Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC)
Abstract
Simple Summary
Abstract
1. Introduction
2. Combination of Immunotherapy with Other Therapies
2.1. Immunotherapy
2.2. Preclinical Stage Combination Immunotherapy
2.3. Clinical Stage Combination Immunotherapy
2.3.1. Pembrolizumab (Anti PD-1 Antibody) Combination
2.3.2. Atezolizumab (Anti-PD-L1 Antibody) Combination
2.3.3. Camrelizumab (Anti-PD-1 Antibody) Combination
2.3.4. Durvalumab (PD-L1 Antibody) Combination
2.3.5. Other Immunotherapeutic Combinations
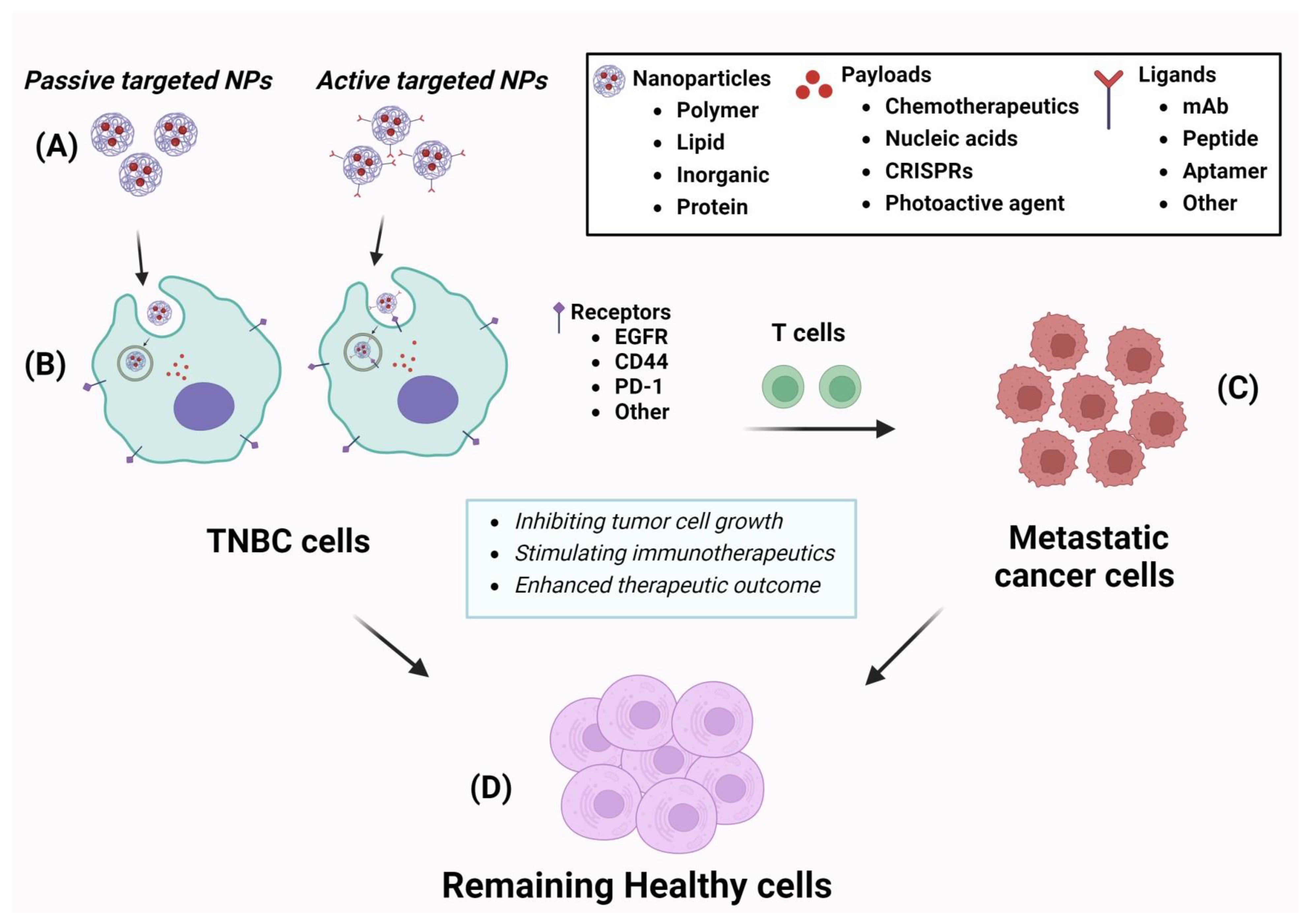
3. Nanotechnology-Based Therapies for TNBC
3.1. Polymer-Based Nanoparticles
3.2. Lipid-Based Nanoparticles
3.3. Inorganic Material-Based Nanoparticle
3.4. Peptide and Protein-Based Nanoparticle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209. [Google Scholar] [CrossRef] [PubMed]
- Schick, J.; Ritchie, R.P.; Restini, C. Breast Cancer Therapeutics and Biomarkers: Past, Present, and Future Approaches. Breast Cancer 2021, 15, 1178223421995854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernandez Hernandez, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy Treatment for Triple Negative Breast Cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Song, B.; Yi, M.; Yan, Y.; Mei, Q.; Wu, K. Recent advances in targeted strategies for triple-negative breast cancer. J. Hematol. Oncol. 2023, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Bhumika; Das, A. Combinatorial drug therapy in cancer—New insights. Life Sci. 2020, 258, 118134. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Immunotherapy in Triple-Negative Breast Cancer. Cancer J. 2021, 27, 59. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, K. Theranostics for Triple-Negative Breast Cancer. Diagnostics 2023, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, F.; Ahmed, A.; Rafiq, M.; Yu, B.; Cong, H.; Shen, Y. Immunotherapy: Cancer immunotherapy and its combination with nanomaterials and other therapies. J. Mater. Chem. B. 2023, 11, 8586. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S. Nanotechnology in medicine. Indian Heart J. 2016, 68, 437. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzi, N.; Kesharwani, P.; Pittala, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Xiong, W.; Yin, B.; Huang, Y.; Chu, J.; Xing, C.; Qian, C.; Du, Y.; Duan, T.; et al. Development of a TCR-like antibody and chimeric antigen receptor against NY-ESO-1/HLA-A2 for cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tokheim, C.; Gu, S.S.; Wang, B.; Tang, Q.; Li, Y.; Traugh, N.; Zeng, Z.; Zhang, Y.; Li, Z.; et al. In vivo CRISPR screens identify the E3 ligase Cop1 as a modulator of macrophage infiltration and cancer immunotherapy target. Cell 2021, 184, 5357. [Google Scholar] [CrossRef] [PubMed]
- Napier, T.S.; Lynch, S.E.; Lu, Y.; Song, P.N.; Burns, A.C.; Sorace, A.G. Molecular Imaging of Oxygenation Changes during Immunotherapy in Combination with Paclitaxel in Triple Negative Breast Cancer. Biomedicines 2023, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- McKnight, B.N.; Kim, S.; Boerner, J.L.; Viola, N.T. Cetuximab PET delineated changes in cellular distribution of EGFR upon dasatinib treatment in triple negative breast cancer. Breast Cancer Res. 2020, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bae, J.H.; Choe, E.J.; Park, J.M.; Park, S.S.; Cho, H.J.; Song, B.J.; Baek, M.C. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics 2022, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Manukian, G.; Kivolowitz, C.; DeAngelis, T.; Shastri, A.A.; Savage, J.E.; Camphausen, K.; Rodeck, U.; Zarif, J.C.; Simone, N.L. Caloric Restriction Impairs Regulatory T cells within the Tumor Microenvironment After Radiation and Primes Effector T cells. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1341. [Google Scholar] [CrossRef] [PubMed]
