Checkpoint Inhibitors in Dogs: Are We There Yet?
Abstract
:Simple Summary
Abstract
1. Introduction
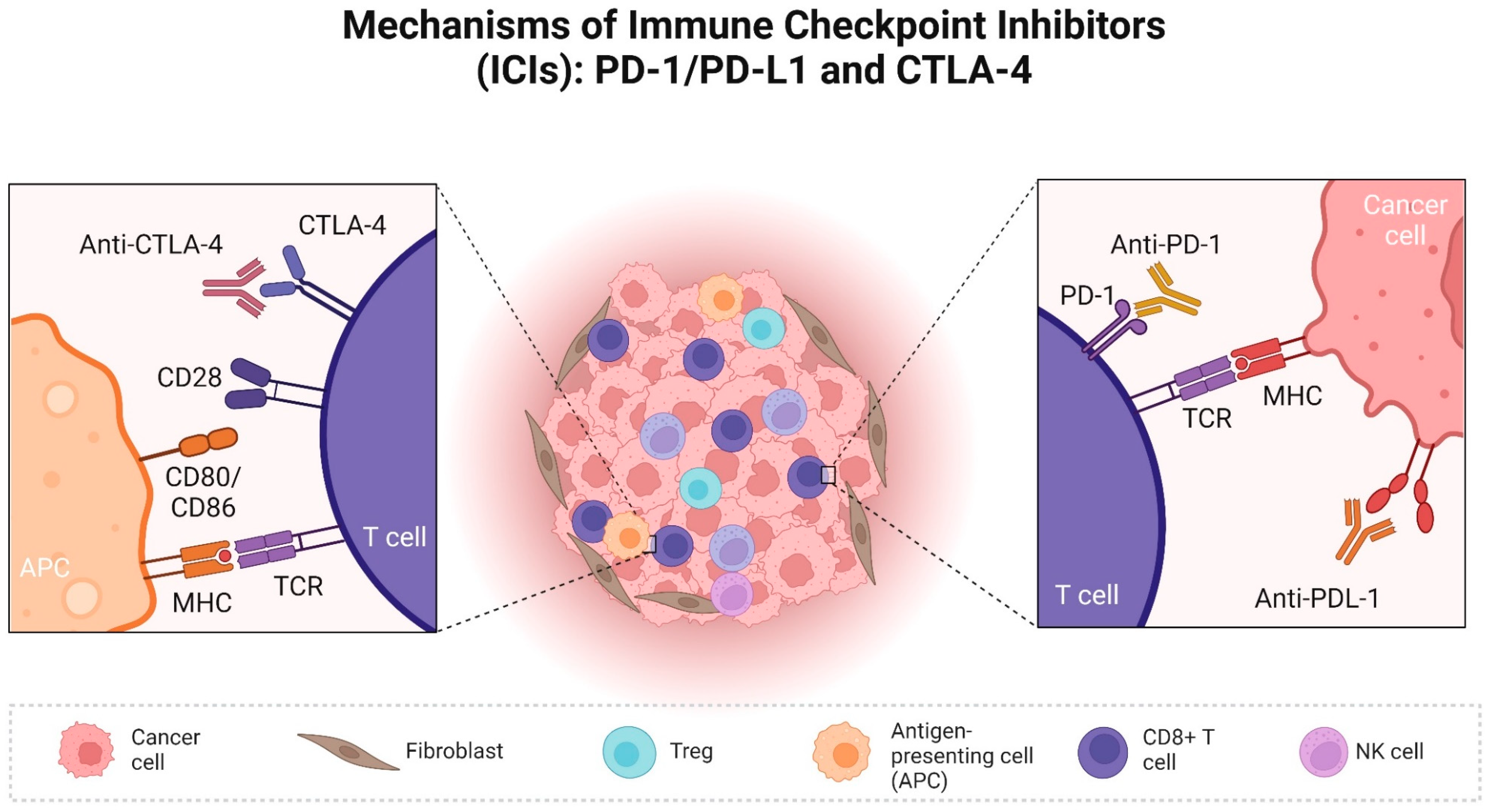
2. Role of Check Point Proteins in Human Cancer and Available ICI
3. The Expression and Clinical Relevance of Immune Checkpoints in Dogs with Cancer
3.1. PD/PD-L1 in Dogs
3.2. CTLA-4
3.3. Clinical Trials of Immune Checkpoint Inhibitors in Dogs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Carreau, N.; Pavlick, A. Revolutionizing treatment of advanced melanoma with immunotherapy. Surg. Oncol. 2022, 42, 101180. [Google Scholar] [CrossRef] [PubMed]
- Marable, J.; Ruiz, D.; Jaiswal, A.K.; Bhattacharya, R.; Pantazes, R.; Agarwal, P.; Suryawanshi, A.S.; Bedi, D.; Mishra, A.; Smith, B.F.; et al. Nanobody-based CTLA4 inhibitors for immune checkpoint blockade therapy of canine cancer patients. Sci. Rep. 2021, 11, 20763. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Hosoya, K.; Kim, S.; Kinoshita, R.; Deguchi, T.; Owaki, R.; Tachibana, Y.; Yokokawa, M.; Takeuchi, H.; et al. Safety and clinical efficacy of an anti-PD-L1 antibody (c4G12) in dogs with advanced malignant tumours. PLoS ONE 2023, 18, e0291727. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Hussein, O.; Sastry, K.S.; Bougarn, S.; Gopinath, N.; Chin-Smith, E.; Sinha, Y.; Korashy, H.M.; Maccalli, C. Deciphering the complexities of cancer cell immune evasion: Mechanisms and therapeutic implications. Adv. Cancer Biol.-Metastasis 2023, 8, 100107. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Cancer Immunother. 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; De Feo, V.; Pietrantuono, G.; Seneca, E.; Mansueto, G.; Villani, O.; La Rocca, F.; D’Auria, F.; Statuto, T.; Valvano, L.; et al. CD200 and Chronic Lymphocytic Leukemia: Biological and Clinical Relevance. Front. Oncol. 2020, 10, 584427. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Torchia, J.A.; Chaudhri, A.; Sharpe, A.H.; Freeman, G.J. Emerging concepts in PD-1 checkpoint biology. Semin. Immunol. 2021, 52, 101480. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Hu-Lieskovan, S. What does PD-L1 positive or negative mean? J. Exp. Med. 2016, 213, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2023, 13, 22–40. [Google Scholar] [CrossRef]
- Keeping, S.; Xu, Y.; Chen, C.I.; Cope, S.; Mojebi, A.; Kuznik, A.; Konidaris, G.; Ayers, D.; Sasane, M.; Allen, R.; et al. Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma. Future Oncol. 2021, 17, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef]
- Raedler, L.A. Opdivo (Nivolumab): Second PD-1 Inhibitor Receives FDA Approval for Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 180–183. [Google Scholar]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.M.; Kaufman, P.A.; Wilks, S.T.; Andreopoulou, E.; et al. Eribulin plus pembrolizumab in patients with metastatic triple-negative breast cancer (ENHANCE 1): A phase Ib/II study. Clin. Cancer Res. 2021, 27, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab KEYNOTE-001: An adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann. Oncol. 