Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics
Abstract
Simple Summary
Abstract
1. Introduction
2. DNA Methylation and Cancer
3. Methylation Detection in Cell-Free DNA
3.1. Methods for Analyzing DNA Methylation Status
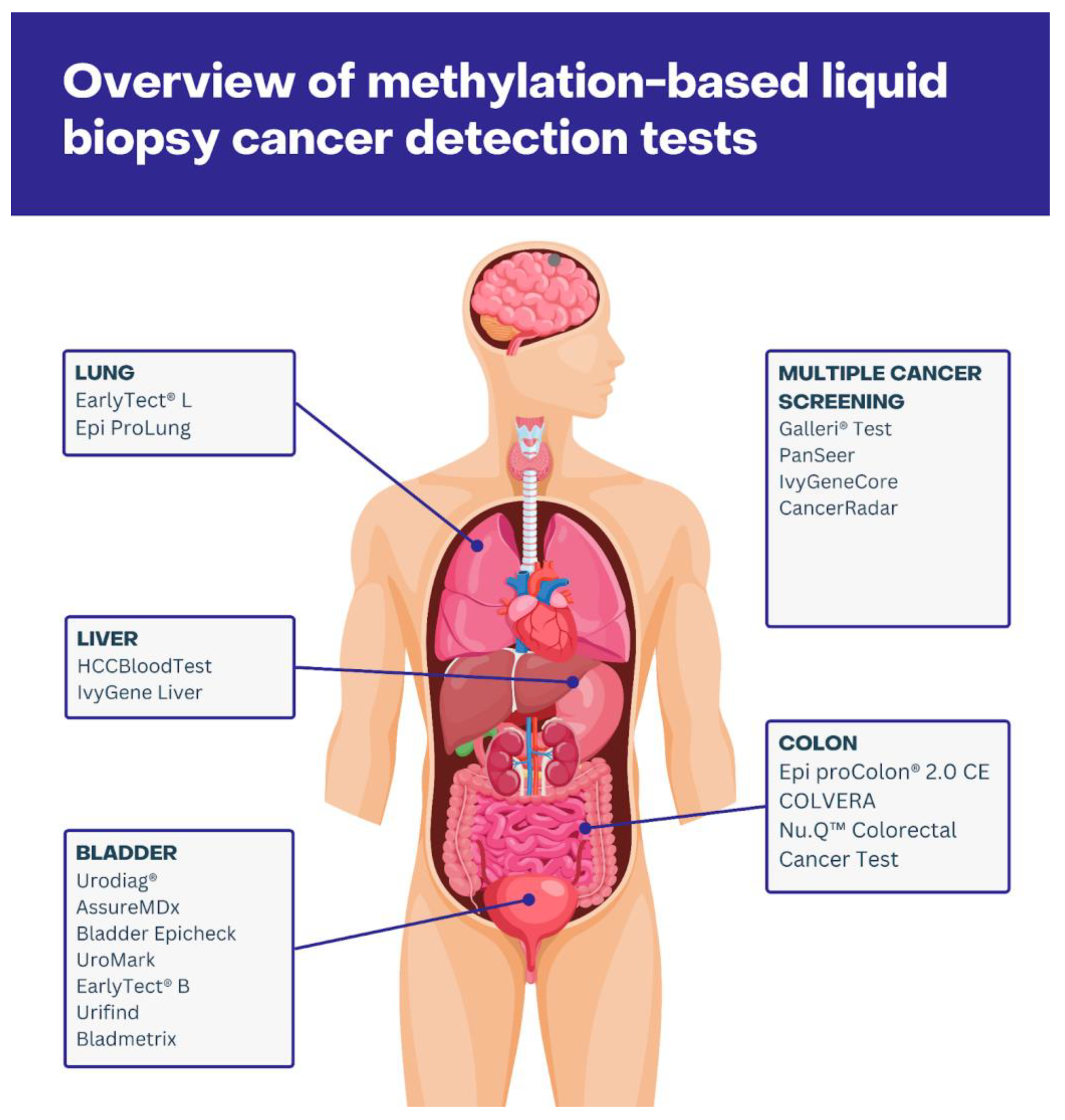
3.2. Clinically Approved Methylation-Based Cancer Screening
3.2.1. Single-Cancer Detection Tests
Colorectal Cancer
Lung Cancer
Bladder Cancer
Liver Cancer
3.2.2. Multi-Cancer Detection Tests
Galleri® Test
PanSeer
IvyGene
CancerRadar
| Test | Cancer Type | Specificity | Sensitivity | Sample Type | Ref. |
|---|---|---|---|---|---|
| Epi proColon® 2.0 CE | Colorectal Cancer | 79.1% | 68.2% | Blood (plasma) | [65] |
| COLVERA | Colorectal Cancer | 89.3% | 73.1% | Blood (plasma) | [69] |
| Nu.Q™ Colorectal Cancer Test | Colorectal Cancer | 70% | 75% | Blood (serum) | [71,72] |
| EarlyTect® L | Lung Cancer | 92.3% | 77.8% | Blood (serum) | [73,74] |
| Epi ProLung | Lung Cancer | 96% | 78% | Blood (plasma) | [75] |
| Urodiag® | Bladder Cancer | 75.9% | 94.5% | Urine | [77] |
| AssureMDx | Bladder Cancer | 83% | 97% | Urine | [78] |
| Bladder Epicheck | Bladder Cancer | 83% | 90% | Urine | [79] |
| UroMark | Bladder Cancer | 97% | 98% | Urine | [81] |
| EarlyTect® B | Bladder Cancer | N/A | N/A | Urine | [70,82] |
| Urifind | Bladder Cancer | 77.3% | 91.7% | Urine | [83] |
| Bladmetrix | Bladder Cancer | 93.3% | 92.1% | Urine | [84] |
| HCCBloodTest | Liver Cancer | 64.1% | 76.7% | Blood | [85] |
| IvyGene Liver | Liver Cancer | 86% | 80% | Blood | [86] |
| Galleri® Test | Multi-cancer Early Detection | N/A * | N/A * | Blood | [96] |
| PanSeer | Multi-cancer Early Detection | 96% | 88% | Blood | [71,100] |
| IvyGeneCORE | Breast, Colon, Liver, and Lung Cancer | 90% | 84% | Blood | [86] |
| CancerRadar | Multi-cancer Early Detection | 97.9% | 85.9% | Blood | [94] |
3.2.3. Methylation-Based Methods in Companion Diagnostics
4. Biological Factors Influencing DNA Methylation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.F.; Wielgosz, A.; et al. Variations in Common Diseases, Hospital Admissions, and Deaths in Middle-Aged Adults in 21 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef]
- Parkinson, D.R.; McCormack, R.T.; Keating, S.M.; Gutman, S.I.; Hamilton, S.R.; Mansfield, E.A.; Piper, M.A.; DeVerka, P.; Frueh, F.W.; Jessup, J.M.; et al. Evidence of Clinical Utility: An Unmet Need in Molecular Diagnostics for Patients with Cancer. Clin. Cancer Res. 2014, 20, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; Antoniou, A.C.; Fruk, L.; Rosenfeld, N. The Future of Early Cancer Detection. Nat. Med. 2022, 28, 666–677. [Google Scholar] [CrossRef]
- Clarke, C.A.; Hubbell, E.; Kurian, A.W.; Colditz, G.A.; Hartman, A.-R.; Gomez, S.L. Projected Reductions in Absolute Cancer-Related Deaths from Diagnosing Cancers Before Metastasis, 2006–2015. Cancer Epidemiol. Biomark. Prev. 2020, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, X.; Jin, P. Developing DNA Methylation-Based Diagnostic Biomarkers. J. Genet. Genom. 2018, 45, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in Cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Vogelstein, B. Hypomethylation Distinguishes Genes of Some Human Cancers from Their Normal Counterparts. Nature 1983, 301, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Reik, W.; Surani, A. Genomic Imprinting; IRL Press: Oxford, UK, 1997; ISBN 9780199636259. [Google Scholar]
- Xie, W.; Barr, C.L.; Kim, A.; Yue, F.; Lee, A.Y.; Eubanks, J.; Dempster, E.L.; Ren, B. Base-Resolution Analyses of Sequence and Parent-of-Origin Dependent DNA Methylation in the Mouse Genome. Cell 2012, 148, 816–831. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A Genome-Wide Analysis of CpG Dinucleotides in the Human Genome Distinguishes Two Distinct Classes of Promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- DNA Methylation and Cancer. Advances in Genetics; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 27–56. [Google Scholar]
