri, D.C.; Rana, R.; Kiyekbayeva, L.; Büsselberg, D.; Samuel, S.M.; Sharifi-Rad, J. Paclitaxel and its semi-synthetic derivatives: Comprehensive insights into chemical structure, mechanisms of action, and anticancer properties. Eur. J. Med. Res. 2024, 29, 90. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.H. On and around microtubules: An overview. Mol. Biotech. 2009, 43, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Haider, M.R.; Shafi, S.; Yar, M.S. Anti-tubulin agents of natural origin: Targeting taxol, vinca, and colchicine binding domains. Eur. J. Med. Chem. 2019, 171, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V. Taxol and related taxanes. I. Taxanes of Taxus brevifolia bark. Pharm. Res. 1993, 10, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Han, J.C.; Zhang, W.; Zou, Y.P.; Li, C.C. Strategies and lessons earned from total synthesis of taxol. Chem. Rev. 2023, 123, 4934–4971. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Luo, Y.F.; Gao, W. Biosynthesis of paclitaxel using synthetic biology. Phytochem. Rev. 2021, 21, 863–877. [Google Scholar] [CrossRef]
- McElroy, C.; Jennewein, S. Taxol biosynthesis and production: From forests to fermenters. In Biotechnology of Natural Products; Springer: Cham, Swizterland, 2018; pp. 145–185. [Google Scholar]
- Faulds, D.; Balfour, J.A.; Chrisp, P.; Langtry, H.D. Mitoxantrone: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs 1991, 41, 400–449. [Google Scholar] [CrossRef] [PubMed]
- Evison, B.J.; Sleebs, B.E.; Watson, K.G.; Phillips, D.R.; Cutts, S.M. Mitoxantrone, more than just another topoisomerase II poison. Med. Res. Rev. 2015, 36, 248–299. [Google Scholar] [CrossRef]
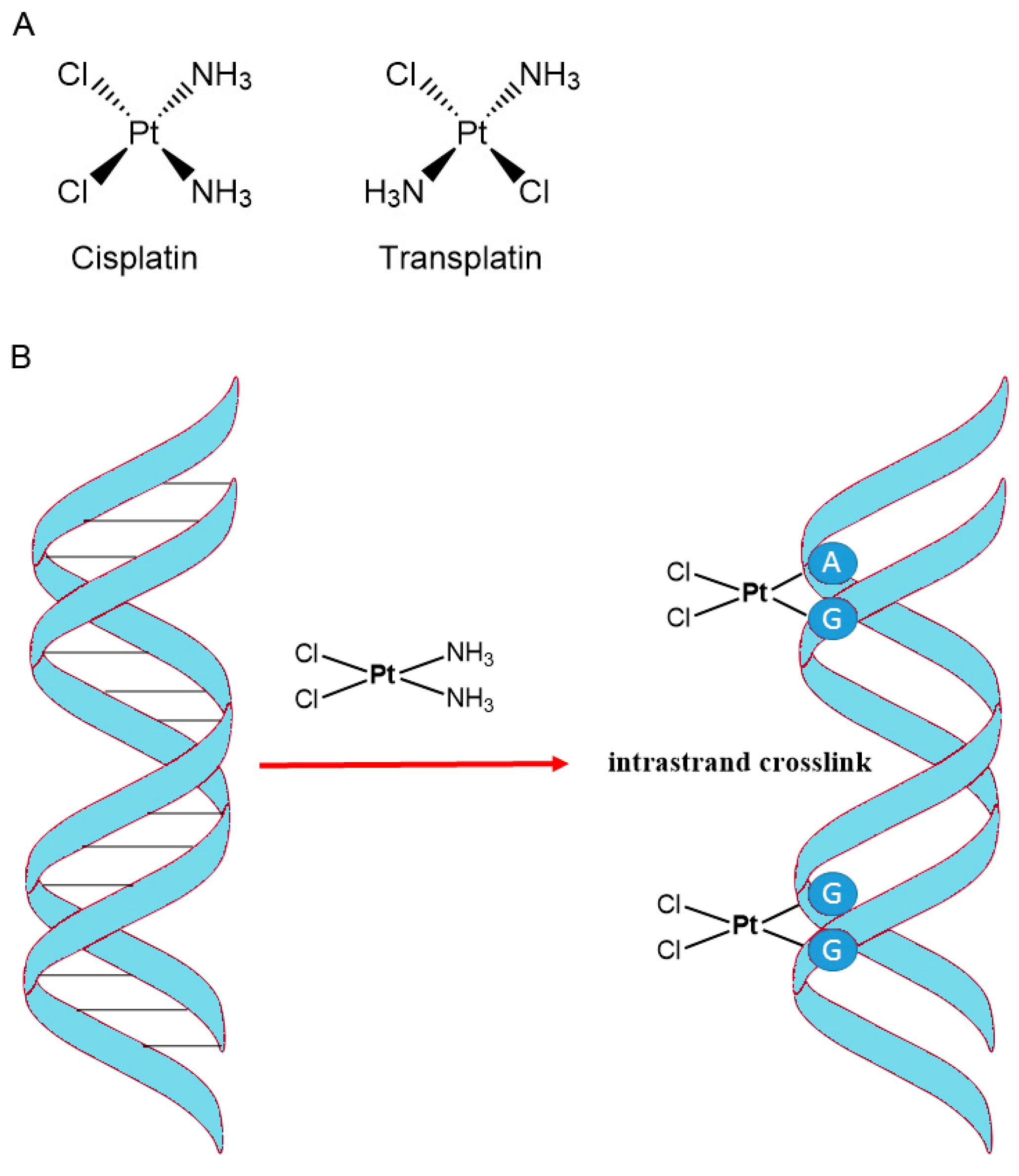
- Alderden, R.A.; Hall, M.D.; Hambley, T.W. The discovery and development of cisplatin. J. Chem. Edu. 2006, 83, 728. [Google Scholar] [CrossRef]
