Simple Summary
For melanoma patients, the most promising curative treatment is immunotherapy. Here, we summarise current immunotherapy strategies and their indications and challenges, and provide future directions of immunotherapy development. Several therapy resistance mechanisms have been described, resulting in primary refractory disease or resistance development in patients. Our increasing knowledge of immune regulation within the tumour microenvironment identifies potential alternate immunotherapy targets, including CD155. CD155 and its receptor, TIGIT, have been shown to be highly expressed in therapy-resistant melanoma cells and interference with both is therefore considered a possible immunotherapeutic strategy. This review describes the immune regulation within the melanoma tumour microenvironment, how and why immunotherapies work, and why CD155 might be an ideal target for the next generation of anti-melanoma immunotherapies.
Abstract
Melanoma is commonly diagnosed in a younger population than most other solid malignancies and, in Australia and most of the world, is the leading cause of skin-cancer-related death. Melanoma is a cancer type with high immunogenicity; thus, immunotherapies are used as first-line treatment for advanced melanoma patients. Although immunotherapies are working well, not all the patients are benefitting from them. A lack of a comprehensive understanding of immune regulation in the melanoma tumour microenvironment is a major challenge of patient stratification. Overexpression of CD155 has been reported as a key factor in melanoma immune regulation for the development of therapy resistance. A more thorough understanding of the actions of current immunotherapy strategies, their effects on immune cell subsets, and the roles that CD155 plays are essential for a rational design of novel targets of anti-cancer immunotherapies. In this review, we comprehensively discuss current anti-melanoma immunotherapy strategies and the immune response contribution of different cell lineages, including tumour endothelial cells, myeloid-derived suppressor cells, cytotoxic T cells, cancer-associated fibroblast, and nature killer cells. Finally, we explore the impact of CD155 and its receptors DNAM-1, TIGIT, and CD96 on immune cells, especially in the context of the melanoma tumour microenvironment and anti-cancer immunotherapies.
1. Introduction
Skin cancer is divided into melanoma and non-melanoma skin cancer, with the latter including basal cell carcinoma and squamous cell carcinoma [1]. While non-melanoma skin cancers have very high incidences, their mortalities are comparably small, whereas melanoma is a major cause of cancer-related death [2], with Australia having the highest melanoma incident worldwide [3,4]. Although melanoma incidence can be significantly reduced by lifestyle interventions such as regular use of sunscreen [5], nevertheless, there is a significant proportion of the population, especially in the 20 to 39 age range, diagnosed every year [4].
Melanocytes are the cells that produce melanin to absorb ultraviolet radiation (UV), and thus provide photoprotection and thermoregulation to the skin [6]. Overexposure to UV is considered the main risk factor for causing malignant transformation of melanocytes [7], which progresses into cutaneous melanoma [6,8]. Early-stage melanoma can be surgically removed, commonly resulting in good outcomes [2], with a 5-year survival rate of stage I melanoma patients of around 97–99% [9]. In contrast, the 5-year survival rate of advanced, metastatic stage IV patients is only 15–20% [9]. Melanoma is associated with the highest rate of genetic mutations amongst cancer types [10] and intertumoural heterogeneity [11], creating challenges for developing advanced melanoma treatments [10]. Common mutations in melanoma are BRAF and NRAS [8,12], but, in addition, approximately 70% of mutations in melanoma involve the mitogen-activated protein kinase (MAPK) pathway [13]. Thus, the BRAF inhibitor vemurafenib is a commonly used targeted therapy and improves melanoma survival rates [14]. However, not all patients with BRAF mutations benefited from the treatment, with some of them displaying initial or acquired drug resistance [14].
Another feature that makes melanoma so aggressive, but has recently been exploited as a therapeutic opportunity, is its high antigenicity [15,16] and immunogenicity [17,18]. Melanoma escapes immune surveillance by manipulating immune checkpoints, such as programmed cell death protein 1 (PD-1) [19], programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein-4 (CTLA4) [20]. One of the recent therapeutic breakthroughs came from the inhibition of these immune checkpoints, which greatly improved advanced melanoma patient survival rates; however, not all the patients obtained a sustained clinical response [18,21,22,23,24], with some patients initially responding but developing immunotherapy resistance [25,26]. It has therefore become a focus to comprehensively understand the details of immune regulation in melanoma in order to provide new potential therapeutic targets to improve patient outcomes.
CD155, also known as poliovirus receptor (PVR), maintains the normal function of immune cells by controlling activating and inhibitory signals via interactions with DNAM-1, CD96, and TIGIT receptors [27]. CD155 is highly expressed in most melanoma cell lines [28] and involved in drug resistance to immune therapy in patients [29,30,31,32], and therefore has been suggested to be a potential target for co-inhibitory immune therapies [33].
Here, we will first discuss clinical outcomes of immune therapy in melanoma patients and the problems that need to be addressed to improve current treatment options. Additionally, we will comprehensively review and discuss immune regulation within the melanoma tumour microenvironment, including specific immune responses triggered in different cell lineages. The specific contributions of these lineages to melanoma immunosurveillance escape will be described, as well as their relative contributions to promoting tumour growth through regulating immune checkpoint proteins. We will also review currently used biomarkers shown to be predicting patient outcomes treated with immune therapies. Finally, we will evaluate how CD155 manipulates immune cells to inhibit anti-tumour immune response, its role in melanoma escape from immune surveillance, and how this knowledge could assist in the clinical management of melanoma patients.
2. Current Melanoma Patient Immunotherapy Strategies
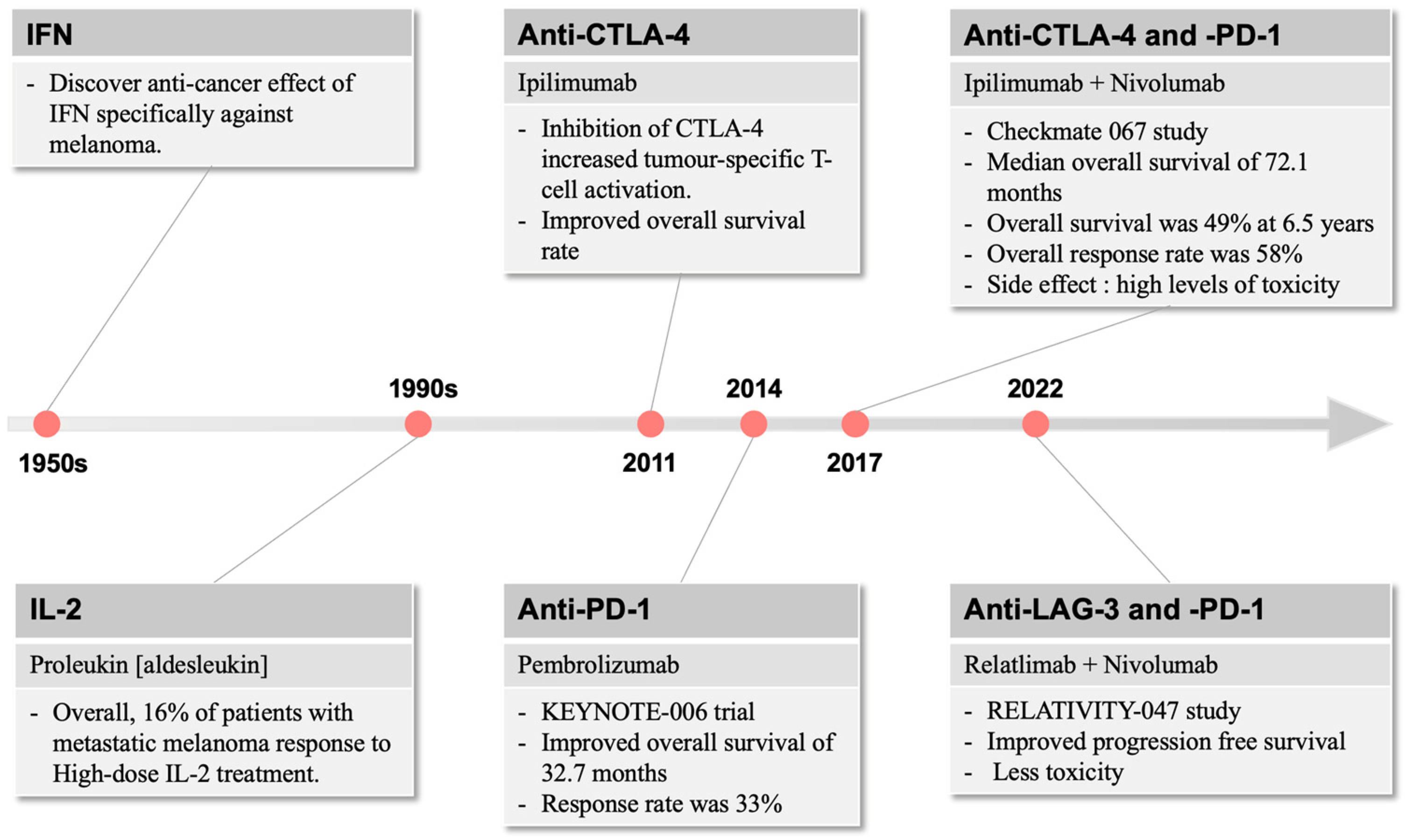
The use of Coley’s toxin over 100 years ago to induce anti-tumour responses provided the inspiration for generations of cancer and immunology researchers to harness the power of the immune system in cancer therapy [34]. Immunotherapy for the treatment of cancer gained momentum in the late 1950s with the discovery by Issacs and Linderman [35] of interferon (IFN), which displayed anti-cancer efficacy, particularly against melanoma (Figure 1). This eventually led to the FDA approval of INF-a2b, which improved relapse-free survival in patients with fully resected melanoma [36]. In parallel, the use of high-dose interleukin (IL)-2 was found to induce objective responses in patients with metastatic melanoma, including complete and durable responses [37], and re-infusion of ex vivo processed and expanded tumour-infiltrating lymphocytes conferred impressive clinical benefit in small studies [38].
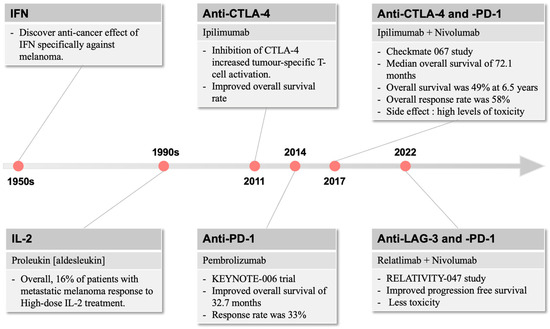
Figure 1.
The development of immunotherapies over time. In melanoma, immune-related therapies were first described in the 1950s, which raised the interest of targeting the immune system for therapeutic use. Since 2011, immunotherapies focus on different immune checkpoint molecules, including CTLA-4 and PD-1, with more recent combination strategies providing more anti-melanoma efficacies with reduced side effect profiles.
While these successes were encouraging, more reliably effective treatments were needed. A better understanding of interactions between the immune system and cancer, particularly mechanisms that inhibit the expansion and activity of cytotoxic T cells and that regulate evasion by cancer cells from immune attack [39], led to the development of more robust treatment strategies. The anti-tumour immune response in the tumour microenvironment is often dysfunctional. Tumour cells respond to immune activation through several mechanisms, including the expression of immunosuppressive moieties like PD-L1, which impairs T-cell responses [40]. T-cell priming may also be inadequate at the point of initial tumour antigen presentation, resulting in impaired initiation of anti-tumour immune responses through inhibitory molecules such as CTLA-4 [41,42].
The first approved therapy in targeting such immune resistance was ipilimumab, which inhibits CTLA-4 and increases generic and tumour-specific T-cell activation [43]. In a paradigm-changing trial in patients with metastatic or unresectable melanoma, ipilimumab demonstrated an improvement in median survival of 10 months compared to 6.4 months for gp100, a peptide vaccine [44]. This resulted in FDA approval of ipilimumab in 2011. While this was a significant improvement over existing standards of care, the response rate to single-agent ipilimumab was low at only 10.9%.