- Insua, Y.V.; De la Cámara, J.; Vázquez, E.B.; Fernández, A.; Rivera, F.V.; Silva, M.J.V.; Barbazán, J.; Muinelo-Romay, L.; Folgar, S.C.; Abalo, A.; et al. Predicting Outcome and Therapy Response in mCRC Patients Using an Indirect Method for CTCs Detection by a Multigene Expression Panel: A Multicentric Prospective Validation Study. Int. J. Mol. Sci. 2017, 18, 1265. [Google Scholar] [CrossRef] [PubMed]
- García-Saenz, J.A.; Ayllón, P.; Laig, M.; Acosta-Eyzaguirre, D.; García-Esquinas, M.; Montes, M.; Sanz, J.; Barquín, M.; Moreno, F.; Garcia-Barberan, V.; et al. Tumor Burden Monitoring Using Cell-Free Tumor DNA Could Be Limited by Tumor Heterogeneity in Advanced Breast Cancer and Should Be Evaluated Together with Radiographic Imaging. BMC Cancer 2017, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Turner, S.T.; Smith, J.A.; Hammond, P.I.; Lazarus, A.; Van De Rostyne, J.L.; Cunningham, J.M.; Kardia, S.L.R. Comparison of the DNA Methylation Profiles of Human Peripheral Blood Cells and Transformed B-Lymphocytes. Hum. Genet. 2010, 127, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Reinius, L.E.; Acevedo, N.; Joerink, M.; Pershagen, G.; Dahlén, S.-E.; Greco, D.; Söderhäll, C.; Scheynius, A.; Kere, J. Differential DNA Methylation in Purified Human Blood Cells: Implications for Cell Lineage and Studies on Disease Susceptibility. PLoS ONE 2012, 7, e41361. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Epigenetics in Cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.T.; Brandes, J.C.; Vertino, P.M. Cancer DNA Methylation: Molecular Mechanisms and Clinical Implications. Clin. Cancer Res. 2009, 15, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Opavsky, R.; Wang, S.-H.; Trikha, P.; Raval, A.; Huang, Y.; Wu, Y.-Z.; Rodriguez, B.; Keller, B.; Liyanarachchi, S.; Wei, G.; et al. CpG Island Methylation in a Mouse Model of Lymphoma Is Driven by the Genetic Configuration of Tumor Cells. PLoS Genet. 2007, 3, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Laurent, L.; Wong, E.; Li, G.; Huynh, T.; Tsirigos, A.; Ong, C.T.; Low, H.M.; Kin Sung, K.W.; Rigoutsos, I.; Loring, J.; et al. Dynamic Changes in the Human Methylome during Differentiation. Genome Res. 2010, 20, 320–331. [Google Scholar] [CrossRef]
- Zane, L.; Sharma, V.; Misteli, T. Common Features of Chromatin in Aging and Cancer: Cause or Coincidence? Trends Cell Biol. 2014, 24, 686–694. [Google Scholar] [CrossRef]
- Miranda, T.B.; Jones, P.A. DNA Methylation: The Nuts and Bolts of Repression. J. Cell. Physiol. 2007, 213, 384–390. [Google Scholar] [CrossRef]
- Costello, J.F.; Frühwald, M.C.; Smiraglia, D.J.; Rush, L.J.; Robertson, G.P.; Gao, X.; Wright, F.A.; Feramisco, J.D.; Peltomäki, P.; Lang, J.C.; et al. Aberrant CpG-Island Methylation Has Non-Random and Tumour-Type-Specific Patterns. Nat. Genet. 2000, 24, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Giannopoulou, E.G.; Park, K.; Mosquera, J.M.; Sboner, A.; Tewari, A.K.; Garraway, L.A.; Beltran, H.; Rubin, M.A.; Elemento, O. Epigenomic Alterations in Localized and Advanced Prostate Cancer. Neoplasia 2013, 15, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, C.; Bastian, P.J.; Bjartell, A.; Carbone, G.M.; Catto, J.W.F.; Clark, S.J.; Henrique, R.; Nelson, W.G.; Shariat, S.F. Epigenetics in Prostate Cancer: Biologic and Clinical Relevance. Eur. Urol. 2011, 60, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Beggs, A.D.; Jones, A.; El-Bahrawy, M.; Abulafi, M.; Hodgson, S.V.; Tomlinson, I.P.M. Whole-Genome Methylation Analysis of Benign and Malignant Colorectal Tumours. J. Pathol. 