2017, 28, 1388–1398. [CrossRef] [PubMed]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Tétu, P.; Mangana, J.; Dummer, R.; Dutriaux, C.; Beneton, N.; Dalle, S.; Meyer, N.; Oriano, B.; Michielin, O.; Lebbe, C. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur. J. Cancer 2018, 93, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2021, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Atezolizumab: First Global Approval. Drugs 2016, 76, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Tagawa, M.; Yamamoto, Y.; Shimbo, G.; Iguchi, A.; Xuan, X.; Tomihari, M.; Miyahara, K. Gene and protein expression of a soluble form of CTLA-4 in a healthy dog. J. Vet. Med. Sci. 2017, 79, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Ma, Z.; Wang, C.; Yang, Q.; Byrne-Steele, M.; Hong, R.; Min, Q.; Zhou, G.; Cheng, Y.; et al. CTLA-4 expression by B-1a B cells is essential for immune tolerance. Nat. Commun. 2021, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Holt, M.P.; Punkosdy, G.A.; Glass, D.D.; Shevach, E.M. TCR Signaling and CD28/CTLA-4 Signaling Cooperatively Modulate T Regulatory Cell Homeostasis. J. Immunol. 2017, 198, 1503–1511. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding lag-3 signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Huard, B.; Prigent, P.; Pagès, F.; Bruniquel, D.; Triebel, F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur. J. Immunol. 1996, 26, 1180–1186. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef]
- Kreidieh, F.Y.; Tawbi, H.A. The introduction of LAG-3 checkpoint blockade in melanoma: Immunotherapy landscape beyond PD-1 and CTLA-4 inhibition. Ther. Adv. Med. Oncol. 2023, 15, 17588359231186027. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Li, Z.; Ju, Z.; Frieri, M. The T-cell immunoglobulin and mucin domain (Tim) gene family in asthma, allergy, and autoimmunity. Allergy Asthma Proc. 2013, 34, 21–26. [Google Scholar] [CrossRef]
- He, Y.; Cao, J.; Zhao, C.; Li, X.; Zhou, C.; Hirsch, F.R. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018, 11, 7005–7009. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, S.; Fan, L.; Zhang, B.; Xu, S. TIM-3: An update on immunotherapy. Int. Immunopharmacol. 2021, 99, 107933. [Google Scholar] [CrossRef]
- Yan, W.; Liu, X.; Ma, H.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015, 64, 1593–1604. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Meyer, N.H.; Schlichtner, S.; Hussain, R.; Siligardi, G.; Casely-Hayford, M.; Fiedler, W.; Wellbrock, J.; Desmet, C.; Calzolai, L.; et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front. Immunol. 2020, 11, 580557. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Giagounidis, A.; Sekeres, M.A.; Xiao, Z.; Sanz, G.F.; Hoef MVan Ma, F.; Hertle, S.; Santini, V. STIMULUS-MDS2 design and rationale: A phase III trial with the anti-TIM-3 sabatolimab (MBG453) + azacitidine in higher risk MDS and CMML-2. Future Oncol. 2022, 19, 631–642. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, S.; Zhang, X.; Jia, K.; Wang, H.; Zhou, C.; He, Y. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. Onco Targets Ther. 2019, 12, 7347–7353. [Google Scholar] [CrossRef]