In 2014, the anti-PD-1 inhibitors pembrolizumab and nivolumab were approved for metastatic melanoma. The KEYNOTE-006 trial demonstrated improved overall survival of 32.7 months for pembrolizumab compared to the historical 15.9 months for ipilimumab [45]. Additionally, response rates to pembrolizumab were 33%, higher than those for ipilimumab, offering clinical meaningful improvements in disease-related symptoms [46] (Figure 1).
A few years later, the landmark Checkmate 067 study of ipilimumab and nivolumab combination therapy compared to nivolumab or ipilimumab alone set a new standard of care for metastatic melanoma [23]. This study demonstrated impressive and durable survival outcomes, with a median overall survival of 72.1 months in the combination arm compared to 39.6 months and 19.9 months for the nivolumab and ipilimumab arms, respectively. Overall survival was 49% at 6.5 years in the combination arm compared to 42% and 23% in the nivolumab and ipilimumab arms, respectively. Overall response rate in the combination was 58%, a significant improvement over single-agent therapy. Similarly to interferon and interleukin therapies, the major downside of this approach was the high levels of toxicity, with nearly all patients experiencing at least minor toxicity and rates of severe toxicity approaching 60%, nearly three times higher than single-agent anti-PD1 therapy in the same trial.
More recent developments have led to efficacious but less toxic immunotherapy combinations in melanoma. Relatlimab, an LAG-3 inhibitor, in combination with nivolumab, demonstrated a superior progression-free survival of 10.1 vs. 4.6 months in the RELATIVITY-047 study compared to nivolumab alone [47]. The toxicity of the relatlimab plus nivolumab combination was substantially lower than that seen in CheckMate 067, with severe toxicity seen in only 18.9% of patients.
The use of immunotherapy has also extended to the treatment of patients with locoregionally advanced disease who undergo curative-intent surgery, nowadays offered in both adjuvant [48,49] and neoadjuvant [50] treatment contexts. Interestingly, while the key studies that tested anti-PD1 in these contexts each showed substantial improvements in relapse-free survival, none of them has to date demonstrated an overall survival benefit compared to placebo treatment. This has raised the possibility that the patients with early-stage disease who benefit from adjuvant anti-PD1 therapy are the same patients who enjoy sustained remission after treatment of their advanced disease. Nevertheless, the strikingly improved relapse-free survival observed in the SWOG 1801 study of the pre-operative neoadjuvant followed by post-operative adjuvant pembrolizumab, compared to adjuvant pembrolizumab alone, suggests that surgery might induce adjuvant immunotherapy resistance in some patients, such that neoadjuvant therapy might eradicate disease in some patients who otherwise would not have responded in a sustained manner had their disease relapsed and metastasised widely [50].
Challenges and opportunities remain, however, in melanoma treatment. Between 40 and 65% of patients treated with immunotherapy may be primarily refractory to this treatment [51], leading to the need for alternative strategies. Later-line treatments for melanoma can be of limited benefit for patients without activating class 1 BRAF mutations, with objective response rates to single-agent ipilimumab following single-agent anti-PD1 therapy as low as 9%, although combination therapy with ipilimumab and nivolumab in this context is more promising, offering response rates of 28% [52]. In another approach, addition of the multi-kinase inhibitor lenvatinib to pembrolizumab demonstrated objective response rates of 21.4% in the phase II LEAP-004 trial, with a median overall survival of 14 months [53].
Immunotherapy is likely to remain a backbone of treatment for metastatic or unresectable melanoma, although many patients do not obtain the deep and durable responses that others enjoy. This highlights the urgent need for ongoing innovation in melanoma treatment through the understanding of primary and secondary mechanisms of resistance to immunotherapy, biomarkers to indicate those early, and strategies to overcome or avert these [54].
3. Immunoregulation Mechanisms within the Melanoma Tumour Microenvironment
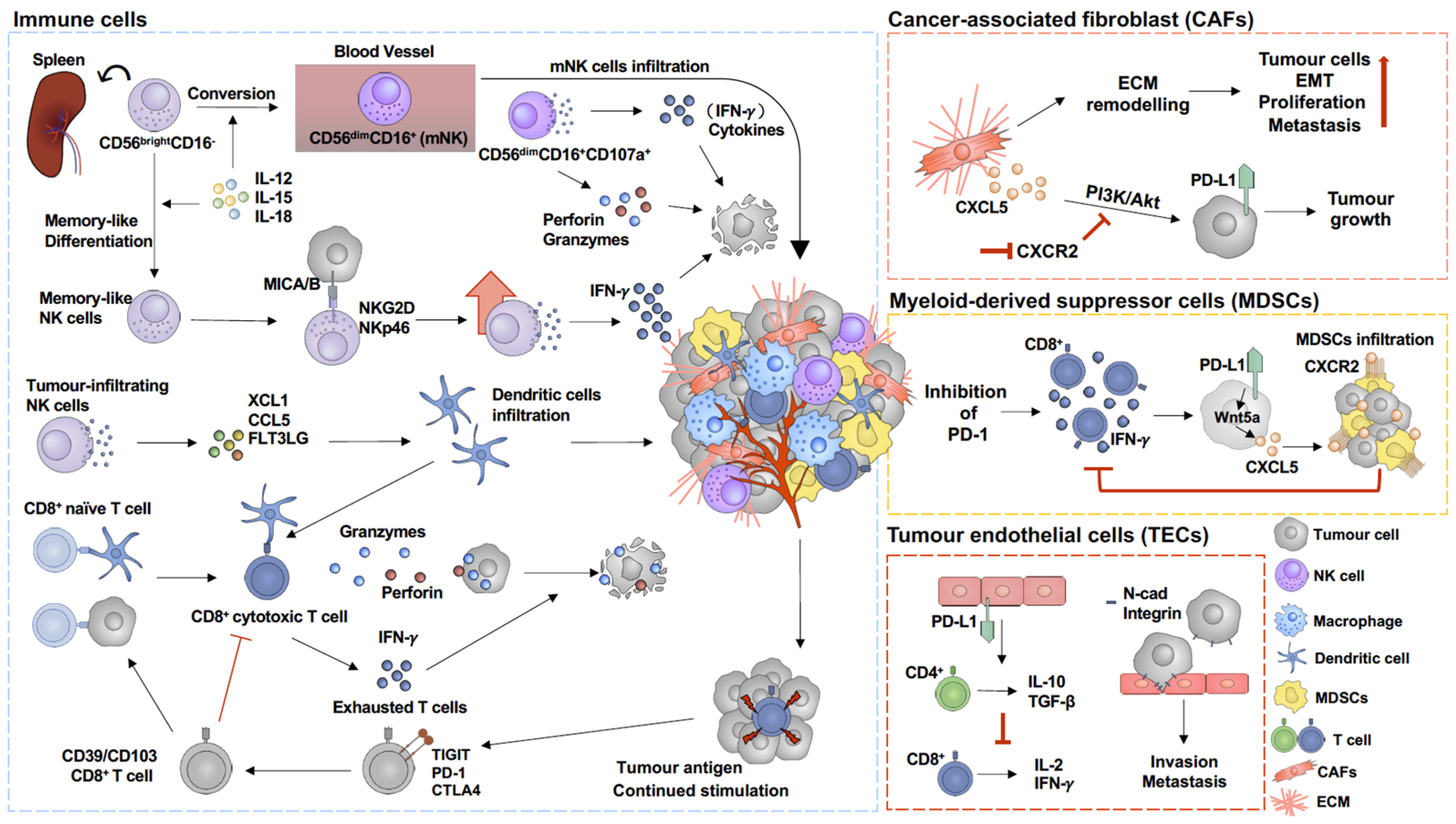
Within the melanoma tumour microenvironment, the most abundant non-malignant cell populations are immune and endothelial cells as well as cancer-associated fibroblasts (CAFs) [55,56]. The expression of immune checkpoint proteins has been observed in a variety of cell types, including tumour endothelial cells (TECs) and myeloid-derived suppressor cells (MDSCs), which are involved in the regulation of immune suppressive signalling [57,58]. Understanding the immune suppressive regulation mechanisms in different cell types might identify potential targets for melanoma therapy (Figure 2).
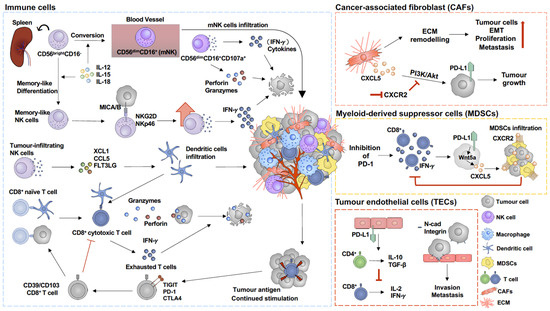
Figure 2.
Immunoregulation in tumour microenvironment of melanoma. Activation of cancer-associated fibroblasts (CAFs) triggers extracellular matrix (ECM) remodelling, which subsequently facilitates tumour cell proliferation [59,60] and epithelial–mesenchymal transition (EMT) [61,62]. In addition, CXCL5, which is secreted by CAFs, upregulates PD-L1 expression on tumour cells through PI3K/Akt pathway activation, which promotes tumour growth and can be prevented by inhibition of CXCR2 [63]. Melanoma cells express adherent molecules, such as N-cadherin (N-cad) and integrin, which allows them to attach to tumour endothelial cells (TECs), increasing their invasion and metastasis capacity [16,64]. In addition, PD-L1 on TECs stimulates suppressive CD4+ T cells and upregulates IL-10 and TGF-β expression, subsequently inhibiting CD8+ T-cell proliferation and cytokine secretion [57]. PD-1 blockade is commonly used in immune therapy for melanoma. However, inhibition of PD-1 has been found to promote myeloid-derived suppressor cell (MDSC) infiltration into the tumour microenvironment [25,26,65]. PD-1 blockade induces CD8+ T-cell activation and IFN-γ secretion, which, in turn, upregulates Wnt5a expression in melanoma cells and further promotes CXCL5 secretion, subsequently promoting MDSC infiltration in a CXCR2/CXCL5-dependent pathway [26]. MDSC infiltration then suppresses CD8+ T-cell activity and IFN-γ secretion [26]. Activated CD107a+ CD56dimCD16+-mNK cells secrete perforin and granzymes and cytokines to target tumour cells in the tumour microenvironment [66]. In addition, memory-like NK cells enhance IFN-γ production to kill melanoma cells in an NKG2D- and NKp46-dependent manner [67]. In addition, MICA/B on tumour cells binding to NKG2D triggers NK cell cytokine production and subsequently promotes tumour cell apoptosis [67,68]. Continued stimulation by tumour antigen on CD8+ T cells increases exhausted T-cell populations, which are considered as dysfunctional CD8+ T cells [69,70]. TIGIT, PD-1, and CTLA4 are commonly expressed on exhausted T cells [71,72].
3.1. Tumour Endothelial Cells (TECs)
Endothelial cells in the tumour microenvironment are essential mediators of neo-angiogenesis, providing the cancer cells with sufficient oxygen and nutrients, thus enabling tumour growth [73,74]. TECs and the resulting cancer vascular system have different phenotypes to normal, physiological endothelial cells and vessels [75,76]. Not only is the cancer vascular system leakier [75], but it also has a different structural morphology, with vessels made mostly of TECs associated with a limited number of pericytes, arranged in a more random fashion, and monocyte-derived cells, phenocopying endothelial cells [75]. TECs in particular trigger immune suppressive signals, resulting in CD8+ T-cell dysfunction [57], which can be overcome by blocking PD-L1, PD-1, or CTLA4 [57,77]. The expression of PD-L1 by TECs induces immune-suppressive CD4+ regulatory T cells (Tregs), which, in turn, upregulate IL-10 and TGF-β expression, resulting in reduced CD8+ T-cell proliferation and pro-inflammatory cytokine production, such as IL-2, TNFα, and IFNγ [57]. Dysfunctional CD8+ T cells then further promote tumour growth [57,77]. However, stimulator of IFN genes (STINGs)-induced IFN-β production by VEGFR2+ CD31+ TECs [77] or inhibition of PD-1 [77] or PD-L1 [57] reverses CD8+ T-cell dysfunction and promotes anti-tumour immune responses. Moreover, melanoma cells express adherent molecules, such as N-cadherin, integrin α4β1, and ALCAM, which allow melanoma cells to attach to TECs, further promoting invasion and metastasis [16,64].