2013, 229, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Salta, S.; Nunes, S.P.; Fontes-Sousa, M.; Lopes, P.; Freitas, M.; Caldas, M.; Antunes, L.; Castro, F.; Antunes, P.; Palma de Sousa, S.; et al. A DNA Methylation-Based Test for Breast Cancer Detection in Circulating Cell-Free DNA. J. Clin. Med. Res. 2018, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Hauge, T.; Jeanmougin, M.; Pharo, H.D.; Kresse, S.H.; Honne, H.; Winge, S.B.; Five, M.-B.; Kumar, T.; Mala, T.; et al. Targeted Genetic and Epigenetic Profiling of Esophageal Adenocarcinomas and Non-Dysplastic Barrett’s Esophagus. Clin. Epigenetics 2022, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Hou, P. Gene Methylation in Gastric Cancer. Clin. Chim. Acta 2013, 424, 53–65. [Google Scholar] [CrossRef]
- Hoang, P.H.; Landi, M.T. DNA Methylation in Lung Cancer: Mechanisms and Associations with Histological Subtypes, Molecular Alterations, and Major Epidemiological Factors. Cancers 2022, 14, 961. [Google Scholar] [CrossRef]
- Rademakers, G.; Massen, M.; Koch, A.; Draht, M.X.; Buekers, N.; Wouters, K.A.D.; Vaes, N.; De Meyer, T.; Carvalho, B.; Meijer, G.A.; et al. Identification of DNA Methylation Markers for Early Detection of CRC Indicates a Role for Nervous System-Related Genes in CRC. Clin. Epigenetics 2021, 13, 80. [Google Scholar] [CrossRef]
- Kisiel, J.B.; Dukek, B.A.; Kanipakam, R.V.S.R.; Ghoz, H.M.; Yab, T.C.; Berger, C.K.; Taylor, W.R.; Foote, P.H.; Giama, N.H.; Onyirioha, K.; et al. Hepatocellular Carcinoma Detection by Plasma Methylated DNA: Discovery, Phase I Pilot, and Phase II Clinical Validation. Hepatology 2019, 69, 1180–1192. [Google Scholar] [CrossRef]
- Stewart, C.M.; Tsui, D.W.Y. Circulating Cell-Free DNA for Non-Invasive Cancer Management. Cancer Genet. 2018, 228–229, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.O.; Alves, B.C.A.; Gehrke, F.d.S.; Kuniyoshi, R.K.; Wroclavski, M.L.; Del Giglio, A.; Fonseca, F.L.A. Characterization of Cell-Free Circulating DNA in Plasma in Patients with Prostate Cancer. Tumour Biol. 2013, 34, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Frattini, M.; Gallino, G.; Signoroni, S.; Balestra, D.; Battaglia, L.; Sozzi, G.; Leo, E.; Pilotti, S.; Pierotti, M.A. Quantitative Analysis of Plasma DNA in Colorectal Cancer Patients: A Novel Prognostic Tool. Ann. N. Y. Acad. Sci. 2006, 1075, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Marsavela, G.; McEvoy, A.C.; Pereira, M.R.; Reid, A.L.; Al-Ogaili, Z.; Warburton, L.; Khattak, M.A.; Abed, A.; Meniawy, T.M.; Millward, M.; et al. Detection of Clinical Progression through Plasma ctDNA in Metastatic Melanoma Patients: A Comparison to Radiological Progression. Br. J. Cancer 2022, 126, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Roadmap Epigenomics Consortium; Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; et al. Integrative Analysis of 111 Reference Human Epigenomes. Nature 2015, 518, 317–330. [Google Scholar]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid Biopsy: A Step Forward towards Precision Medicine in Urologic Malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef] [PubMed]
- Shames, D.S.; Girard, L.; Gao, B.; Sato, M.; Lewis, C.M.; Shivapurkar, N.; Jiang, A.; Perou, C.M.; Kim, Y.H.; Pollack, J.R.; et al. A Genome-Wide Screen for Promoter Methylation in Lung Cancer Identifies Novel Methylation Markers for Multiple Malignancies. PLoS Med. 2006, 3, e486. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Jensen, T.; Oshiro, M.M.; Watts, G.S.; Kim, C.J.; Futscher, B.W. Agglomerative Epigenetic Aberrations Are a Common Event in Human Breast Cancer. Cancer Res. 2008, 68, 8616–8625. [Google Scholar] [CrossRef]