- Zanker, D.J.; Spurling, A.J.; Brockwell, N.K.; Owen, K.L.; Zakhour, J.M.; Robinson, T.; Duivenvoorden, H.M.; Hertzog, P.J.; Mullins, S.R.; Wilkinson, R.W.; et al. Intratumoral administration of the Toll-like receptor 7/8 agonist 3M-052 enhances interferon-driven tumor immunogenicity and suppresses metastatic spread in preclinical triple-negative breast cancer. Clin. Transl. Immunol. 2020, 9, e1177. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lee, N.; Pedroza, D.A.; Bado, I.L.; Hamor, C.; Zhang, L.; Aguirre, S.; Hu, J.; Shen, Y.; Xu, Y.; et al. Chemotherapy Coupled to Macrophage Inhibition Induces T-cell and B-cell Infiltration and Durable Regression in Triple-Negative Breast Cancer. Cancer Res. 2022, 82, 2281. [Google Scholar] [CrossRef] [PubMed]
- Stuber, T.; Monjezi, R.; Wallstabe, L.; Kuhnemundt, J.; Nietzer, S.L.; Dandekar, G.; Wockel, A.; Einsele, H.; Wischhusen, J.; Hudecek, M. Inhibition of TGF-beta-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J. Immunother. Cancer 2020, 8, e000676. [Google Scholar] [CrossRef]
- Tentler, J.J.; Lang, J.; Capasso, A.; Kim, D.J.; Benaim, E.; Lee, Y.B.; Eisen, A.; Bagby, S.M.; Hartman, S.J.; Yacob, B.W.; et al. RX-5902, a novel beta-catenin modulator, potentiates the efficacy of immune checkpoint inhibitors in preclinical models of triple-negative breast Cancer. BMC Cancer 2020, 20, 1063. [Google Scholar] [CrossRef]
- Wu, F.T.H.; Xu, P.; Chow, A.; Man, S.; Kruger, J.; Khan, K.A.; Paez-Ribes, M.; Pham, E.; Kerbel, R.S. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br. J. Cancer 2019, 120, 196. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Woodcock, M.G.; Van Swearingen, A.E.D.; Moore, D.T.; Sambade, M.J.; Laurie, S.; Robeson, A.; Kolupaev, O.; Cuaboy, L.A.; Garrett, A.L.; et al. Evaluating the efficacy of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer. J. Immunother. Cancer 2022, 10, e003427. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Inoue, K.; Kaneko, K.; Ito, Y.; Tsugawa, K.; Hasegawa, A.; Nakagawa, S.; Kuratomi, H.; Tamura, K. Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn. J. Clin. Oncol. 2019, 49, 1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Li, Y.; Li, Q.; Su, F.; Yao, H.; Su, S.; Wang, Q.; Jin, L.; Wang, Y.; et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J. Immunother. Cancer 2020, 8, e000696. [Google Scholar] [CrossRef] [PubMed]
- Foldi, J.; Kahn, A.; Silber, A.; Qing, T.; Reisenbichler, E.; Fischbach, N.; Persico, J.; Adelson, K.; Katoch, A.; Chagpar, A.; et al. Clinical Outcomes and Immune Markers by Race in a Phase I/II Clinical Trial of Durvalumab Concomitant with Neoadjuvant Chemotherapy in Early-Stage TNBC. Clin. Cancer Res. 2022, 28, 3720. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Canc. Netw. 2020, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511. [Google Scholar] [CrossRef]
- Zeng, G.; Touloukian, C.E.; Wang, X.; Restifo, N.P.; Rosenberg, S.A.; Wang, R.F. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. J. Immunol. 2000, 165, 1153. [Google Scholar] [CrossRef]