3.2. Cancer-Associated Fibroblast (CAF)
Under physiological conditions, the activation of fibroblasts occurs in the process of wound healing and decreases once wound healing is completed [78]. Sustained activation of fibroblasts is observed under pathological conditions, such as cancer and chronic fibrosis [79]. These activated fibroblasts, also termed myofibroblasts, are spindle-shaped cells and highly express α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) [78]. Myofibroblast activation has been suggested to enhance contraction and promote extracellular matrix (ECM) remodelling, which results in fibrosis [78]. Similar features of remodelling the ECM can be found in cancer-derived fibroblasts (CAFs) [80], which trigger mechanotransduction signalling pathways, such as the hippo pathway, to promote cancer cell proliferation [59,60] and epithelial–mesenchymal transition (EMT) [61,62]. In addition, the secretome of CAFs has been shown to promote tumour growth [63] and induce drug resistance to therapeutic BRAF inhibition [81]. CAF-secreted CXCL5 causes PI3K/Akt pathway activation in cancer cells, resulting in PD-L1 expression, which can be blocked by the inhibition of the CXCL5 receptor CXCR2 [63]. Together, these data suggest that CAFs are suitable therapeutic target cell lineages in melanoma [82].
3.3. Myeloid-Derived Suppressor Cells (MDSCs)
Under normal conditions, myeloid cells are critical innate immune cells, involved in activating adaptive immunity in response to pathogen-associated molecular signals, which trigger the differentiation of myeloid progenitors to mature monocytes and granulocytes to protect the host [83]. In the tumour microenvironment, tumour-associated factors such as VEGF, TGFβ, IL-6, and PD-1 impair myeloid cell functions, resulting in inhibition of the anti-tumour immune response, which further promotes tumour growth [84,85]. Downregulation of granulocyte macrophage progenitor differentiation increases PD-1 expression on myeloid cells, which suppress CD8+ T-cell activity and promote tumour growth [58]. This effect can be reversed by specifically targeting PD-1 on myeloid cells [58]. Although the inhibition of PD-1 restores CD8+ T-cell activity and enhances anti-tumour immune regulation, drug resistance has been found in advanced melanoma patients [26,86]. Multiple pathways drive resistance to PD-1 blockade by promoting MDSCs infiltration into the tumour microenvironment [25,26,65], which is one of the major causes for the inhibition of the anti-tumour immune response [26,85,87]. Tumour-derived exosomes released by various tumour cells induce the expansion and immunosuppression of MDSCs through exosomal proteins, such as PGE2, TGFβ, Hsp72, IL-10, or IL-16 [88,89]. Moreover, high MDSC abundance is correlated with poor treatment responses to the inhibition of immune checkpoint therapy in patients with advanced melanoma [86,90]. MDSCs are mainly divided into two sub-populations, monocytic-MDSCs (M-MDSCs) and polymorphonuclear-MDSCs (PMN-MDSCs) [91,92]. Both M-MDSCs [85] and PMN-MDSCs [26] are involved in immune-suppressive regulation in melanoma [87]. M-MDSCs are abundant in tumour tissues, suppressing both an antigen-specific and non-specific T-cell response, mainly relying on arginase 1 (Arg1), nitric oxide (NO), and immunosuppressive cytokines. PMN-MDSCs predominately locate to the peripheral lymphoid organs and activate antigen-specific suppression, and their function depends largely on reactive oxygen species (ROS) and peroxynitrite (PNT) [93]. M-MDSCs promote immune escape by the inhibition of CD8+ T-cell infiltration into tumour tissues, depending on CCR2-mediated signalling [94] or suppression of CD8+ T-cell proliferation, including using CCR5-related pathway signalling [95]. M-MDSCs have more potent suppressive effects on antigen-specific CD8+ T-cell proliferation than PMN-MDSCs, but PMN-MDSCs are the major immunosuppressor in cancer patients [96,97]. PMN-MDSCs accumulate in tumour tissues and represent a key adaptive resistance pathway to PD-1 blockade after CD8+ T-cell activation in CXCR2/CXCL5-dependent manners [26]. Several inflammatory cytokines, including CCL2, CCL5, and CXCL5, have been found to promote MDSCs infiltration, and correlate with a poor treatment outcome [26,98,99,100]. Furthermore, high CXCL cytokine family expression is also found in patients with different cancer types [101,102]. In a BRAFV600E/PTEN−/− melanoma model, the inhibition of PD-1 increases CD8+ T-cell activity and IFN-γ secretion, which, in turn, increases Wnt5a expression in melanoma cells. Activation of Wnt5a triggers CXCL5 secretion through a YAP-dependent pathway, subsequently promoting PMN-MDSCs infiltration [26]. However, anti-PD-1 therapy using pembrolizumab decreases the level of PMN-MDSC (CD11b+CD33+HLA-DRlo/−CD15+) in melanoma patient PBMCs [103]. In melanoma-bearing mice, blocking CCR5/CCL5 interaction by mCCR5-Ig [104] reduces the migration and immunosuppressive potential of PMN-MDSCs (CD11b+Ly6G+Ly6Clo), resulting in increased survival [95]. IL-33 also has a role in the anti-tumour response to melanoma by not only increasing the secretion of INFγ and granzyme B from CD8+ T and NK cells as well as proliferation of CD4+ T cells [105,106] but also reducing the differentiation of PMN-MDSCs from bone marrow cells [106]. The capacity of MDSCs to induce Tregs is in turn inhibited by IL-33 treatment [106].
3.4. Cytotoxic T Cells (CD8+ T Cells)
When naïve CD8+ T cells recognise antigens on antigen-presenting cells (APCs), such as dendritic cells or macrophages in secondary lymphoid organs, they are activated and then differentiate into cytotoxic effector CD8+ T cells to control the antigens or pathogens [107]. Once antigens or pathogens are cleared, cytotoxic effector T cells undergo either of two fates: either apoptosis or differentiation into central memory T cells (TCMs) and effector memory T cells (TEMs) for a rapid response against subsequent infection [107]. TEMs, which migrate between blood and peripheral organs, have more immediate effects on immune surveillance than TCMs, but are not sustained longer term. In contrast, TCMs sustain a more durable response by high rates of proliferation through IL-2 production in secondary lymphoid organs [108,109].
Upon tumour initiation, tumour antigens trigger cytotoxic CD8+ T-cell activation, which eliminates tumour cells by two main pathways: death ligands and granule exocytosis. Various death ligands, such as tumour necrosis factor (TNF)-α, Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL), are expressed on the membrane of CD8+ T cells or secreted from these cells and induce tumour cell apoptosis [110]. Granzymes (Gzms) are released from activated cytotoxic CD8+ T cells together with perforin, which induces pore formation in the plasma membrane of target cells to deliver Gzms into them, including into tumour cells [111,112]. Granzyme B (GzmB), the most potent pro-apoptotic serine protease, is commonly found in the granules of cytotoxic CD8+ T cells [113]. Anti-tumour responses are enhanced by IFN-γ secreted from activated CD8+ T cells through an increase in the expression of MHC class I antigens in tumour cells, thereby making these cancer cells a better target for CD8+ T cells [114]. In tumour tissues, the abundance of GzmB, perforin, TNF-α, and IFN-γ is significantly decreased in CD4+, CD8+ T cells, and NK cells compared to non-tumour tissues [115].
The tumour environment is characterised by a persistent antigen stimulation as well as chronic inflammatory conditions, which result in T cells progressing into a state of “dysfunction” or “exhaustion” [69,70]. Dysfunctional (also commonly named as exhausted) CD8+ T cells lose part of their function to kill tumour cells and appear in large amounts in tumour tissues alongside cytotoxic CD8+ T cells [69,71]. Especially in melanoma, dysfunctional CD8+ T-cell populations occur in high abundances of 5–80% of total tumour-infiltrating lymphocytes (TILs) [71]. Dysfunctional CD8+ TILs are not connected to effector cytotoxic CD8+ T cells, as minimal overlapping transcripts and TCR repertoires between cytotoxic and dysfunctional CD8+ T-cell pools exist [116]. Dysfunctional CD8+ T cells are characterised by expressing different inhibitory molecules, such as LAG3, PDCD1 (encoding PD-1), CXCL13, HAVCR2 (encoding Tim3), ENTPD1 (encoding CD39), CTLA4, and TIGIT, which are all less expressed in memory-like T cells in melanoma [71,72]. However, dysfunctional CD8+ T cells still have the capacity of tumour antigen recognition, discovered in autologous single-cell melanoma digests [70]. This cell compartment highly expresses CD39 (ENTPD1) and CD103 (ITGAE), whereas cytotoxic effector KLRG1 is absent [70]. CD39/CD103-double-positive CD8+ TILs are found in high abundances in cancer tissues isolated from patients with melanoma, head and neck squamous cell carcinoma, lung, ovarian, and rectal cancer. Both CD39 and CD103 are upregulated by TGF-β and TCR stimulation, suggesting that CD39+/CD103+ CD8+ TILs are enriched in the tumour microenvironment [117]. PD-1 is highly co-expressed with CD103, one of the tissue-resident markers on intra-epithelial CD8+ TIL in human ovarian cancer and non-small-cell lung cancer [118,119]. PD-1 expression relies on the persistence of antigen exposure to CD8+ T cells by its TCR stimulation [117,118]. PD-1 negatively regulates the activation of the co-stimulatory molecule CD28 by dephosphorylation in T cells after interaction with PD-L1 [120], and PD-1+Tim3+CD8+ TILs lose CD28 expression [121]. The blockade of the immune checkpoint PD-1 by anti-PD-1/PD-L1 induces the clinical benefit of tumour patients [122,123]. Anti-PD-1 treatment increases the transfer of Tcf1+Tim3- progenitor exhausted (dysfunctional) CD8+ T cells into the B16 melanoma tumour, but not of their Tcf1-Tim3+ terminally exhausted counter parts [124]. In addition, PD-1/PD-L1 blockade induces the selective expansion of PD-1IntCD44Hi exhausted CD8+ T cells in LCMV chronic infection [125]. Anti-PD-L1 therapy is sensitive to the proliferation of CD28-positive cells among CD8+ T cells in lung cancer [121].
TIGIT, which is one of the CD155 receptors, has been found to be highly expressed in exhausted T cells [126]. After binding to CD155, TIGIT downregulates DNAM-1 expression, which prevents cytotoxic T cells from transforming into exhausted T cells, to promote cytotoxic T-cell dysfunction and suppress cytotoxic T-cell proliferation (Figure 2). TIGIT+CD8+ T cells are highly expressed in PBMCs from patients with cervical cancer [126], but no differences have been found in PBMCs from melanoma patients compared with healthy donors [127]. However, TIGIT-positive populations are high among NY-ESO-1 (TA; tumour antigen)-specific CD8+ T cells from PMBCs [127]. In addition, TIGIT expression is higher in CD8+ TILs than circulating CD8+ T cells in PBMCs in melanoma patients [127]. TIGIT downregulates the expression of TNF-α and IFN-γ in CD8+ T cells [126,127]. Especially, high numbers of infiltrating TIGIT+CD8+ T cells into the tumour diminish the anti-tumour immunity, associated with a low expression of TNF-α, IFN-γ, and GzmB [128].