- Heeke, S.; Gay, C.M.; Estecio, M.R.; Tran, H.; Morris, B.B.; Zhang, B.; Tang, X.; Raso, M.G.; Rocha, P.; Lai, S.; et al. Tumor- and Circulating-Free DNA Methylation Identifies Clinically Relevant Small Cell Lung Cancer Subtypes. Cancer Cell 2024, 42, 225–237.e5. [Google Scholar] [CrossRef]
- Pham, T.M.Q.; Phan, T.H.; Jasmine, T.X.; Tran, T.T.T.; Huynh, L.A.K.; Vo, T.L.; Nai, T.H.T.; Tran, T.T.; Truong, M.H.; Tran, N.C.; et al. Multimodal Analysis of Genome-Wide Methylation, Copy Number Aberrations, and End Motif Signatures Enhances Detection of Early-Stage Breast Cancer. Front. Oncol. 2023, 13, 1127086. [Google Scholar] [CrossRef]
- Brenne, S.S.; Madsen, P.H.; Pedersen, I.S.; Hveem, K.; Skorpen, F.; Krarup, H.B.; Giskeødegård, G.F.; Laugsand, E.A. Colorectal Cancer Detected by Liquid Biopsy 2 Years prior to Clinical Diagnosis in the HUNT Study. Br. J. Cancer 2023, 129, 861–868. [Google Scholar] [CrossRef]
- Dillinger, T.; Sheibani-Tezerji, R.; Pulverer, W.; Stelzer, I.; Hassler, M.R.; Scheibelreiter, J.; Pérez Malla, C.U.; Kuroll, M.; Domazet, S.; Redl, E.; et al. Identification of Tumor Tissue-Derived DNA Methylation Biomarkers for the Detection and Therapy Response Evaluation of Metastatic Castration Resistant Prostate Cancer in Liquid Biopsies. Mol. Cancer 2022, 21, 7. [Google Scholar] [CrossRef]
- Cheng, F.; Su, L.; Qian, C. Circulating Tumor DNA: A Promising Biomarker in the Liquid Biopsy of Cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Pajares, M.J.; Palanca-Ballester, C.; Urtasun, R.; Alemany-Cosme, E.; Lahoz, A.; Sandoval, J. Methods for Analysis of Specific DNA Methylation Status. Methods 2021, 187, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Martisova, A.; Holcakova, J.; Izadi, N.; Sebuyoya, R.; Hrstka, R.; Bartosik, M. DNA Methylation in Solid Tumors: Functions and Methods of Detection. Int. J. Mol. Sci. 2021, 22, 4247. [Google Scholar] [CrossRef]
- Perry, N.; Wasko, K.; Cheng, J.; Tabbaa, D.; Marco, E.; Giannoukos, G.; Albright, C.F.; Borges, C.M. Methylation-Sensitive Restriction Enzyme Quantitative Polymerase Chain Reaction Enables Rapid, Accurate, and Precise Detection of Methylation Status of the Regulatory T Cell (Treg)-Specific Demethylation Region in Primary Human Tregs. J. Immunol. 2021, 206, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; Paul, C.L. A Genomic Sequencing Protocol That Yields a Positive Display of 5-Methylcytosine Residues in Individual DNA Strands. Proc. Natl. Acad. Sci. USA 1992, 89, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Mikeska, T.; Krypuy, M.; Dobrovic, A. Sensitive Melting Analysis after Real Time- Methylation Specific PCR (SMART-MSP): High-Throughput and Probe-Free Quantitative DNA Methylation Detection. Nucleic Acids Res. 2008, 36, e42. [Google Scholar] [CrossRef] [PubMed]
- Sigalotti, L.; Covre, A.; Colizzi, F.; Fratta, E. Quantitative Methylation-Specific PCR: A Simple Method for Studying Epigenetic Modifications of Cell-Free DNA. Methods Mol. Biol. 2019, 1909, 137–162. [Google Scholar]
- Esteller, M. The Necessity of a Human Epigenome Project. Carcinogenesis 2006, 27, 1121–1125. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Repetto, M.; Crimini, E.; Valenza, C.; Belli, C.; Criscitiello, C.; Marra, A.; Subbiah, V.; Curigliano, G. Variant Allele Frequency: A Decision-Making Tool in Precision Oncology? Trends Cancer Res. 2023, 9, 1058–1068. [Google Scholar] [CrossRef]
- Leitão, E.; Beygo, J.; Zeschnigk, M.; Klein-Hitpass, L.; Bargull, M.; Rahmann, S.; Horsthemke, B. Locus-Specific DNA Methylation Analysis by Targeted Deep Bisulfite Sequencing. Methods Mol. Biol. 2018, 1767, 351–366. [Google Scholar] [PubMed]