- Monneret, C. Platinum anticancer drugs. From serendipity to rational design. Ann. Pharm. Fr. 2011, 69, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D. Cisplatin: Forty Years of a Serendipitous Discovery for Cancer. Resonance 2022, 27, 659–666. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- Kishimoto, T.; Yoshikawa, Y.; Yoshikawa, K.; Komeda, S. Different effects of cisplatin and transplatin on the higher-order structure of DNA and gene expression. Int. J. Mol. Sci. 2019, 21, 34. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, A.J.; Kerwood, D.J.; Shi, Y.; Goodisman, J.; Dabrowiak, J.C. Stability of carboplatin and oxaliplatin in their infusion solutions is due to self-association. Dalton Trans. 2011, 40, 4821–4825. [Google Scholar] [CrossRef]
- Farrell, N.P. Multi-platinum anti-cancer agents. Substitution-inert compounds for tumor selectivity and new targets. Chem. Soc. Rev. 2015, 44, 8773–8785. [Google Scholar] [CrossRef]
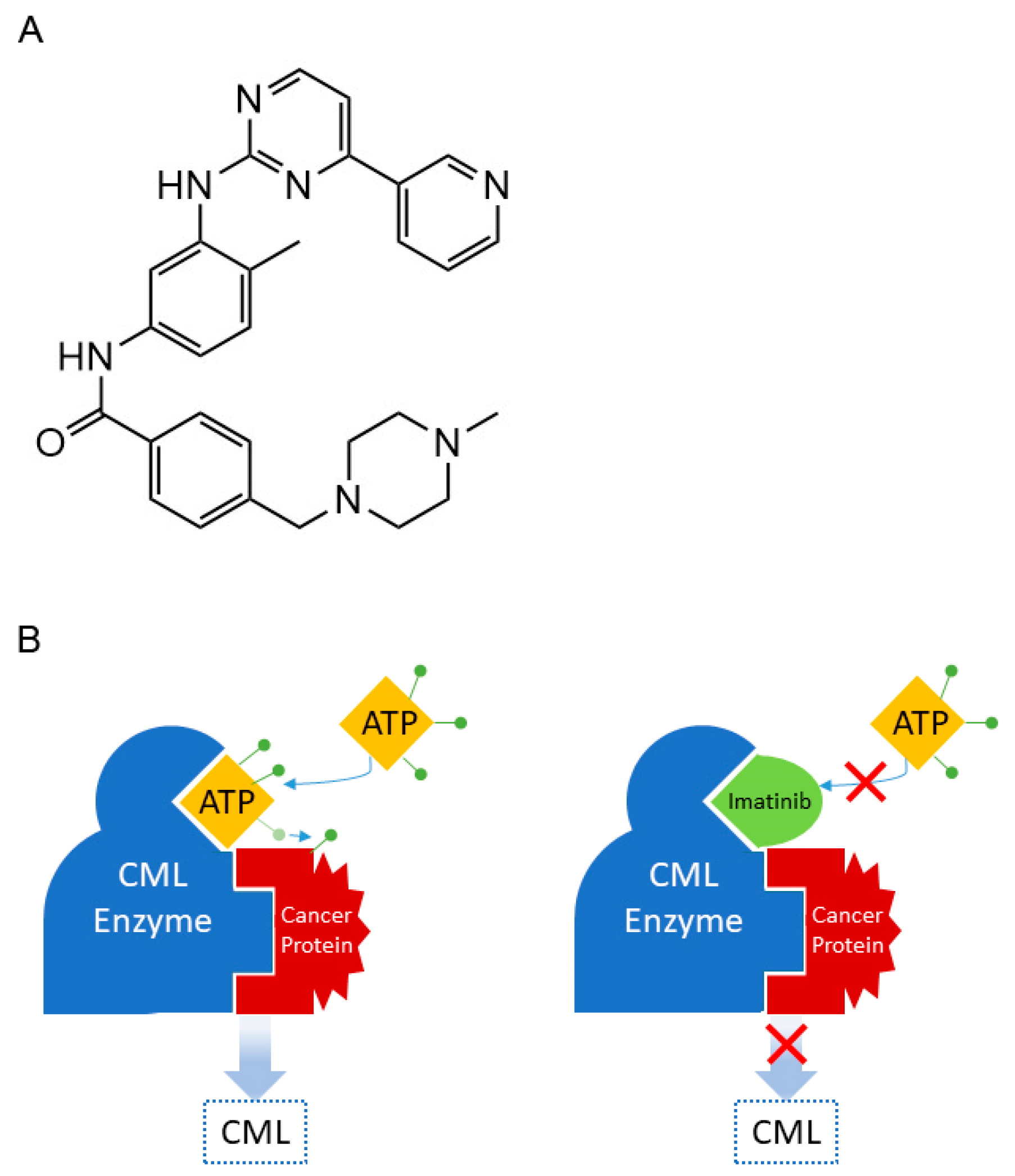
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Disc. 2002, 1, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.J.; Liu, Y.F.; Xu, L.Z.; Long, Z.J.; Huang, D.; Yang, Y.; Liu, Q. The Philadelphia chromosome in leukemogenesis. Chin. J. Cancer 2016, 35, 48. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Nsairat, H.; Matalka, I.I.; Lee, Y.F.; Rizzo, M.; Aljabali, A.A.; Mishra, V.; Mishra, Y.; Hromić-Jahjefendić, A.; Tambuwala, M.M. The impact of the BCR-ABL oncogene in the pathology and treatment of chronic myeloid leukemia. Pathol. Res. Pract. 2024, 254, 155161. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.K.; Duhme-Klair, A.K.; Morgan, T.; Ramjee, M.K. The background, discovery and clinical development of BCR-ABL inhibitors. Drug Disc. Today 2013, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schiöth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Druker, B.J.; Lydon, N.B. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Investig. 2000, 105, 3–7. [Google Scholar] [CrossRef]