3.5. Natural Killer Cells (NK Cells)
Natural killer cells (NK cells) are innate lymphoid cells that can directly kill pathogens or target cells without requiring triggers by other innate immune cells [129]. The appearance of CD56 on human NK cell surfaces indicates the differentiation from immature NK (iNK) cells to mature NK (mNK) cells [130]. CD56-negative iNK cells mostly differentiate into CD56dim-mNK cells that are converted through CD56bright-mNK cells, or directly [130,131,132,133]. The maturation of NK cells is also tightly associated with CD16 expression [131,133,134]. CD56bright mNK cells, which have a high inflammatory cytokine secretion capacity, are mainly found in lymphoid tissues [133,135,136]. CD56dimCD16+-mNK cells, which are mostly found in peripheral blood, have higher cytolytic activity [131,135,137]. Similar subsets of NK cells were found in mice, with immature NK cells being associated with CD27+CD11b− phenotypes and terminally mature NK cells with CD27−CD11b+KLRG1+ [130,134]. The functionally activated CD107a+ NK cells use perforin and GzmB as granule-mediated cytotoxicity against target tumour cells as an early stage of effect, while NK cells subsequently switch to CD95L death-receptor-mediated cytotoxicity as a final stage of cell killing [66]. CD107a protects against the perforin-mediated self-destruction in NK cells by reducing perforin binding during target cell lysis [138]. The expression of CD107a is higher on CD56dimCD16−NK cells than CD56dimCD16bright-NK cells [139], supporting that CD107a is supposed to decrease alongside CD16 in CD56dim-NK cells after targeting tumour cells. Upon pathogen infection or tumour formation, NK cells migrate to these sides and release cytokines to enhance the functional killing effect against target cells [140]. Cytokines, such as IL-15 and IL-18, have been shown to be essential for NK cell maturation and function [140]. IL-15 promotes NK cell maturation and enhances NK cell cytokine production [67,141]. IL-18 facilitate NK cell cytokine production and cytolysis [142,143]. Moreover, IL-15 and IL-18 have been found to promote NK cell activation and their undergoing of memory-like differentiation in melanoma [67]. IL-12 enhances the proliferation of NK cells because this cytokine binds to the α chain of the IL-2 receptor and induces CD25 [140]. IL-12 also promotes IFN-γ production and IL-2, IL-15, and IL-18 signalling in a positive loop with each IL receptor (i.e., IL-2R, IL-12R, IL-15R, and IL-18R) in NK cells [144]. Memory-like NK cells increase IFN-γ production to kill melanoma cells in an NKG2D- and NKp46-dependent manner [67].
Within the immune cell population, there are only small amounts of NK cells infiltrating into the tumour microenvironment [145]. Despite the low abundance, NK cell infiltration has been shown to significantly affect cancer prognosis [146,147]. Protein signatures of tumour-infiltrating NK cells are also quite different from those of circulating blood NK cells in melanoma patients [147,148]. Some cytokines, including XCL1 and XCL2, were found to be highly expressed specifically in tumour-infiltrating NK cells [148]. NK cells secrete cytokines, such as XCL1 [149], CCL5 [149] and FLT3LG [149], to stimulate dendritic cell (DC) infiltration into the tumour microenvironment and interact with cytotoxic CD8+ T cells to improve patient responses to immunotherapy [149,150,151]. Thus, the infiltration of DCs and NK cells is associated with the positive response to immunotherapy in melanoma patients [150]. In addition, NK-cell-based immunotherapy is a promising approach for treating advanced melanoma patients who are resistant to T-cell-based immunotherapy because NK cells target the HLA class-I-reduced tumour [67].
The expression of receptors on NK cells also determines its function and activity [67,152]. When activating receptors, such as DNAM-1, NKG2D (Rae1δ in the murine system), and NKp46, bind to their ligands CD155 and MICA/B on cancer cells, it increases NK cell cytokine production, and further promotes cancer cell apoptosis [67,68]. It has also been shown that cancer cells shed MIC, which, in turn, results in the binding of NKG2D of NK cells to the soluble MIC, causing the degradation of NKG2D and overall decreasing NK cell activity [153]. Moreover, cancer cells tend to inhibit activating receptor expression on NK cells and upregulate inhibitory receptor expression, such as TIGIT, on NK cells [67]. CD155 has been shown to be highly expressed in melanoma cells [154], and the overexpression of CD155 in cancer cells induces DNAM-1 internalised in NK cells, resulting in reduced NK cell activity [155] (Figure 2).
4. Processes of Resistance to Immunotherapy Responses
Unlike in targeted therapies, it is more challenging to predict treatment responses and outcomes of immune therapies solely based on several markers, such as PD-L1, CTLA-4, T-cell abundances, or phenotypes. A better understanding of the mechanisms underlying melanoma responses to immunotherapy would greatly benefit the specific therapy selection for a patient and improve overall treatment outcomes. Biomarkers, which are associated with immune responses and/or cancer cell malignant features, may be used to predict treatment outcome [156].
4.1. Tertiary Lymphoid Structures (TLSs)
Tertiary lymphoid structures (TLSs), which are formed by CD20+ B cells, CD4+ T cells, CD8+ T cells, and DCs, are lymphoid-like structures found in non-lymphoid tissue under pathological conditions, such as chronic inflammation and cancer [157,158]. TLS formation increases antigen presentation and cytokine-regulating signalling, which are associated with improvement of prognosis in cancer patients [159]. Moreover, TLSs promote immunotherapy response in melanoma [158,160,161], with high TLS formation found in those individuals that respond to immunotherapy compared to refractory patients [161]. Moreover, intratumourally injected low-dose STING in murine melanoma B16F10 tumour-bearing mice upregulates infiltrating CD8+ T cells and DCs that associate with TLS formation, resulting in delayed tumour growth [162]. Thus, it has been suggested that the induction of TLS formation can be a therapeutic target for improving the response to immunotherapy [157,162].
4.2. Therapy-Resistant Cancer Cells
In the tumour microenvironment, cancer cells tend to express and secrete proteins that trigger different mechanisms to suppress immune responses. One example is the migratory inhibition factor (MIF), which correlates with poor prognosis [163], and its receptor, CD74, found expressed on tumour-associated macrophages, DCs and MDSC [164]. MIF-CD74 signalling suppresses CD8+ T-cell infiltration and promotes tumour growth, which can be overcome by the blockade of MIF-CD74 signalling [164,165]. In addition, MIF also induces HIF-1α and PD-L1 expression on melanoma cells, thereby inducing further mechanisms of resistance to immunotherapies [165].
Activin-A, part of the TGF-β signalling pathway, is found to be highly expressed in tumour-associated macrophages and cancer cells in melanoma patients with metastatic disease [166]. Melanoma-derived activin-A suppresses CXCL9-CXCR3 signalling, which in turn inhibits CD8+ T-cell proliferation and infiltration, overall promoting tumour growth [167]. As a consequence, melanoma patients with high activin-A expression commonly fail to respond to immunotherapies [167].
4.3. Cytoskeleton Remodelling
A large proportion of mutations found in melanoma involve the MAPK pathway, which can promote cytoskeleton contraction and induce therapy-resistant cells [168,169]. MAPK pathway reactivation after BRAF inhibition or anti-PD-1 therapies is commonly found in therapy-resistant cancer cells [169,170]. Re-activation of MAPK signalling induces downstream events, such as Rho-associated protein kinase (ROCK) non-muscle myosin II (NMII) [171], which contributes to cytoskeleton remodelling and promotes the proliferation of the therapy-resistant cells [170]. Within the resistant tumour microenvironment, ROCK-NMII increases the abundance of CD206+ macrophages and FOXP3+ Tregs, resulting in immunosuppression [170]. Moreover, the EMT marker ZEB1 is also found to be highly expressed in the melanoma cancer cells and is associated with resistance to MAPK inhibitors [172] and immunotherapy [173], in which the latter is associated with a lower secretion of IFN-γ and TNF-α and induction of TGF-β2 secretion [173].
4.4. Immune Responses
Typically, melanoma patients with higher TILs, especially CD8+ T cells, generally have a better response to immunotherapies [174,175]. However, CD8+ T cells with higher exhausted T-cell marker abundance, such as TIGIT, are more likely to fail immunotherapies, despite high overall CD8+ T abundance; however, therapeutic efficacy can be improved by combining immunotherapies with TIGIT inhibitor treatments [29]. High expression of IFN-γ was found in patients who respond to immunotherapy [176]. IFN-γ upregulates MHC-I and MHC-II expression on cancer cells, which facilitates cancer cell recognition by immune cells and a subsequent induction of cytotoxic responses in the cancer cells [176,177]. Thus, the loss of MHC-I and MHC-II on melanoma cells prevents the triggering of an immune response, causing the cells to escape immune surveillance. MHC-II-positive cancer cells in melanoma patients correlate to positive anti-PD-1/PD-L1 therapeutic responses [177]. On the other hand, the expression of tumour-specific MHC-I is required for responding to anti-CTLA4 therapy [176]. Other immune cell factors, such as B-cell markers, are also important for the response to immunotherapies. Compared to non-responders in melanoma patients, B-cell-associated gene expression, such as MZB1 and IGLL5, is significantly higher in therapy responders [161]. B cells have been shown to promote T-cell functions in the cancer microenvironment [161].
5. The Role of CD155 in the Melanoma Microenvironment and Its Potential as Immunotherapy Target
CD155 is highly expressed on melanoma cells [28] and plays a unique and emerging role in immunotherapy resistance [29]. As such, CD155 has been proposed as a potential target for immune therapy [33]; thus, understanding the role of CD155 in cancer immunoregulation might be beneficial and improve immunotherapy outcomes (Figure 3).
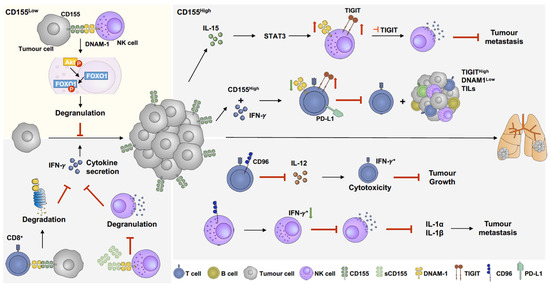
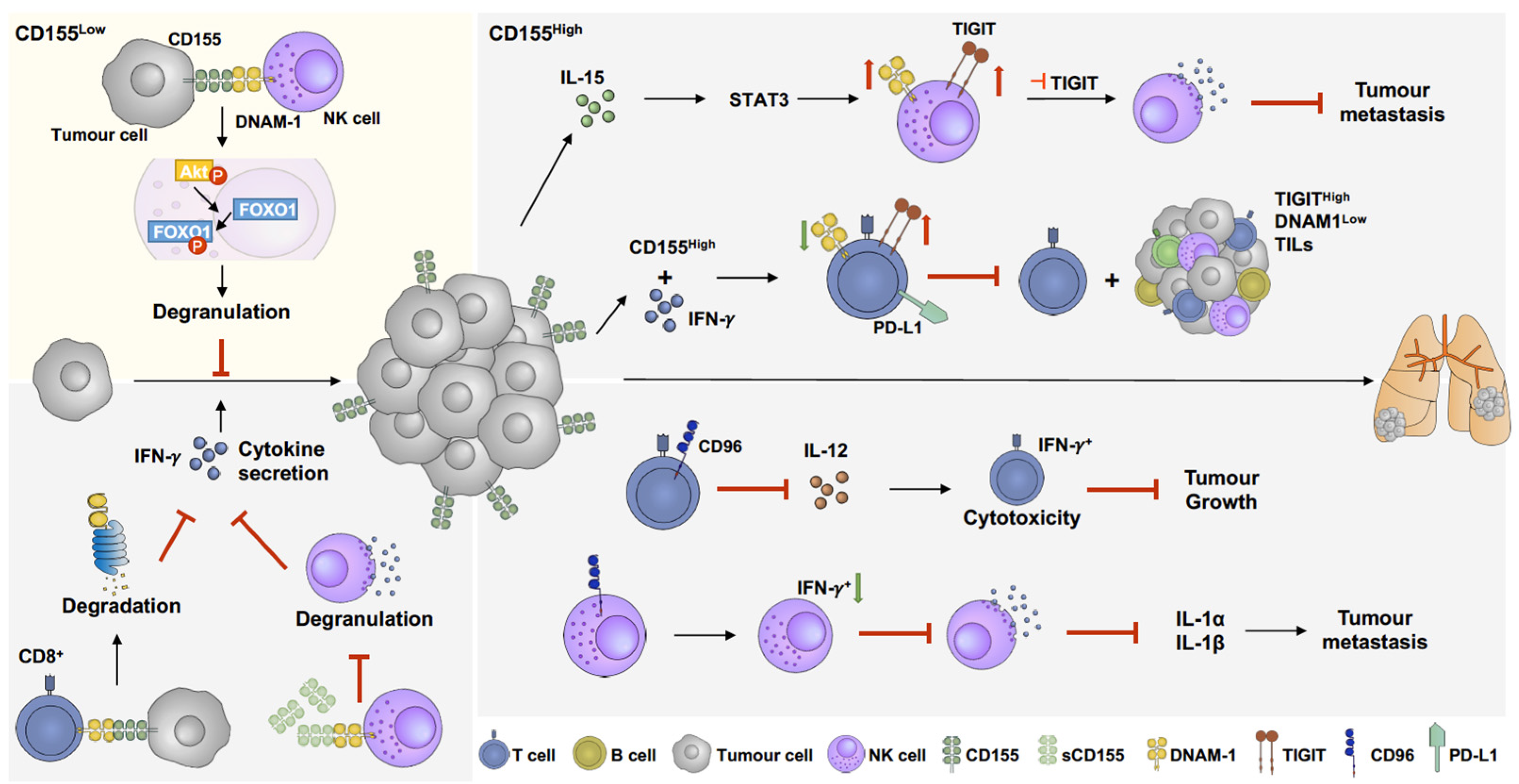
Figure 3.