- Söderhäll, C.; Reinius, L.E.; Salmenperä, P.; Gentile, M.; Acevedo, N.; Konradsen, J.R.; Nordlund, B.; Hedlin, G.; Scheynius, A.; Myllykangas, S.; et al. High-Resolution Targeted Bisulfite Sequencing Reveals Blood Cell Type-Specific DNA Methylation Patterns in IL13 and ORMDL3. Clin. Epigenetics 2021, 13, 106. [Google Scholar] [CrossRef]
- Schenk, D.; Song, G.; Ke, Y.; Wang, Z. Amplification of Overlapping DNA Amplicons in a Single-Tube Multiplex PCR for Targeted next-Generation Sequencing of BRCA1 and BRCA2. PLoS ONE 2017, 12, e0181062. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, Y.A.; Ho, D.; Poon, P.; Poh, Z.W.; Wong, P.M.; Gan, A.; Chang, M.M.; Kleftogiannis, D.; Lau, Y.T.; et al. Tissue-Specific Cell-Free DNA Degradation Quantifies Circulating Tumor DNA Burden. Nat. Commun. 2021, 12, 2229. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.M.; Borsu, L.; Cankovic, M.; Earle, J.S.L.; Gocke, C.D.; Hameed, M.; Jordan, D.; Lopategui, J.R.; Pullambhatla, M.; Reuther, J.; et al. Recommendations for Cell-Free DNA Assay Validations: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2023, 25, 876–897. [Google Scholar] [CrossRef]
- Chen, Z.; Li, C.; Zhou, Y.; Yao, Y.; Liu, J.; Wu, M.; Su, J. Liquid Biopsies for Cancer: From Bench to Clinic. MedComm (2020) 2023, 4, e329. [Google Scholar] [CrossRef]
- Warren, J.D.; Xiong, W.; Bunker, A.M.; Vaughn, C.P.; Furtado, L.V.; Roberts, W.L.; Fang, J.C.; Samowitz, W.S.; Heichman, K.A. Septin 9 Methylated DNA Is a Sensitive and Specific Blood Test for Colorectal Cancer. BMC Med. 2011, 9, 133. [Google Scholar] [CrossRef]
- Onieva-García, M.Á.; Llanos-Méndez, A.; Baños-Álvarez, E.; Isabel-Gómez, R. A Systematic Review of the Clinical Validity of the CologuardTM Genetic Test for Screening Colorectal Cancer. Rev. Clin. Esp. 2015, 215, 527–536.
- Ned, R.M.; Melillo, S.; Marrone, M. Fecal DNA Testing for Colorectal Cancer Screening: The ColoSureTM Test. PLoS Curr. 2011, 3, RRN1220. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.T.; Hurban, P.; White, M.N.; Whitlock, K.D.; Lofton-Day, C.E.; Tetzner, R.; Koenig, T.; Quigley, N.B.; Weiss, G. Validation of a Real-Time PCR-Based Qualitative Assay for the Detection of Methylated SEPT9 DNA in Human Plasma. Clin. Chem. 2014, 60, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N.; Dhillon, S. Epi proColon® 2.0 CE: A Blood-Based Screening Test for Colorectal Cancer. Mol. Diagn. Ther. 2017, 21, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Kang, Q.; Wang, X.; Yang, L.; Yu, Y.; Li, N.; He, Y.-Q.; Han, X.; Hang, J.; Zhang, J.; et al. Performance of a Second-Generation Methylated SEPT9 Test in Detecting Colorectal Neoplasm. J. Gastroenterol. Hepatol. 2015, 30, 830–833. [Google Scholar] [CrossRef]
- Vucetic, Z.; Loayza, N.; Pedersen, S.K.; Tuck, M.; LaPointe, L.C. Clinical Performance of Methylation-Based Liquid Biopsy Test COLVERA after Optimization of Test Interpretation Rules. J. Clin. Oncol. 2021, 39, 3546. [Google Scholar] [CrossRef]
- Murray, D.H.; Baker, R.T.; Gaur, S.; Young, G.P.; Pedersen, S.K. Validation of a Circulating Tumor-Derived DNA Blood Test for Detection of Methylated BCAT1 and IKZF1 DNA. J. Appl. Lab. Med. 2017, 2, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Ladd-Acosta, C.; Wen, B.; Wu, Z.; Montano, C.; Onyango, P.; Cui, H.; Gabo, K.; Rongione, M.; Webster, M.; et al. The Human Colon Cancer Methylome Shows Similar Hypo- and Hypermethylation at Conserved Tissue-Specific CpG Island Shores. Nat. Genet. 2009, 41, 178–186. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Dharuman, Y.; Standop, J.; Trimpop, N.; Herzog, M.; Hettwer, K.; Simon, K.; Uhlig, S.; Micallef, J. Novel Serum Nucleosomics Biomarkers for the Detection of Colorectal Cancer. Anticancer. Res. 2014, 34, 2357–2362. [Google Scholar]