- Curti, B.D.; Kovacsovics-Bankowski, M.; Morris, N.; Walker, E.; Chisholm, L.; Floyd, K.; Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar] [CrossRef]
- D’arena, G.; Vitale, C.; Coscia, M.; Lamorte, D.; Pietrantuono, G.; Perutelli, F.; D’auria, F.; Statuto, T.; Valvano, L.; Tomasso, A.; et al. Cd200 baseline serum levels predict prognosis of chronic lymphocytic leukemia. Cancers 2021, 13, 4329. [Google Scholar] [CrossRef]
- Olin, M.R.; Ampudia-Mesias, E.; Pennell, C.A.; Sarver, A.; Chen, C.C.; Moertel, C.L.; Hunt, M.A.; Pluhar, G.E. Treatment combining CD200 immune checkpoint inhibitor and tumor-lysate vaccination after surgery for pet dogs with high-grade glioma. Cancers 2019, 11, 137. [Google Scholar] [CrossRef]
- Coy, J.; Caldwell, A.; Chow, L.; Guth, A.; Dow, S. PD-1 expression by canine T cells and functional effects of PD-1 blockade. Vet. Comp. Oncol. 2017, 15, 1487–1502. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Muscatello, L.V.; Gobbo, F.; Avallone, G.; Innao, M.; Benazzi, C.; D’Annunzio, G.; Romaniello, D.; Orioles, M.; Lauriola, M.; Sarli, G. PDL1 immunohistochemistry in canine neoplasms: Validation of commercial antibodies, standardization of evaluation, and scoring systems. Vet. Pathol. 2024, 61, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Aresu, L.; Marconato, L.; Martini, V.; Fanelli, A.; Licenziato, L.; Foiani, G.; Melchiotti, E.; Nicoletti, A.; Vascellari, M. Prognostic value of pd-l1, pd-1 and cd8a in canine diffuse large b-cell lymphoma detected by rnascope. Vet. Sci. 2021, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- O’Malley, D.P.; Yang, Y.; Boisot, S.; Sudarsanam, S.; Wang, J.F.; Chizhevsky, V.; Zhao, G.; Arain, S.; Weiss, L.M. Immunohistochemical detection of PD-L1 among diverse human neoplasms in a reference laboratory: Observations based upon 62,896 cases. Mod. Pathol. 2019, 32, 929–942. [Google Scholar] [CrossRef]
- Pinard, C.J.; Hocker, S.E.; Poon, A.C.; Inkol, J.M.; Matsuyama, A.; Wood, R.D.; Wood, G.A.; Woods, J.P.; Mutsaers, A.J. Evaluation of PD-1 and PD-L1 expression in canine urothelial carcinoma cell lines. Vet. Immunol. Immunopathol. 2022, 243, 110367. [Google Scholar] [CrossRef]
- Reis, I.B.; Tibo, L.H.S.; de Souza, B.R.; Durán, N.; Fávaro, W.J. OncoTherad® is an immunomodulator of biological response that downregulate RANK/RANKL signaling pathway and PD-1/PD-L1 immune checkpoint in non-muscle invasive bladder cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 5025–5036. [Google Scholar] [CrossRef]
- Sommer, B.C.; Dhawan, D.; Ratliff, T.L.; Knapp, D.W. Naturally-Occurring Canine Invasive Urothelial Carcinoma: A Model for Emerging Therapies. Bladder Cancer 2018, 4, 149–159. [Google Scholar] [CrossRef]