The role of CD155 in melanoma immunoregulation. CD155 manipulates immune response by activating or suppressing immune responses. When CD155 level slightly increases, the interaction between CD155 and DNAM-1 facilitates immune response by activating pAkt-FOXO1 pathway, results in NK cell degranulation and further suppression of tumour growth [178] (yellow panel). Overexpression of CD155 is commonly found in melanoma, which suppresses activation of CD8 T cells and NK cells. Overexpression of CD155 induces DNAM-1 degradation in CD8 T cells [32], which downregulates cytokine secretion and promotes tumour growth. In addition, soluble CD155 (sCD155), which has higher binding affinity to DNAM-1, inhibits NK cell degranulation and cytokine secretion [179,180]. IL-15 enhances both TIGIT and DNAM-1 expression on NK cells through STAT3 pathways. Combination of IL-15 and TIGIT inhibition facilitates NK cell degranulation and further suppresses tumour metastasis [180]. Furthermore, IFN-γ upregulates CD155 expression and PD-L1+/TIGIT+ CD8 T-cell populations, which suppresses DNAM-1 activation on CD8 T cells and further inhibits CD8 T-cell cytotoxicity and increases TIGIThighDNAM-1low tumour-infiltrating lymphocytes (TILs) [154]. CD96, another immune-suppressing receptor, abolishes IL-12-induced CD8 T-cell activation and further suppresses cytokine secretion and promotes tumour growth [181]. Moreover, CD96 decreases IFN+ NK cell populations, inhibiting NK cell degranulation and downregulating IL-1α and IL-1β expression, resulting in metastasis promotion [182].
Two CD155 isoforms have been identified: the membrane-bound version of CD155 (mCD155) and soluble CD155 (sCD155) [183]. sCD155 is encoded by the alternative splicing isoforms CD155β and CD155γ [183], whereas mCD155 is encoded by CD155α and CD155δ [179]. Unlike mCD155, sCD155 lacks a transmembrane domain [179], which could result in the triggering of different functional pathways. CD155 is the ligand of three immunoglobulin-superfamily receptors: DNAM-1, TIGIT, and CD96 [27]. The main structural difference of the three receptors is the number of extracellular immunoglobulin-like domains. CD96 [184], DNAM-1 [185], and TIGIT [186] have three, two, and one extracellular immunoglobulin-like domains, respectively. Between these receptors, TIGIT has the highest binding affinity to CD155; thus, TIGIT has a dominant role in immune regulation [187,188,189]. Furthermore, these receptors are mainly expressed by NK cells, CD4+ T cells, and CD8+ T cells [190,191], and trigger both activating and inhibitory immune responses [27]. Briefly, when CD155 binds to TIGIT on cytokine-induced killer cells, which are a heterogeneous population of CD3+CD56+ NK T cells [192], it inhibits the production and secretion of cytokines, thereby suppressing cell-mediated immune responses [193]. On the contrary, when CD155 binds to DNAM-1 on NK cells, phosphorylation of the DNAM-1 intracellular domain induces LFA-1-dependent intracellular signalling, which enhances the cytokine secretion and cytotoxicity of NK cells [194]. During cancer development, immune cells lose the capacity to eliminate cancer cells, thereby contributing to cancer progression [195]. Under physiological conditions, the expression of CD155 is low, but significantly increases in cancer [154,196,197,198], which makes it a relevant possible cause for resistance to immunotherapy [29,30,31,32]. Thus, understanding immune regulation mechanisms triggered by CD155 in melanoma will provide a rational for its specific targeting to improve anti-cancer immunotherapies.
5.1. DNAM-1(CD226)
DNAM-1 is the only CD155 receptor that induces NK and T-cell cytotoxic activity. Upon CD155 binding to DNAM-1 on NK cells, pAkt promotes FOXO1 phosphorylation and its cytosolic translocation, resulting in NK cell activation [178]. FOXO1, a negative regulator of NK cell activation, undergoes degradation after phosphorylation [199]. The percentages of tumour-infiltrating immune cells, including CD4+ T cells, CD8+ T cells, and NK cells, are significantly reduced in DNAM-1−/− mice [178], suggesting that DNAM-1 plays an essential role as activating receptor for immunosurveillance in the tumour microenvironment [200]. Furthermore, DNAM-1/CD155 interactions also promote the IL-2, IL-12, and IL-21 cytokine-induced suppression of lung metastasis in melanoma [191]. However, this metastasis-suppressive effect by inhibiting DNAM-1 only happens within three days of melanoma injections into mice, whereas, later, this inhibition is not observed, suggesting the DNAM-1-mediated NK cell activation to only be effective during early cancer growth stages [191]. In the tumour microenvironment, overexpression of CD155 suppresses DNAM-1 on NK cells and CD8+ T cells, resulting in NK and CD8+ T-cell inactivation, resulting in uninhibited cancer cell proliferation [32,179,180,197]. It has also been shown that CD155 promotes CBL-B binding to DNAM-1, which further facilitates DNAM-1 degradation in CD8+ T cells [32]. In melanoma patients, a low DNAM-1 CD8+ T-cell abundance is associated with a poor response to immunotherapy treatment [32]. Moreover, the abundance of sCD155 in the serum is also increased in cancer patients [201]. Given that sCD155 has a higher binding affinity to DNAM-1 compared to TIGIT and CD96 [179,202], sCD155 might have systemic effects, including regulation of immune responses toward circulating cancer cells or micrometastatic deposits. sCD155 is highly capable of suppressing DNAM-1 on NK cells, leading to the inhibition of degranulation and cytokine secretion, further enhancing tumour growth and metastasis [179,180]. Furthermore, there is a significant increase in DNAM-1 abundance on CD4+ T cells, CD8+ T cells, and NK cells in CD155−/− mice compared to WT mice [179]. Overall, these data suggest that CD155 suppresses anti-tumour immune responses by blocking DNAM-1 via various mechanisms on both NK and CD8+ T cells.
5.2. T-Cell Immunoreceptor with Ig and ITIM Domains (TIGIT)
TIGIT, a complementary costimulatory receptor of DNAM-1, is expressed by NK cells and TILs in melanoma [28,154], capable of triggering immune suppressive responses [189]. Upon binding to CD155, TIGIT inhibits T-cell proliferation and activation, including suppressing T-cell activation markers, CD69 and CD25 [27,188,203]. In melanoma, TILs have higher TIGIT and lower DNAM-1 expression compared to peripheral blood T cells [127,154]. TIGIT has been shown to inhibit DNAM-1 expression in cytotoxic T lymphocytes and NK cells to suppress their cytotoxic activity [180], which can be reversed by TIGIT blockade [28,154]. One mechanism used by TIGIT to downregulate DNAM-1 is inhibiting DNAM-1 homodimerization, which was demonstrated in colon cancer and breast cancer models [188] and provides a potential, testable DNAM-1 inhibition mechanism in melanoma. Additionally, PD-L1 is highly co-expressed with TIGIT in T cells [154]. Moreover, IFN-γ has been shown to upregulate CD155 expression and PD-L1+/TIGIT+ T-cell populations, subsequently resulting in T-cell dysfunction and TIL suppression [154]. Co-inhibition of PD-L1 and TIGIT recovers cytotoxic T-cell activity and the suppression of tumour growth [154]. However, this effect is abolished by the inhibition of DNAM-1 expression, which suggests that TIGIT suppresses cytotoxic T-cell activity through a DNAM-1-dependent mechanism [154,180]. In metastatic melanoma, both TIGIT and DNAM-1 expression are downregulated in NK cells. Furthermore, the inhibition of TIGIT failed to prevent NK cell dysfunction, potentially due to the low initial expression levels of DNAM-1 in NK cells [180]. Cytokines, such as IL-15, promote DNAM-1 and TIGIT expression in NK cells through the STAT3 pathway [180]. Thus, IL-15 induction combined with TIGIT inhibition promotes NK cell degranulation and impedes tumour metastasis [180]. Taken together, the data published so far demonstrate that CD155 induces an imbalance of TIGIT and DNAM-1 expression in the tumour microenvironment, further suppressing T-cell and NK cell activity, consequently promoting tumour growth and metastasis.
5.3. CD96 (TACTILE)
The expression of CD96 is upregulated in melanoma [204], and the protein is also found to be expressed by NK cells and CD8+ T cells [182,184], capable of further tuning the activity of these cells [181,182,205,206]. CD96 prevents IL-12-induced CD8+ T-cell-dependent anti-tumour effects, especially by downregulating cytokine secretion [181]. Upon inhibition of CD96, the population of IFN-γ+ T cells and their cytokine secretion significantly increases, resulting in tumour suppression [181]. However, this effect is not observed in IL-12−/− mice, suggesting that IL-12 is required for the CD96-mediated inhibition of anti-tumour immune responses [181]. Similar to TIGIT, CD96 has been shown to be co-expressed with PD-1 on CD8+ T cells [181]. Co-inhibition of PD-1 and CD96 improves CD8+ T-cell activity and reduces tumour growth [181]. CD96 has also been shown to directly suppress NK cell activation and limit cytokine secretion [182]. CD96 downregulates IFN-γ+ NK cells, which, in contrast, were found and recoverable in CD96-deficient mice [182], whereas IL-1β and IL-1α were upregulated in CD96−/− mice [182]. Together, the effect of the absence of CD96 is a strong suppression of melanoma lung metastasis [182]. Opposite to TIGIT, CD96 mainly limits NK and CD8+ T-cell cytokine secretion instead of their cytotoxicity [182]. Intriguingly, and unlike DNAM-1 and TIGIT, there is a rather limited number of reports on the role of CD96 in immunoregulation.