- Herzog, M.; Eccleston, M.; Micallef, J.; Pamart, D.; Cuvelier, B.; Josseaux, E.; Terrell, J.; Christensen, I.; Nielsen, H. Validation of Nu.QTM Colorectal Cancer Screening Triage Test to Identify FIT Positive Individuals at Low Risk of Screen Relevant Neoplasia. Ann. Oncol. 2017, 28 (Suppl. S3), iii146. [Google Scholar] [CrossRef]
- Jeong, I.B.; Yoon, Y.S.; Park, S.Y.; Cha, E.J.; Na, M.J.; Kwon, S.J.; Kim, J.H.; Oh, T.J.; An, S.; Park, C.R.; et al. Methylation Biomarker in Bronchial Washing Specimens as an Adjunctive Diagnostic Tool to Bronchoscopy in Lung Cancer. Oncol. Lett. 2018, 16, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- EarlyTect® Lung Cancer. Available online: https://genomictree.com/eng/products/earlytect-lung-cancer/ (accessed on 25 March 2024).
- Weiss, G.; Schlegel, A.; Kottwitz, D.; König, T.; Tetzner, R. Validation of the SHOX2/PTGER4 DNA Methylation Marker Panel for Plasma-Based Discrimination between Patients with Malignant and Nonmalignant Lung Disease. J. Thorac. Oncol. 2017, 12, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Roperch, J.-P.; Hennion, C. A Novel Ultra-Sensitive Method for the Detection of FGFR3 Mutations in Urine of Bladder Cancer Patients—Design of the Urodiag® PCR Kit for Surveillance of Patients with Non-Muscle-Invasive Bladder Cancer (NMIBC). BMC Med. Genet. 2020, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Roperch, J.P.; Grandchamp, B.; Desgrandchamps, F. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer 2016, 16, 704. [Google Scholar] [CrossRef]
- van Kessel, K.E.M.; Van Neste, L.; Lurkin, I.; Zwarthoff, E.C.; Van Criekinge, W. Evaluation of an Epigenetic Profile for the Detection of Bladder Cancer in Patients with Hematuria. J. Urol. 2016, 195, 601–607. [Google Scholar] [CrossRef]
- Wasserstrom, A.; Frumkin, D.; Dotan, Z.; Bukin, E.; Gadish, T.; Hanuka, S.; Knirsh, R.; Darawsha, A.E.; Leibovitch, I. Mp13-15 Molecular Urine Cytology—Bladder Epicheck Is a Novel Molecular Diagnostic Tool for Monitoring of Bladder Cancer Patients. J. Urol. 2016, 195, e140. [Google Scholar] [CrossRef][Green Version]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheckTM Methylation Test for Patients Under Surveillance for Non–muscle-Invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Feber, A.; Dhami, P.; Dong, L.; de Winter, P.; Tan, W.S.; Martínez-Fernández, M.; Paul, D.S.; Hynes-Allen, A.; Rezaee, S.; Gurung, P.; et al. UroMark-a Urinary Biomarker Assay for the Detection of Bladder Cancer. Clin. Epigenetics 2017, 9, 8. [Google Scholar] [CrossRef]
- EarlyTect® Bladder Cancer. Available online: https://genomictree.com/eng/products/earlytect-bladder-cancer/ (accessed on 26 March 2024).
- Chen, X.; Zhang, J.; Ruan, W.; Huang, M.; Wang, C.; Wang, H.; Jiang, Z.; Wang, S.; Liu, Z.; Liu, C.; et al. Urine DNA Methylation Assay Enables Early Detection and Recurrence Monitoring for Bladder Cancer. J. Clin. Invest. 2020, 130, 6278–6289. [Google Scholar] [CrossRef]
- Pharo, H.D.; Jeanmougin, M.; Ager-Wick, E.; Vedeld, H.M.; Sørbø, A.K.; Dahl, C.; Larsen, L.K.; Honne, H.; Brandt-Winge, S.; Five, M.-B.; et al. BladMetrix: A Novel Urine DNA Methylation Test with High Accuracy for Detection of Bladder Cancer in Hematuria Patients. Clin. Epigenetics 2022, 14, 115. [Google Scholar] [CrossRef]
- Lewin, J.; Kottwitz, D.; Aoyama, J.; deVos, T.; Garces, J.; Hasinger, O.; Kasielke, S.; Knaust, F.; Rathi, P.; Rausch, S.; et al. Plasma Cell Free DNA Methylation Markers for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Case Control Study. BMC Gastroenterol. 2021, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- The IvyGene Liver Test—IvyGene, Non-Invasive Early Cancer Detection Test. Available online: https://www.ivygenelabs.co.za/the-ivygene-liver-test/ (accessed on 11 March 2024).