- Du, F.Y.; Zhou, Q.F.; Sun, W.J.; Chen, G.L. Targeting cancer stem cells in drug discovery: Current state and future perspectives. World J. Stem Cells. 2019, 11, 398–420. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Bellot, G.L.; Hirpara, J.L.; Pervaiz, S. Understanding the cancer stem cell phenotype: A step forward in the therapeutic management of cancer. Biochem. Pharmacol. 2019, 162, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Low, J.A.; Marsters, J.C., Jr.; Robarge, K.; Rubin, L.L.; de Sauvage, F.J.; Sutherlin, D.P.; Wong, H.; Yauch, R.L. Discovery and preclinical development of vismodegib. Expert Opin. Drug Discov. 2014, 9, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B. Sonidegib: First Global Approval. Drugs 2015, 75, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Glasdegib: First Global Approval. Drugs 2019, 79, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimer’s Dement. TRCI 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 7, 3049–3062. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Aggarwal, B.B. Serendipity in cancer drug discovery: Rational or coincidence? Trends Pharmacol. Sci. 2016, 37, 435–450. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gach-Janczak, K.; Drogosz-Stachowicz, J.; Janecka, A.; Wtorek, K.; Mirowski, M. Historical Perspective and Current Trends in Anticancer Drug Development. Cancers 2024, 16, 1878. https://doi.org/10.3390/cancers16101878
Gach-Janczak K, Drogosz-Stachowicz J, Janecka A, Wtorek K, Mirowski M. Historical Perspective and Current Trends in Anticancer Drug Development. Cancers. 2024; 16(10):1878. https://doi.org/10.3390/cancers16101878
Chicago/Turabian StyleGach-Janczak, Katarzyna, Joanna Drogosz-Stachowicz, Anna Janecka, Karol Wtorek, and Marek Mirowski. 2024. "Historical Perspective and Current Trends in Anticancer Drug Development" Cancers 16, no. 10: 1878. https://doi.org/10.3390/cancers16101878
APA StyleGach-Janczak, K., Drogosz-Stachowicz, J., Janecka, A., Wtorek, K., & Mirowski, M. (2024). Historical Perspective and Current Trends in Anticancer Drug Development. Cancers, 16(10), 1878. https://doi.org/10.3390/cancers16101878