Recently, CD155 has been identified and described as a potential novel target for immunotherapy [207]. Supporting this idea, the absence of CD155 in cancer cells decreases cell proliferation [208], invasion [209], and migration [179]. Furthermore, the inhibition of PD-L1 and CTLA4 in CD155−/− mice resulted in strongly increased anti-tumour effects compared to those of PD-L1/CTLA4 blockade in WT mice [210]. Moreover, targeting CD155 by using a non-neurovirulent rhinovirus/poliovirus chimera (PVSRIPO) showed promising anti-tumour effects in a melanoma phase I clinical trial [211]. These observations highlight that the inhibition of CD155 might act additively or synergistically with the blockade of PD-L1 or CTLA4, which supports the idea that CD155 is a potential novel target for immunotherapy. So far, there are some clinical trial studies that target CD155 or its receptors in combination with current immune therapy targets (Table 1).

Table 1.
Clinical trials targeting CD155 and its receptors.
6. Overall Summary
Melanoma is a cancer type with high immunogenicity and our developing understanding of the immune regulation within the melanoma lesion will benefit the development of more effective treatments for patients. In this review, we discuss how the immune regulation involves different cell populations and how recent findings on the role of CD155 might contribute to the immune suppression in melanoma. One of the major challenges of immunotherapy is the difficulty of predicting treatment responses, especially in the case in which advanced patients failed other, previous therapies. Although a variety of contributing factors, such as TIL populations [174] and TIGIT expression on CD8+ T cells [154], can modulate immunotherapy outcomes, a comprehensive understanding of resistance pathways is required to overcome these limitations. Thus, future work will describe more detailed ideal combinations of existing and emerging immunotherapy targets required to achieve a sustained and complete response in melanoma patients with limited toxicities.
Author Contributions
L.-Y.W. and A.M. designed the manuscript; L.-Y.W., S.-H.P. and H.J. wrote the manuscript. L.-Y.W. designed the figures; L.-Y.W., S.-H.P., H.J., M.S. and A.M. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cancer Council Queensland, Australia (ACCR027), the Innovation and Technology Commission, Hong Kong SAR (PiH/048-050/22GS), the Global STEM scheme (GSP153), and the Hong Kong Jockey Club Charities Trust to A.M. The APC was funded by internal funding of the Department of Otorhinolaryngology, Chinese University of Hong Kong.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer. Adv. Exp. Med. Biol. 2017, 996, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Karimkhani, C.; Green, A.C.; Nijsten, T.; Weinstock, M.A.; Dellavalle, R.P.; Naghavi, M.; Fitzmaurice, C. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017, 177, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef] [PubMed]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef]
- O’Neill, C.H.; Scoggins, C.R. Melanoma. J. Surg. Oncol. 2019, 120, 873–881. [Google Scholar] [CrossRef]
- Andor, N.; Graham, T.A.; Jansen, M.; Xia, L.C.; Aktipis, C.A.; Petritsch, C.; Ji, H.P.; Maley, C.C. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat. Med. 2016, 22, 105–113. [Google Scholar] [CrossRef]
- Ng, M.F.; Simmons, J.L.; Boyle, G.M. Heterogeneity in Melanoma. Cancers 2022, 14, 3030. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef]
- Scolyer, R.A.; Long, G.V.; Thompson, J.F. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol. Oncol. 2011, 5, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Ghafouri-Fard, S. Melanoma: A prototype of cancer-testis antigen-expressing malignancies. Immunotherapy 2017, 9, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Braeuer, R.R.; Watson, I.R.; Wu, C.J.; Mobley, A.K.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Why is melanoma so metastatic? Pigment. Cell Melanoma Res. 2014, 27, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef]
- Liu, D.; Schilling, B.; Liu, D.; Sucker, A.; Livingstone, E.; Jerby-Arnon, L.; Zimmer, L.; Gutzmer, R.; Satzger, I.; Loquai, C.; et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 2019, 25, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Uhara, H.; Kiyohara, Y.; Uehara, J.; Fujisawa, Y.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Fukushima, S.; Minami, H.; Hatsumichi, M.; et al. Five-year survival with nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. J. Dermatol. 2021, 48, 592–599. [Google Scholar] [CrossRef]
- Neubert, N.J.; Schmittnaegel, M.; Bordry, N.; Nassiri, S.; Wald, N.; Martignier, C.; Tille, L.; Homicsko, K.; Damsky, W.; Maby-El Hajjami, H.; et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci. Transl. Med. 2018, 10, eaan3311. [Google Scholar] [CrossRef] [PubMed]
- Theivanthiran, B.; Evans, K.S.; DeVito, N.C.; Plebanek, M.; Sturdivant, M.; Wachsmuth, L.P.; Salama, A.K.; Kang, Y.; Hsu, D.; Balko, J.M.; et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J. Clin. Investig. 2020, 130, 2570–2586. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.R.; Readler, J.M.; Sharma, P.; Excoffon, K. Poliovirus Receptor: More than a simple viral receptor. Virus Res. 2017, 242, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hoogi, S.; Eisenberg, V.; Mayer, S.; Shamul, A.; Barliya, T.; Cohen, C.J. A TIGIT-based chimeric co-stimulatory switch receptor improves T-cell anti-tumor function. J. Immunother. Cancer 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Inozume, T.; Kawazu, M.; Ueno, T.; Nagasaki, J.; Tanji, E.; Honobe, A.; Ohnuma, T.; Kawamura, T.; Umeda, Y.; et al. TIGIT/CD155 axis mediates resistance to immunotherapy in patients with melanoma with the inflamed tumor microenvironment. J. Immunother. Cancer 2021, 9, e003134. [Google Scholar] [CrossRef]
- Lepletier, A.; Madore, J.; O’Donnell, J.S.; Johnston, R.L.; Li, X.Y.; McDonald, E.; Ahern, E.; Kuchel, A.; Eastgate, M.; Pearson, S.A.; et al. Tumor CD155 Expression Is Associated with Resistance to Anti-PD1 Immunotherapy in Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 3671–3681. [Google Scholar] [CrossRef]
- Ito, M.; Mimura, K.; Nakajima, S.; Saito, K.; Min, A.K.T.; Okayama, H.; Saito, M.; Momma, T.; Saze, Z.; Ohtsuka, M.; et al. Immune escape mechanism behind resistance to anti-PD-1 therapy in gastrointestinal tract metastasis in malignant melanoma patients with multiple metastases. Cancer Immunol. Immunother. 2022, 71, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Aguilera, A.R.; Sundarrajan, A.; Corvino, D.; Stannard, K.; Krumeich, S.; Das, I.; Lima, L.G.; Meza Guzman, L.G.; Li, K.; et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8(+) T Cells. Immunity 2020, 53, 805–823.e15. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, K.; Enk, A.H. TIGIT-CD155 Interactions in Melanoma: A Novel Co-Inhibitory Pathway with Potential for Clinical Intervention. J. Investig. Dermatol. 2016, 136, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Lindenmann, J.; Burke, D.C.; Isaacs, A. Studies on the production, mode of action and properties of interferon. Br. J. Exp. Pathol. 1957, 38, 551–562. [Google Scholar]
- Kirkwood, J.M.; Strawderman, M.H.; Ernstoff, M.S.; Smith, T.J.; Borden, E.C.; Blum, R.H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996, 14, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yannelli, J.R.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Weber, J.S.; Parkinson, D.R.; Seipp, C.A.; Einhorn, J.H.; White, D.E. Treatment of Patients with Metastatic Melanoma with Autologous Tumor-Infiltrating Lymphocytes and Interleukin 2. JNCI J. Natl. Cancer Inst. 1994, 86, 1159–1166. [Google Scholar] [CrossRef]
- Vesely, M.D.; Chen, L. Normalization Cancer Immunotherapy for Melanoma. J. Investig. Dermatol. 2020, 140, 1134–1142. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Overcoming immunologic tolerance to melanoma: Targeting CTLA-4 with ipilimumab (MDX-010). Oncologist 2008, 13 (Suppl. 4), 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Carlino, M.S.; McNeil, C.; Ribas, A.; Grob, J.-J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J. Clin. Oncol. 2023, 41, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Amaral, T.; Seeber, O.; Mersi, E.; Sanchez, S.; Thomas, I.; Meiwes, A.; Forschner, A.; Leiter, U.; Eigentler, T.; Keim, U.; et al. Primary Resistance to PD-1-Based Immunotherapy-A Study in 319 Patients with Stage IV Melanoma. Cancers 2020, 12, 1027. [Google Scholar] [CrossRef]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Arance, A.; Cruz-Merino, L.d.l.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Márquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma with Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2023, 41, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Zimmer, L.; Sickmann, T.; Eigentler, T.K.; Meier, F.; Mohr, P.; Pukrop, T.; Roesch, A.; Vordermark, D.; Wendl, C.; et al. Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition. Cancers 2023, 15, 3448. [Google Scholar] [CrossRef]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., 2nd; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lacina, L.; Kodet, O.; Dvorankova, B.; Szabo, P.; Smetana, K., Jr. Ecology of melanoma cell. Histol. Histopathol. 2018, 33, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Onoe, T.; Yoshida, T.; Yamashita, Y.; Tanaka, Y.; Ohdan, H. Tumor Endothelial Cell-Mediated Antigen-Specific T-cell Suppression via the PD-1/PD-L1 Pathway. Mol. Cancer Res. 2020, 18, 1427–1440. [Google Scholar] [CrossRef]
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Chang, Y.C.; Wu, J.W.; Wang, C.W.; Jang, A.C. Hippo Signaling-Mediated Mechanotransduction in Cell Movement and Cancer Metastasis. Front. Mol. Biosci. 2019, 6, 157. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, J.; Zhang, J.; Li, S.; Wang, H.; Du, J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer 2019, 145, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Haass, N.K.; Smalley, K.S.; Li, L.; Herlyn, M. Adhesion, migration and communication in melanocytes and melanoma. Pigment. Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Fleming, V.; Hu, X.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandstrom, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.D.; Krasnick, B.A.; Becker-Hapak, M.; Conant, L.; Goedegebuure, S.P.; Berrien-Elliott, M.M.; Robbins, K.J.; Foltz, J.A.; Foster, M.; Wong, P.; et al. Memory-like Differentiation Enhances NK Cell Responses to Melanoma. Clin. Cancer Res. 2021, 27, 4859–4869. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Miller, J.S. Promoting T and NK cell attack: Preserving tumor MICA/B by vaccines. Cell Res. 2022, 32, 961–962. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef]
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.J.; van den Braber, M.; Rozeman, E.A.; Haanen, J.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789.e18. [Google Scholar] [CrossRef]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hochheiser, K.; Gyorki, D.E.; Gebhardt, T. Tumor reactivity of CD8(+) T cells favors acquisition of dysfunctional states in human melanoma. Immunol. Cell Biol. 2021, 99, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Niewiarowska, J. Endothelial Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Annan, D.A.; Hida, Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 1272. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am. J. Pathol. 2003, 162, 1747–1757. [Google Scholar] [CrossRef]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Benedicto, A.; Hernandez-Unzueta, I.; Sanz, E.; Marquez, J. Ocoxin Increases the Antitumor Effect of BRAF Inhibition and Reduces Cancer Associated Fibroblast-Mediated Chemoresistance and Protumoral Activity in Metastatic Melanoma. Nutrients 2021, 13, 686. [Google Scholar] [CrossRef]