- Lin, H.; Chen, T.-C.; Chang, T.-C.; Cheng, Y.-M.; Chen, C.-H.; Chu, T.-Y.; Hsu, S.-T.; Liu, C.-B.; Yeh, L.-S.; Wen, K.-C.; et al. Methylated ZNF582 Gene as a Marker for Triage of Women with Pap Smear Reporting Low-Grade Squamous Intraepithelial Lesions—A Taiwanese Gynecologic Oncology Group (TGOG) Study. Gynecol. Oncol. 2014, 135, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N.; Wentzensen, N.; Maurer, M.J.; Hawthorne, K.M.; Voss, J.S.; Kroneman, T.N.; Famuyide, A.O.; Clayton, A.C.; Halling, K.C.; Kerr, S.E.; et al. Detection of Endometrial Cancer via Molecular Analysis of DNA Collected with Vaginal Tampons. Gynecol. Oncol. 2015, 137, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-García, J.; Osca-Verdegal, R.; Mena-Mollá, S.; García-Giménez, J.L. Epigenetic IVD Tests for Personalized Precision Medicine in Cancer. Front. Genet. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Loomans-Kropp, H.A.; Umar, A.; Minasian, L.M.; Pinsky, P.F. Multi-Cancer Early Detection Tests: Current Progress and Future Perspectives. Cancer Epidemiol. Biomark. Prev. 2022, 31, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Klein, E.A.; Seiden, M.V.; Hubbell, E.; Venn, O.; Jamshidi, A.; Zhang, N.; Beausang, J.F.; Gross, S.; Kurtzman, K.N.; et al. Simultaneous Multi-Cancer Detection and Tissue of Origin (TOO) Localization Using Targeted Bisulfite Sequencing of Plasma Cell-Free DNA (cfDNA). Ann. Oncol. 2019, 30, v912. [Google Scholar] [CrossRef]
- Blood Test for Cancer Screening. Available online: https://www.galleri.com/ (accessed on 25 March 2024).
- Hackshaw, A.; Clarke, C.A.; Hartman, A.-R. New Genomic Technologies for Multi-Cancer Early Detection: Rethinking the Scope of Cancer Screening. Cancer Cell 2022, 40, 109–113. [Google Scholar] [CrossRef]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical Validation of a Targeted Methylation-Based Multi-Cancer Early Detection Test Using an Independent Validation Set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H., 3rd; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-Based Tests for Multicancer Early Detection (PATHFINDER): A Prospective Cohort Study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Neal, R.D.; Johnson, P.; Clarke, C.A.; Hamilton, S.A.; Zhang, N.; Kumar, H.; Swanton, C.; Sasieni, P. Cell-Free DNA-Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers 2022, 14, 4818. [Google Scholar] [CrossRef]
- Al-Obeidee, M.; Al-Obeidee, E. Exploring the Potential of Multi-Cancer Early Detection Tests as Triage Tools in Urgent Referrals: Insights from Recent Clinical Trial. Postgrad. Med. J. 2024, 140, qgae033. [Google Scholar] [CrossRef] [PubMed]
- Pons-Belda, O.D.; Fernandez-Uriarte, A.; Diamandis, E.P. Multi Cancer Early Detection by Using Circulating Tumor DNA-The Galleri Test. Reply to Klein et Al. The Promise of Multicancer Early Detection. Comment on “Pons-Belda et al. Can Circulating Tumor DNA Support a Successful Screening Test for Early Cancer Detection? The Grail Paradigm. 2021, 11, 2171”. Diagnostics 2022, 12, 1244. [Google Scholar] [CrossRef]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-Invasive Early Detection of Cancer Four Years before Conventional Diagnosis Using a Blood Test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Locke, W.J.; Guanzon, D.; Ma, C.; Liew, Y.J.; Duesing, K.R.; Fung, K.Y.C.; Ross, J.P. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Front. Genet. 2019, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Luo, H.; Krawczyk, M.; Wei, W.; Wang, W.; Wang, J.; Flagg, K.; Hou, J.; Zhang, H.; Yi, S.; et al. DNA Methylation Markers for Diagnosis and Prognosis of Common Cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 7414–7419. [Google Scholar] [CrossRef] [PubMed]
- CancerRadar. Available online: https://earlydx.com/product/cancer-radar/ (accessed on 25 March 2024).