- Zeng, G.; Li, Y.; El-Gamil, M.; Sidney, J.; Sette, A.; Wang, R.F.; Rosenberg, S.A.; Robbins, P.F. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: A new strategy for vaccine design. Cancer Res. 2002, 62, 3630. [Google Scholar]
- He, J.; McLaughlin, R.P.; van der Noord, V.; Foekens, J.A.; Martens, J.W.M.; van Westen, G.; Zhang, Y.; van de Water, B. Multi-targeted kinase inhibition alleviates mTOR inhibitor resistance in triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 178, 263. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Michel, L.L.; Mobus, V.; Tesch, H.; Klare, P.; Hahnen, E.; Denkert, C.; Kast, K.; Pohl-Rescigno, E.; Hanusch, C.; et al. Survival analysis of the randomised phase III GeparOcto trial comparing neoadjuvant chemotherapy of intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for patients with high-risk early breast cancer. Eur. J. Cancer 2022, 160, 100. [Google Scholar]
- Criscitiello, C.; Marra, A.; Morganti, S.; Zagami, P.; Gandini, S.; Esposito, A.; Curigliano, G. Clinical outcomes of patients with metastatic breast cancer enrolled in phase I clinical trials. Eur. J. Cancer 2021, 157, 40. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Raman, S.S.; Chan, A.; Kalinsky, K.; Baurain, J.F.; Jimenez, M.M.; Garcia, M.M.; Berger, M.D.; Lauer, U.M.; Khattak, A.; et al. Phase Ib study of talimogene laherparepvec in combination with atezolizumab in patients with triple negative breast cancer and colorectal cancer with liver metastases. ESMO Open 2023, 8, 100884. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tian, Z.; Lin, Y.; Li, H.; Zhu, Z.; Liu, Q.; Su, S.; Zeng, Y.; Jia, W.; et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat. Commun. 2022, 13, 3011. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, B.; Tong, Z.; Ouyang, Q.; Wang, Y.; Xu, G.; Li, S.; Li, H. A phase Ib study of camrelizumab in combination with apatinib and fuzuloparib in patients with recurrent or metastatic triple-negative breast cancer. BMC Med. 2022, 20, 321. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xu, Y.; Chen, L.; Fan, L.; Ma, X.Y.; Zhao, S.; Song, X.Q.; Hu, X.; Yang, W.T.; Chai, W.J.; et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: Concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol. Cancer 2022, 21, 84. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.S.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712. [Google Scholar] [CrossRef]
- Chick, R.C.; Clifton, G.T.; Hale, D.F.; Vreeland, T.J.; Hickerson, A.T.; Kemp Bohan, P.M.; McCarthy, P.M.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; et al. Subgroup analysis of nelipepimut-S plus GM-CSF combined with trastuzumab versus trastuzumab alone to prevent recurrences in patients with high-risk, HER2 low-expressing breast cancer. Clin. Immunol. 2021, 225, 108679. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.F.; Bakri, H.M.; Abdelfattah, O.N.; Eid, S. Does bevacizumab carry a hope for metastatic triple-negative breast cancer in the era of immunotherapy? Anti-Cancer Drug 2022, 33, E604. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Tan, A.R.; Rugo, H.S.; Aftimos, P.; Andric, Z.; Beelen, A.; Zhang, J.; Yi, J.S.; Malik, R.; O’Shaughnessy, J. Trilaciclib prior to gemcitabine plus carboplatin for metastatic triple-negative breast cancer: Phase III PRESERVE 2. Future Oncol. 2022, 18, 3701. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, J.; Zhang, J.; Yan, Y.; Jiang, N.; Yu, J.; Di, L.; Song, G.; Che, L.; Jia, J.; et al. Prospective study of cyclophosphamide, thiotepa, carboplatin combined with adoptive DC-CIK followed by metronomic cyclophosphamide therapy as salvage treatment for triple negative metastatic breast cancers patients (aged < 45). Clin. Transl. Oncol. 2016, 18, 82. [Google Scholar] [PubMed]