- Tsang, M.; Quesnel, K.; Vincent, K.; Hutchenreuther, J.; Postovit, L.M.; Leask, A. Insights into Fibroblast Plasticity: Cellular Communication Network 2 Is Required for Activation of Cancer-Associated Fibroblasts in a Murine Model of Melanoma. Am. J. Pathol. 2020, 190, 206–221. [Google Scholar] [CrossRef]
- Kawamoto, H.; Minato, N. Myeloid cells. Int. J. Biochem. Cell Biol. 2004, 36, 1374–1379. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Singh, L.; Muise, E.S.; Bhattacharya, A.; Grein, J.; Javaid, S.; Stivers, P.; Zhang, J.; Qu, Y.; Joyce-Shaikh, B.; Loboda, A.; et al. ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells. Mol. Cancer Res. 2021, 19, 702–716. [Google Scholar] [CrossRef]
- Weber, J.; Gibney, G.; Kudchadkar, R.; Yu, B.; Cheng, P.; Martinez, A.J.; Kroeger, J.; Richards, A.; McCormick, L.; Moberg, V.; et al. Phase I/II Study of Metastatic Melanoma Patients Treated with Nivolumab Who Had Progressed after Ipilimumab. Cancer Immunol. Res. 2016, 4, 345–353. [Google Scholar] [CrossRef]
- Douglass, S.M.; Fane, M.E.; Sanseviero, E.; Ecker, B.L.; Kugel, C.H., 3rd; Behera, R.; Kumar, V.; Tcyganov, E.N.; Yin, X.; Liu, Q.; et al. Myeloid-Derived Suppressor Cells Are a Major Source of Wnt5A in the Melanoma Microenvironment and Depend on Wnt5A for Full Suppressive Activity. Cancer Res. 2021, 81, 658–670. [Google Scholar] [CrossRef]
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019, 12, 84. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Kanterman, J.; Klieger, Y.; Ish-Shalom, E.; Olga, M.; Saragovi, A.; Shtainberg, H.; Lotem, M.; Baniyash, M. Clinical Significance of Circulating CD33+CD11b+HLA-DR- Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 5661–5672. [Google Scholar] [CrossRef]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Hohl, T.M.; Kitano, S.; Cortez, C.; Hirschhorn-Cymerman, D.; Avogadri, F.; Rizzuto, G.A.; Lazarus, J.J.; Pamer, E.G.; Houghton, A.N.; et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012, 72, 876–886. [Google Scholar] [CrossRef]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5(+) Myeloid-Derived Suppressor Cells Are Enriched and Activated in Melanoma Lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Ma, P.; Beatty, P.L.; McKolanis, J.; Brand, R.; Schoen, R.E.; Finn, O.J. Circulating Myeloid Derived Suppressor Cells (MDSC) That Accumulate in Premalignancy Share Phenotypic and Functional Characteristics with MDSC in Cancer. Front. Immunol. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Joseph, R.; Soundararajan, R.; Vasaikar, S.; Yang, F.; Allton, K.L.; Tian, L.; den Hollander, P.; Isgandarova, S.; Haemmerle, M.; Mino, B.; et al. CD8(+) T cells inhibit metastasis and CXCL4 regulates its function. Br. J. Cancer 2021, 125, 176–189. [Google Scholar] [CrossRef]
- Payne, A.S.; Cornelius, L.A. The role of chemokines in melanoma tumor growth and metastasis. J. Investig. Dermatol. 2002, 118, 915–922. [Google Scholar] [CrossRef]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.; Jin, R.; Huang, Z. The role of CXCL chemokine family in the development and progression of gastric cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 484–492. [Google Scholar] [PubMed]
- Paczek, S.; Lukaszewicz-Zajac, M.; Gryko, M.; Mroczko, P.; Kulczynska-Przybik, A.; Mroczko, B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2040. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Benner, B.; Savardekar, H.; Lapurga, G.; Good, L.; Abood, D.; Nagle, E.; Duggan, M.; Stiff, A.; DiVincenzo, M.J.; et al. Effect of Immune Checkpoint Blockade on Myeloid-Derived Suppressor Cell Populations in Patients with Melanoma. Front. Immunol. 2021, 12, 740890. [Google Scholar] [CrossRef]
- Sapir, Y.; Vitenshtein, A.; Barsheshet, Y.; Zohar, Y.; Wildbaum, G.; Karin, N. A fusion protein encoding the second extracellular domain of CCR5 arrests chemokine-induced cosignaling and effectively suppresses ongoing experimental autoimmune encephalomyelitis. J. Immunol. 2010, 185, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef]
- Lim, H.X.; Choi, S.; Cho, D.; Kim, T.S. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol. Cell Biol. 2017, 95, 99–107. [Google Scholar] [CrossRef]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.D.; Ely, K.H.; Woodland, D.L. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005, 202, 123–133. [Google Scholar] [CrossRef]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Lopez, J.A.; Susanto, O.; Jenkins, M.R.; Lukoyanova, N.; Sutton, V.R.; Law, R.H.; Johnston, A.; Bird, C.H.; Bird, P.I.; Whisstock, J.C.; et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013, 121, 2659–2668. [Google Scholar] [CrossRef]
- D’Angelo, M.E.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.A.; Sutton, V.R. Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519. [Google Scholar] [CrossRef]
- Tsukumo, S.I.; Yasutomo, K. Regulation of CD8(+) T Cells and Antitumor Immunity by Notch Signaling. Front. Immunol. 2018, 9, 101. [Google Scholar] [CrossRef]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-gamma by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef]
- Yost, K.E.; Satpathy, A.T.; Wells, D.K.; Qi, Y.; Wang, C.; Kageyama, R.; McNamara, K.L.; Granja, J.M.; Sarin, K.Y.; Brown, R.A.; et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019, 25, 1251–1259. [Google Scholar] [CrossRef]
- Duhen, T.; Duhen, R.; Montler, R.; Moses, J.; Moudgil, T.; de Miranda, N.F.; Goodall, C.P.; Blair, T.C.; Fox, B.A.; McDermott, J.E.; et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018, 9, 2724. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Nelson, B.H. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 926–935. [Google Scholar] [CrossRef]
- Djenidi, F.; Adam, J.; Goubar, A.; Durgeau, A.; Meurice, G.; de Montpreville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef]
- Pena-Asensio, J.; Calvo, H.; Torralba, M.; Miquel, J.; Sanz-de-Villalobos, E.; Larrubia, J.R. Anti-PD-1/PD-L1 Based Combination Immunotherapy to Boost Antigen-Specific CD8(+) T Cell Response in Hepatocellular Carcinoma. Cancers 2021, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Freeman, G.J.; Wherry, E.J. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl. Acad. Sci. USA 2008, 105, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, A.; Liu, X.; Han, S.; Sun, Y.; Zhang, J.; Guo, L.; Zhang, Y. Blocking TIGIT/CD155 signalling reverses CD8(+) T cell exhaustion and enhances the antitumor activity in cervical cancer. J. Transl. Med. 2022, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Q.; Wang, Z.; Zhang, H.; Zeng, H.; Huang, Q.; Chen, Y.; Jiang, W.; Lin, Z.; Qu, Y.; et al. Intratumoral TIGIT(+) CD8(+) T-cell infiltration determines poor prognosis and immune evasion in patients with muscle-invasive bladder cancer. J. Immunother. Cancer 2020, 8, e000978. [Google Scholar] [CrossRef] [PubMed]
- Ortaldo, J.R.; Oldham, R.K.; Cannon, G.C.; Herberman, R.B. Specificity of natural cytotoxic reactivity of normal human lymphocytes against a myeloid leukemia cell line. J. Natl. Cancer Inst. 1977, 59, 77–82. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Bi, J.; Wang, X. Molecular Regulation of NK Cell Maturation. Front. Immunol. 2020, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Crinier, A.; Milpied, P.; Escaliere, B.; Piperoglou, C.; Galluso, J.; Balsamo, A.; Spinelli, L.; Cervera-Marzal, I.; Ebbo, M.; Girard-Madoux, M.; et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 2018, 49, 971–986.e5. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Hintzen, G.; Kemper, A.; Beul, K.; Kempf, S.; Behrens, G.; Sykora, K.W.; Schmidt, R.E. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 2001, 31, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, N.K.; Riese, P.; Heuts, F.; Andersson, S.; Fauriat, C.; Ivarsson, M.A.; Bjorklund, A.T.; Flodstrom-Tullberg, M.; Michaelsson, J.; Rottenberg, M.E.; et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010, 116, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Cohnen, A.; Chiang, S.C.; Stojanovic, A.; Schmidt, H.; Claus, M.; Saftig, P.; Janssen, O.; Cerwenka, A.; Bryceson, Y.T.; Watzl, C. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 2013, 122, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Amand, M.; Iserentant, G.; Poli, A.; Sleiman, M.; Fievez, V.; Sanchez, I.P.; Sauvageot, N.; Michel, T.; Aouali, N.; Janji, B.; et al. Human CD56(dim)CD16(dim) Cells As an Individualized Natural Killer Cell Subset. Front. Immunol. 2017, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Mujal, A.M.; Delconte, R.B.; Sun, J.C. Natural Killer Cells: From Innate to Adaptive Features. Annu. Rev. Immunol. 2021, 39, 417–447. [Google Scholar] [CrossRef]
- Kamimura, Y.; Lanier, L.L. Homeostatic control of memory cell progenitors in the natural killer cell lineage. Cell Rep. 2015, 10, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tsutsui, H.; Yoshimoto, T.; Adachi, O.; Yoshida, N.; Kishimoto, T.; Okamura, H.; Nakanishi, K.; Akira, S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 1998, 8, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Chaix, J.; Tessmer, M.S.; Hoebe, K.; Fuseri, N.; Ryffel, B.; Dalod, M.; Alexopoulou, L.; Beutler, B.; Brossay, L.; Vivier, E.; et al. Cutting edge: Priming of NK cells by IL-18. J. Immunol. 2008, 181, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, H.; Diao, Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. Int. J. Mol. Sci. 2019, 20, 317. [Google Scholar] [CrossRef] [PubMed]
- Cozar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Sconocchia, G.; Eppenberger, S.; Spagnoli, G.C.; Tornillo, L.; Droeser, R.; Caratelli, S.; Ferrelli, F.; Coppola, A.; Arriga, R.; Lauro, D.; et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology 2014, 3, e952197. [Google Scholar] [CrossRef] [PubMed]
- Cursons, J.; Souza-Fonseca-Guimaraes, F.; Foroutan, M.; Anderson, A.; Hollande, F.; Hediyeh-Zadeh, S.; Behren, A.; Huntington, N.D.; Davis, M.J. A Gene Signature Predicting Natural Killer Cell Infiltration and Improved Survival in Melanoma Patients. Cancer Immunol. Res. 2019, 7, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.F.; Lu, Y.; Luoma, A.; Ito, Y.; Pan, D.; Pyrdol, J.W.; Yoon, C.H.; Yuan, G.C.; Wucherpfennig, K.W. Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 2019, 4, e133103. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Ji, S.; Lee, J.; Lee, E.S.; Kim, D.H.; Sin, J.I. B16 melanoma control by anti-PD-L1 requires CD8+ T cells and NK cells: Application of anti-PD-L1 Abs and Trp2 peptide vaccines. Hum. Vaccin. Immunother. 2021, 17, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Sung, H.M.; Ickes, C.; Gibhardt, C.S.; Vultur, A.; Bhat, H.; Hu, Z.; Brafford, P.; Denger, A.; Stejerean-Todoran, I.; et al. Protein Signatures of NK Cell-Mediated Melanoma Killing Predict Response to Immunotherapies. Cancer Res. 2021, 81, 5540–5554. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Inozume, T.; Yaguchi, T.; Furuta, J.; Harada, K.; Kawakami, Y.; Shimada, S. Melanoma Cells Control Antimelanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase. J. Investig. Dermatol. 2016, 136, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Suarez-Gosalvez, J.; Voigt, C.; Markota, A.; Giannou, A.D.; Schubel, M.; Jobst, J.; Zhang, T.; Dorr, J.; Markl, F.; et al. T cell-derived interleukin-22 drives the expression of CD155 by cancer cells to suppress NK cell function and promote metastasis. Immunity 2023, 56, 143–161.e11. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, J.; Xiao, Y.; Ming, J.; Zhou, J.; Dong, F.; Zhou, X.; Xu, Z.; Zhao, X.; Lei, P.; et al. CD20(+)CD22(+)ADAM28(+) B Cells in Tertiary Lymphoid Structures Promote Immunotherapy Response. Front. Immunol. 2022, 13, 865596. [Google Scholar] [CrossRef] [PubMed]