- Knapp, D.W.; Dhawan, D.; Ramos-Vara, J.A.; Ratliff, T.L.; Cresswell, G.M.; Utturkar, S.; Sommer, B.C.; Fulkerson, C.M.; Hahn, N.M. Naturally-Occurring Invasive Urothelial Carcinoma in Dogs, a Unique Model to Drive Advances in Managing Muscle Invasive Bladder Cancer in Humans. Front. Oncol. 2020, 9, 1493. [Google Scholar] [CrossRef]
- Tagawa, M.; Kurashima, C.; Takagi, S.; Maekawa, N.; Konnai, S.; Shimbo, G.; Matsumoto, K.; Inokuma, H.; Kawamoto, K.; Miyahara, K. Evaluation of costimulatory molecules in dogs with B cell high grade lymphoma. PLoS ONE 2018, 13, e0201222. [Google Scholar] [CrossRef]
- Ariyarathna, H.; Thomson, N.A.; Aberdein, D.; Perrott, M.R.; Munday, J.S. Increased programmed death ligand (PD-L1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) expression is associated with metastasis and poor prognosis in malignant canine mammary gland tumours. Vet. Immunol. Immunopathol. 2020, 230, 110142. [Google Scholar] [CrossRef] [PubMed]
- Minoli, L.; Licenziato, L.; Kocikowski, M.; Cino, M.; Dziubek, K.; Iussich, S.; Fanelli, A.; Morello, E.; Martano, M.; Hupp, T.; et al. Development of Monoclonal Antibodies Targeting Canine PD-L1 and PD-1 and Their Clinical Relevance in Canine Apocrine Gland Anal Sac Adenocarcinoma. Cancers 2022, 14, 6188. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Maekawa, N.; Konnai, S.; Takagi, S. Evaluation of costimulatory molecules in peripheral blood lymphocytes of canine patients with histiocytic sarcoma. PLoS ONE 2016, 11, e0150030. [Google Scholar] [CrossRef]
- Porcellato, I.; Brachelente, C.; Cappelli, K.; Menchetti, L.; Silvestri, S.; Sforna, M.; Mecocci, S.; Iussich, S.; Leonardi, L.; Mechelli, L. FoxP3, CTLA-4, and IDO in Canine Melanocytic Tumors. Vet. Pathol. 2021, 58, 42–52. [Google Scholar] [CrossRef]
- Wong, C.; Darby, J.M.; Murphy, P.R.; Pinfold, T.L.; Lennard, P.R.; Woods, G.M.; Lyons, A.B.; Flies, A.S. Tasmanian devil CD28 and CTLA4 capture CD80 and CD86 from adjacent cells. Dev. Comp. Immunol. 2021, 115, 103882. [Google Scholar] [CrossRef]
- Simone, R.; Pesce, G.; Antola, P.; Rumbullaku, M.; Bagnasco, M.; Bizzaro, N.; Saverino, D. The soluble form of CTLA-4 from serum of patients with autoimmune diseases regulates T-cell responses. Biomed. Res. Int. 2014, 2014, 215763. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Faro, T.A.S.; de Oliveira, E.H.C. Canine transmissible venereal tumor—From general to molecular characteristics: A review. Anim. Genet. 2023, 54, 82–89. [Google Scholar] [CrossRef]
- Wilson, R.A.M.; Evans, T.R.J.; Fraser, A.R.; Nibbs, R.J.B. Immune checkpoint inhibitors: New strategies to checkmate cancer. Clin. Exp. Immunol. 2018, 191, 133–148. [Google Scholar] [CrossRef]
- Singh, P.; de Souza, P.; Scott, K.F.; Hall, B.M.; Verma, N.D.; Becker, T.M.; Toh, J.W.T.; Sajinovic, M.; Spring, K.J. Biomarkers in immune checkpoint inhibition therapy for cancer patients: What is the role of lymphocyte subsets and PD1/PD-L1? Transl. Med. Commun. 2019, 4, 2. [Google Scholar] [CrossRef]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci. Rep. 2020, 10, 18311. [Google Scholar] [CrossRef]
- Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers 2023, 15, 3013. [Google Scholar] [CrossRef]
- De Velasco, G.; Krajewski, K.M.; Albiges, L.; Awad, M.M.; Bellmunt, J.; Hodi, F.S.; Choueiri, T.K. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol. Res. 2016, 4, 12–17. [Google Scholar] [CrossRef]
- Kawabe, M.; Mori, T.; Ito, Y.; Murakami, M.; Sakai, H.; Yanai, T.; Maruo, K. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Cunha, S.C.; Corgozinho, K.B.; Silva, F.B.F.; da Silva, K.V.G.C.; Ferreira, A.M.R. Radiation therapy for oral melanoma in dogs: A retrospective study. Cienc. Rural. 2018, 48, e20160396. [Google Scholar] [CrossRef]
- Igase, M.; Inanaga, S.; Tani, K.; Nakaichi, M.; Sakai, Y.; Sakurai, M.; Kato, M.; Tsukui, T.; Mizuno, T. Long-term survival of dogs with stage 4 oral malignant melanoma treated with anti-canine therapeutic antibody: A follow-up case report. Vet. Comp. Oncol. 2022, 20, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Asano, Y.; Sajiki, Y.; Deguchi, T.; Okagawa, T.; Watari, K.; Takeuchi, H.; Takagi, S.; Hosoya, K.; et al. Exploration of serum biomarkers in dogs with malignant melanoma receiving anti-PD-L1 therapy and potential of COX-2 inhibition for combination therapy. Sci. Rep. 2022, 12, 9265. [Google Scholar] [CrossRef] [PubMed]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Inanaga, S.; Nishibori, S.; Itamoto, K.; Sunahara, H.; Nemoto, Y.; Tani, K.; Horikirizono, H.; Nakaichi, M.; Baba, K.; et al. Proof-of-concept study of the caninized anti-canine programmed death 1 antibody in dogs with advanced non-oral malignant melanoma solid tumors. J. Vet. Sci. 2024, 25, e15. [Google Scholar] [CrossRef]
- Morsey, M.; Stock, M.; Boeckh, A.; Lehmann, H. An Anti-Canine PD-1 Monoclonal Antibody for Immunotherapy of Cancer in Dogs. In 2021 ACVIM Forum Research Abstract Program. J. Vet. Intern. Med. 2021, 35, 3020–3021. [Google Scholar]
- Pantelyushin, S.; Ranninger, E.; Guerrera, D.; Hutter, G.; Maake, C.; Markkanen, E.; Bettschart-Wolfensberger, R.; Bley, C.R.; Läubli, H.; Vom Berg, J. Cross-reactivity and functionality of approved human immune checkpoint blockers in dogs. Cancers 2021, 13, 785. [Google Scholar] [CrossRef]
- Bergeron, L.M.; McCandless, E.E.; Dunham, S.; Dunkle, B.; Zhu, Y.; Shelly, J.; Lightle, S.; Gonzales, A.; Bainbridge, G. Comparative functional characterization of canine IgG subclasses. Vet. Immunol. Immunopathol. 2014, 157, 31–41. [Google Scholar] [CrossRef]
| Pathology | Maekawa et al. (2021) [4] | Muscatello et al. (2023) [59] | ||
|---|---|---|---|---|
| Positive Immunolabeling | TPS (≥50%) | Positive Immunolabeling | TPS | |
| Melanoma | 19/20 (95%) | 18/20 (90%) | 17/17 (100%) | 1–14% |
| Squamous cell carcinoma | 18/20 (90%) | 16/20 (80%) | 3/17 (18%) | 17–64% |
| Mammary gland carcinoma | 20/20 (100%) | 20/20 (100%) | 1/31 (3%) | 9% |