- Jørgensen, J.T.; Hersom, M. Companion Diagnostics—A Tool to Improve Pharmacotherapy. Ann. Transl. Med. 2016, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Garcia-Foncillas, J.; Andion, E.; Goodman, S.N.; Hidalgo, O.F.; Vanaclocha, V.; Baylin, S.B.; Herman, J.G. Inactivation of the DNA-Repair Gene MGMT and the Clinical Response of Gliomas to Alkylating Agents. N. Engl. J. Med. 2000, 343, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT Promoter Methylation Status Testing to Guide Therapy for Glioblastoma: Refining the Approach Based on Emerging Evidence and Current Challenges. Neuro. Oncol. 2018, 21, 167–178. [Google Scholar] [CrossRef]
- Mastoraki, S.; Strati, A.; Tzanikou, E.; Chimonidou, M.; Politaki, E.; Voutsina, A.; Psyrri, A.; Georgoulias, V.; Lianidou, E. Methylation: A Liquid Biopsy-Based Epigenetic Assay for the Follow-up of Patients with Metastatic Breast Cancer Receiving Endocrine Treatment. Clin. Cancer Res. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Hafazalla, K.; Sahgal, A.; Jaja, B.; Perry, J.R.; Das, S. Procarbazine, CCNU and Vincristine (PCV) versus Temozolomide Chemotherapy for Patients with Low-Grade Glioma: A Systematic Review. Oncotarget 2018, 9, 33623–33633. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Erdem-Eraslan, L.; Idbaih, A.; de Rooi, J.; Eilers, P.H.C.; Spliet, W.G.M.; den Dunnen, W.F.A.; Tijssen, C.; Wesseling, P.; Sillevis Smitt, P.A.E.; et al. MGMT-STP27 Methylation Status as Predictive Marker for Response to PCV in Anaplastic Oligodendrogliomas and Oligoastrocytomas—A Report from EORTC Study 26951. Clin. Cancer Res. 2013, 19, 5513–5522. [Google Scholar] [CrossRef] [PubMed]
- Gusyatiner, O.; Hegi, M.E. Glioma Epigenetics: From Subclassification to Novel Treatment Options. Semin. Cancer Biol. 2018, 51, 50–58. [Google Scholar] [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid Biopsy and Primary Brain Tumors. Cancers 2021, 13, 5429. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, N.C.; Pierron, G.; Klughammer, J.; Datlinger, P.; Schönegger, A.; Schuster, M.; Hadler, J.; Surdez, D.; Guillemot, D.; Lapouble, E.; et al. DNA Methylation Heterogeneity Defines a Disease Spectrum in Ewing Sarcoma. Nat. Med. 2017, 23, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Garrett-Bakelman, F.E.; Chung, S.S.; Sanders, M.A.; Hricik, T.; Rapaport, F.; Patel, J.; Dillon, R.; Vijay, P.; Brown, A.L.; et al. Distinct Evolution and Dynamics of Epigenetic and Genetic Heterogeneity in Acute Myeloid Leukemia. Nat. Med. 2016, 22, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Mazor, T.; Pankov, A.; Song, J.S.; Costello, J.F. Intratumoral Heterogeneity of the Epigenome. Cancer Cell 2016, 29, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Chen, D.; Lang, J.E. The Current Status of the Clinical Utility of Liquid Biopsies in Cancer. Expert. Rev. Mol. Diagn. 2019, 19, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the Bioactive Diet Components on the Gene Expression Regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Kaushik, A.; Chaudhary, V.; Longkumer, I.; Saraswathy, K.N.; Jain, S. Sex-Specific Variations in Global DNA Methylation Levels with Age: A Population-Based Exploratory Study from North India. Front. Genet. 2023, 14, 1038529. [Google Scholar] [CrossRef] [PubMed]
- Yusipov, I.; Bacalini, M.G.; Kalyakulina, A.; Krivonosov, M.; Pirazzini, C.; Gensous, N.; Ravaioli, F.; Milazzo, M.; Giuliani, C.; Vedunova, M.; et al. Age-Related DNA Methylation Changes Are Sex-Specific: A Comprehensive Assessment. Aging 2020, 12, 24057–24080. [Google Scholar] [CrossRef] [PubMed]
- Golden, L.C.; Itoh, Y.; Itoh, N.; Iyengar, S.; Coit, P.; Salama, Y.; Arnold, A.P.; Sawalha, A.H.; Voskuhl, R.R. Parent-of-Origin Differences in DNA Methylation of X Chromosome Genes in T Lymphocytes. Proc. Natl. Acad. Sci. USA 2019, 116, 26779–26787. [Google Scholar] [CrossRef] [PubMed]
- Dayeh, T.; Volkov, P.; Salö, S.; Hall, E.; Nilsson, E.; Olsson, A.H.; Kirkpatrick, C.L.; Wollheim, C.B.; Eliasson, L.; Rönn, T.; et al. Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion. PLoS Genet. 2014, 10, e1004160. [Google Scholar] [CrossRef]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aïssi, D.; Wahl, S.; Meduri, E.; Morange, P.-E.; Gagnon, F.; Grallert, H.; et al. DNA Methylation and Body-Mass Index: A Genome-Wide Analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendek, T.; Pos, O.; Duranova, T.; Saade, R.; Budis, J.; Repiska, V.; Szemes, T. Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics. Cancers 2024, 16, 2001. https://doi.org/10.3390/cancers16112001
Rendek T, Pos O, Duranova T, Saade R, Budis J, Repiska V, Szemes T. Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics. Cancers. 2024; 16(11):2001. https://doi.org/10.3390/cancers16112001
Chicago/Turabian StyleRendek, Tomas, Ondrej Pos, Terezia Duranova, Rami Saade, Jaroslav Budis, Vanda Repiska, and Tomas Szemes. 2024. "Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics" Cancers 16, no. 11: 2001. https://doi.org/10.3390/cancers16112001
APA StyleRendek, T., Pos, O., Duranova, T., Saade, R., Budis, J., Repiska, V., & Szemes, T. (2024). Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics. Cancers, 16(11), 2001. https://doi.org/10.3390/cancers16112001