- Sautes-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chelvanambi, M.; Fecek, R.J.; Taylor, J.L.; Storkus, W.J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 2021, 9, e001906. [Google Scholar] [CrossRef]
- Ekmekcioglu, S.; Davies, M.A.; Tanese, K.; Roszik, J.; Shin-Sim, M.; Bassett, R.L., Jr.; Milton, D.R.; Woodman, S.E.; Prieto, V.G.; Gershenwald, J.E.; et al. Inflammatory Marker Testing Identifies CD74 Expression in Melanoma Tumor Cells, and Its Expression Associates with Favorable Survival for Stage III Melanoma. Clin. Cancer Res. 2016, 22, 3016–3024. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.R.; Azevedo, R.A.; Mousdell, S.; Resende-Lara, P.T.; Ireland, L.; Santos, A.; Girola, N.; Cunha, R.; Schmid, M.C.; Polonelli, L.; et al. Blockade of MIF-CD74 Signalling on Macrophages and Dendritic Cells Restores the Antitumour Immune Response Against Metastatic Melanoma. Front. Immunol. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, R.A.; Shoshan, E.; Whang, S.; Markel, G.; Jaiswal, A.R.; Liu, A.; Curran, M.A.; Travassos, L.R.; Bar-Eli, M. MIF inhibition as a strategy for overcoming resistance to immune checkpoint blockade therapy in melanoma. Oncoimmunology 2020, 9, 1846915. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Seijo, A.; Garcia-Martinez, E.; Barrio-Alonso, C.; Parra-Blanco, V.; Aviles-Izquierdo, J.A.; Sanchez-Mateos, P.; Samaniego, R. Activin A Sustains the Metastatic Phenotype of Tumor-Associated Macrophages and Is a Prognostic Marker in Human Cutaneous Melanoma. J. Investig. Dermatol. 2022, 142, 653–661.e2. [Google Scholar] [CrossRef] [PubMed]
- Pinjusic, K.; Dubey, O.A.; Egorova, O.; Nassiri, S.; Meylan, E.; Faget, J.; Constam, D.B. Activin-A impairs CD8 T cell-mediated immunity and immune checkpoint therapy response in melanoma. J. Immunother. Cancer 2022, 10, e004533. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.; Hong, H.; Lee, S.H.; Lee, J.K.; Jung, E.; Kim, J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016, 35, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Barreno, A.; Orgaz, J.L. Cytoskeletal Remodelling as an Achilles’ Heel for Therapy Resistance in Melanoma. Cells 2022, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Orgaz, J.L.; Crosas-Molist, E.; Sadok, A.; Perdrix-Rosell, A.; Maiques, O.; Rodriguez-Hernandez, I.; Monger, J.; Mele, S.; Georgouli, M.; Bridgeman, V.; et al. Myosin II Reactivation and Cytoskeletal Remodeling as a Hallmark and a Vulnerability in Melanoma Therapy Resistance. Cancer Cell 2020, 37, 85–103.e9. [Google Scholar] [CrossRef]
- Wu, L.Y.; Han, C.L.; Lin, H.H.; Tang, M.J. Ha-Ras(V12)-Induced Multilayer Cellular Aggregates Is Mediated by Rac1 Activation Rather Than YAP Activation. Biomedicines 2022, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Dalle, S.; Monet, M.A.; Ligier, M.; Boespflug, A.; Pommier, R.M.; de la Fouchardiere, A.; Perier-Muzet, M.; Depaepe, L.; Barnault, R.; et al. ZEB1-mediated melanoma cell plasticity enhances resistance to MAPK inhibitors. EMBO Mol. Med. 2016, 8, 1143–1161. [Google Scholar] [CrossRef]
- Plaschka, M.; Benboubker, V.; Grimont, M.; Berthet, J.; Tonon, L.; Lopez, J.; Le-Bouar, M.; Balme, B.; Tondeur, G.; de la Fouchardiere, A.; et al. ZEB1 transcription factor promotes immune escape in melanoma. J. Immunother. Cancer 2022, 10, e003484. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Schmidt, H.; Nissan, A.; Ridolfi, L.; Aamdal, S.; Hansson, J.; Guida, M.; Hyams, D.M.; Gomez, H.; Bastholt, L.; et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 2011, 9, 204. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; de Almeida, P.; Manieri, N.; de Almeida Nagata, D.; Wu, T.D.; Harden Bowles, K.; Arumugam, V.; Schartner, J.; Cubas, R.; Mittman, S.; et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1. Proc. Natl. Acad. Sci. USA 2018, 115, E11731–E11740. [Google Scholar] [CrossRef]
- Okumura, G.; Iguchi-Manaka, A.; Murata, R.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J. Exp. Med. 2020, 217, e20191290. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.Y.; Ferrone, S.; et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma. Clin. Cancer Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef]
- Mittal, D.; Lepletier, A.; Madore, J.; Aguilera, A.R.; Stannard, K.; Blake, S.J.; Whitehall, V.L.J.; Liu, C.; Bettington, M.L.; Takeda, K.; et al. CD96 Is an Immune Checkpoint That Regulates CD8(+) T-cell Antitumor Function. Cancer Immunol. Res. 2019, 7, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Baury, B.; Masson, D.; McDermott, B.M., Jr.; Jarry, A.; Blottiere, H.M.; Blanchardie, P.; Laboisse, C.L.; Lustenberger, P.; Racaniello, V.R.; Denis, M.G. Identification of secreted CD155 isoforms. Biochem. Biophys. Res. Commun. 2003, 309, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; O’Farrell, S.; Clayberger, C.; Krensky, A.M. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J. Immunol. 1992, 148, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Rovis, T.L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J.; Jonjic, S.; Mandelboim, O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 2013, 43, 2138–2150. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Fuhrman, C.A.; Yeh, W.I.; Seay, H.R.; Saikumar Lakshmi, P.; Chopra, G.; Zhang, L.; Perry, D.J.; McClymont, S.A.; Yadav, M.; Lopez, M.C.; et al. Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226. J. Immunol. 2015, 195, 145–155. [Google Scholar] [CrossRef]
- Chan, C.J.; Andrews, D.M.; McLaughlin, N.M.; Yagita, H.; Gilfillan, S.; Colonna, M.; Smyth, M.J. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J. Immunol. 2010, 184, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mi, Y.; Guo, N.; Xu, H.; Xu, L.; Gou, X.; Jin, W. Cytokine-Induced Killer Cells as Pharmacological Tools for Cancer Immunotherapy. Front. Immunol. 2017, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.F.; Smyth, M.J.; Martinet, L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol. Cell Biol. 2014, 92, 237–244. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M.G. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001, 49, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015, 35, 2287–2297. [Google Scholar]
- Deng, Y.; Kerdiles, Y.; Chu, J.; Yuan, S.; Wang, Y.; Chen, X.; Mao, H.; Zhang, L.; Zhang, J.; Hughes, T.; et al. Transcription factor Foxo1 is a negative regulator of natural killer cell maturation and function. Immunity 2015, 42, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanth, T.; Burke, S.; Ali, T.H.; Kimpfler, S.; Ursini, F.; Ruggeri, L.; Capanni, M.; Umansky, V.; Paschen, A.; Sucker, A.; et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J. Clin. Investig. 2009, 119, 1251–1263. [Google Scholar] [CrossRef]
- Iguchi-Manaka, A.; Okumura, G.; Kojima, H.; Cho, Y.; Hirochika, R.; Bando, H.; Sato, T.; Yoshikawa, H.; Hara, H.; Shibuya, A.; et al. Increased Soluble CD155 in the Serum of Cancer Patients. PLoS ONE 2016, 11, e0152982. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 2012, 188, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cai, X.; Liu, F. CD96 as a Potential Diagnostic Biomarker and New Target for Skin Cutaneous Melanoma. Contrast Media Mol. Imaging 2022, 2022, 6409376. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Madore, J.; Li, X.Y.; Smyth, M.J. Tumor intrinsic and extrinsic immune functions of CD155. Semin. Cancer Biol. 2020, 65, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.J.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.C.; Mittal, D.; Aguilera, A.R.; Miles, J.J.; Lutzky, V.P.; de Andrade, L.F.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Kucan Brlic, P.; Lenac Rovis, T.; Cinamon, G.; Tsukerman, P.; Mandelboim, O.; Jonjic, S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell. Mol. Immunol. 2019, 16, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Kakunaga, S.; Ikeda, W.; Shingai, T.; Fujito, T.; Yamada, A.; Minami, Y.; Imai, T.; Takai, Y. Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J. Biol. Chem. 2004, 279, 36419–36425. [Google Scholar] [CrossRef]
- Sloan, K.E.; Eustace, B.K.; Stewart, J.K.; Zehetmeier, C.; Torella, C.; Simeone, M.; Roy, J.E.; Unger, C.; Louis, D.N.; Ilag, L.L.; et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer 2004, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Das, I.; Lepletier, A.; Addala, V.; Bald, T.; Stannard, K.; Barkauskas, D.; Liu, J.; Aguilera, A.R.; Takeda, K.; et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Investig. 2018, 128, 2613–2625. [Google Scholar] [CrossRef]
- Beasley, G.M.; Nair, S.K.; Farrow, N.E.; Landa, K.; Selim, M.A.; Wiggs, C.A.; Jung, S.H.; Bigner, D.D.; True Kelly, A.; Gromeier, M.; et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 2021, 9, e002203. [Google Scholar] [CrossRef]
- Rasco, D.; Dumbrava, E.; Sharma, M.; Shepard, D.; Vaena, D.; Fleming, G.; Chmielowski, B.; Hamilton, E.; Sullivan, R.; Papadopoulos, K.; et al. 659 COM701 plus nivolumab demonstrates preliminary antitumor activity and immune modulation of tumor microenvironment in patients with metastatic MSS-CRC and liver metastases. J. Immunother. Cancer 2022, 10, A690. [Google Scholar] [CrossRef]
- Obeidat, A.; Atieh, A.; Vitenshtein, A.; Cinamon, G.; Paz, K.; Roviš, T.; Brilc, P.; Hirsl, L.; Mandelboim, O.; Jonjic, S.; et al. 474 First-in-class anti-PVR mAb NTX1088 restores expression of DNAM1 and augments antitumor immunity. J. Immunother. Cancer 2022, 10, A494. [Google Scholar] [CrossRef]
- Long, G.V.; Eggermont, A.M.; Gershenwald, J.E.; Schadendorf, D.; Ascierto, P.A.; Dummer, R.; Hauschild, A.; Carlino, M.S.; Ribas, A.; Robert, C.; et al. KEYVIBE-010: Adjuvant coformulated vibostolimab with pembrolizumab versus adjuvant pembrolizumab in patients with high-risk stage II-IV melanoma. J. Clin. Oncol. 2023, 41, TPS9611. [Google Scholar] [CrossRef]
- Dummer, R.; Long, G.V.; Pavlick, A.; Postow, M.; Ribas, A.; Robert, C.; Scolyer, R.A.; Taube, J.; Tetzlaff, M.; Liao, J.; et al. 426 MK-3475-U02: Phase 1/2 study of investigational agents with or without pembrolizumab versus pembrolizumab monotherapy in melanoma. J. Immunother. Cancer 2020, 8, A259. [Google Scholar] [CrossRef]
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.W.; Nagrial, A.; Voskoboynik, M.; Chung, H.C.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 2022, 33, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Rasco, D.W.; Vaena, D.A.; Fleming, G.F.; Dumbrava, E.E.; Yeku, O.O.; Sharma, M.; Papadopoulos, K.P.; Sullivan, R.J.; Gaillard, S.; Adewoye, A.H.; et al. Preliminary antitumor activity of the combination of COM701 + BMS-986207 + nivolumab in patients with recurrent, metastatic MSS endometrial cancer. J. Clin. Oncol. 2023, 41, 5595. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).