| Apocrine gland anal sac adenocarcinoma | 19/20 (95%) | 15/20 (75%) | - | - |
| Nasal adenocarcinoma | 20/20 (100%) | 18/20 (90%) | - | - |
| Pulmonary carcinoma | - | - | 2/20 (10%) | 78–97% |
| Renal carcinoma | - | - | 4/17 (24%) | 16–91% |
| Urothelial carcinoma | 20/20 (100%) | 20/20 (100%) | 2/18 (11%) | 29–69% |
| Gastric carcinoma | 4/5 (80%) | 3/5 (60%) | 0/10 | - |
| Intestinal carcinoma | - | - | 0/24 | - |
| Lymphoma | 17/20 (85%) | 12/20 (60%) | 2/14 (31%) | 1–13% |
| Transmissible venereal tumour | 0/4 (0%) | - | - | - |
| Soft-tissue sarcoma | 14/20 (70%) | 12/20 (60%) | - | - |
| Histiocytic sarcoma | 18/20 (90%) | 6/20 (30%) | - | - |
| Osteosarcoma | 20/20 (100%) | 16/20 (80%) | - | - |
| Sample | B Cell High Grade Lymphoma | Mammary Gland Tumour | Melanoma |
|---|---|---|---|
| Tumour cells | Unknown | IHC | IHC and RT-qPCR |
| Serum | Unknown | Unknown | Unknown |
| TIL CD4+ | Flow cytometry | Unknown | Unknown |
| TIL CD8+ | Flow cytometry | Unknown | Unknown |
| Serum CD4+ | Flow cytometry | Unknown | Unknown |
| Serum CD8+ | Flow cytometry | Unknown | Unknown |
| References | Tagawa et al., 2018 [68] | Ariyarathna et al., 2020 [69] | Porcellato et al., 2021 [72] |
| Study | ICI Agent | Number of Dogs | Response Rate | Median Survival Time | Clinical Staging (WHO) |
|---|---|---|---|---|---|
| Deguchi et al. (2023) [80] | Chimeric rat–dog anti-PD-L1 | 39 (TG) | CR in 5/34, PR/SD in 4/34 *, and PD in 25/34 | 129 days (TG) and 48 days (HCG) | Stage IV (all with pulmonary metastases) |
| Maekawa et al. (2021) [4] | Chimeric rat–dog anti-PD-L1 | 29 (TG) and 15 (HCG) | CR in 1/10 and PD in 9/10 | 143 days (TG) and 54 (HCG) | Stage IV (all with pulmonary metastases) |
| Igase et al. (2020) [79] | Chimeric rat–dog anti-PD-1 and caninized anti-PD-1 | 21 (TG, 15 in the chimeric ICI group and 6 in the caninized ICI group) and 23 (HCG) | PR in 4/15, SD in 1/15, and PD in 10/15 | 166 days (TG) and 55 days (HCG) | Stage III (4) and stage IV (17) |
| Maekawa et al. (2017) [5] | Chimeric rat–dog anti-PD-L1 | 7 (TG) and 17 (HCG) | PR in 1/7 and PD in 6/7 | 93.5 days (TG) and 54 days (HCG) | Stage II (1), stage II (2), and stage IV (4 with pulmonary metastases) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, A.; Pimentel, P.A.B.; Horta, R.S. Checkpoint Inhibitors in Dogs: Are We There Yet? Cancers 2024, 16, 2003. https://doi.org/10.3390/cancers16112003
Giuliano A, Pimentel PAB, Horta RS. Checkpoint Inhibitors in Dogs: Are We There Yet? Cancers. 2024; 16(11):2003. https://doi.org/10.3390/cancers16112003
Chicago/Turabian StyleGiuliano, Antonio, Pedro A. B. Pimentel, and Rodrigo S. Horta. 2024. "Checkpoint Inhibitors in Dogs: Are We There Yet?" Cancers 16, no. 11: 2003. https://doi.org/10.3390/cancers16112003
APA StyleGiuliano, A., Pimentel, P. A. B., & Horta, R. S. (2024). Checkpoint Inhibitors in Dogs: Are We There Yet? Cancers, 16(11), 2003. https://doi.org/10.3390/cancers16112003