- Jiang, Y.Z.; Liu, Y.; Xiao, Y.; Hu, X.; Jiang, L.; Zuo, W.J.; Ma, D.; Ding, J.; Zhu, X.; Zou, J.; et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: The FUTURE trial. Cell Res. 2021, 31, 178. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.E.; Adler, B.L.; Makdisi, J.; Schairer, D.; Rosen, J.; Landriscina, A.; Navati, M.; Alfieri, A.; Friedman, J.M.; Nosanchuk, J.D.; et al. Nanoparticle-Encapsulated Doxorubicin Demonstrates Superior Tumor Cell Kill in Triple Negative Breast Cancer Subtypes Intrinsically Resistant to Doxorubicin. Precis. Nanomed. 2018, 1, 173. [Google Scholar] [PubMed]
- Li, Y.; Liu, L.; Shang, H.; Feng, X.; Fan, N.; Wang, J.; Wu, Y.; Chen, Y.; Chu, X.; Zhong, M.; et al. Self-Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment. Pharmaceutics 2022, 14, 2461. [Google Scholar] [CrossRef]
- Sulaiman, A.; McGarry, S.; El-Sahli, S.; Li, L.; Chambers, J.; Phan, A.; Cote, M.; Cron, G.O.; Alain, T.; Le, Y.; et al. Co-targeting Bulk Tumor and CSCs in Clinically Translatable TNBC Patient-Derived Xenografts via Combination Nanotherapy. Mol. Cancer Ther. 2019, 18, 1755. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, H.; Yu, J.; Wang, L.; Sun, Y.; Zhang, J.; Duan, Y. Asynchronous blockade of PD-L1 and CD155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials 2021, 275, 120988. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, D.M.; Day, E.S. Dual Regulation of miR-34a and Notch Signaling in Triple-Negative Breast Cancer by Antibody/miRNA Nanocarriers. Mol. Ther. Nucleic Acids 2020, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, D.M.; Dang, M.N.; Day, E.S. IR820-loaded PLGA nanoparticles for photothermal therapy of triple-negative breast cancer. J. Biomed. Mater. Res. A 2019, 107, 1702. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, D.M.; Dang, M.N.; Scully, M.A.; Day, E.S. Nanoparticle-Mediated Co-Delivery of Notch-1 Antibodies and ABT-737 as a Potent Treatment Strategy for Triple-Negative Breast Cancer. ACS Nano 2020, 14, 3378. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Pittala, V.; Haider, M.; Greish, K. Enhanced Anticancer Activity of Nanoformulation of Dasatinib against Triple-Negative Breast Cancer. J. Pers. Med. 2021, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Nabil, G.; Alzhrani, R.; Alsaab, H.O.; Atef, M.; Sau, S.; Iyer, A.K.; Banna, H.E. CD44 Targeted Nanomaterials for Treatment of Triple-Negative Breast Cancer. Cancers 2021, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, I.M.; Pittala, V.; Eltayeb, D.; Greish, K. Selective Targeting of Breast Cancer by Tafuramycin A Using SMA-Nanoassemblies. Molecules 2021, 26, 3532. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Lu, H.; Wang, S. Photo-responsive prodrug nanoparticles for efficient cytoplasmic delivery and synergistic photodynamic-chemotherapy of metastatic triple-negative breast cancer. Acta Biomater. 2021, 126, 421. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019, 14, 388. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Li, K.K.; Zang, X.L.; Xie, Y.; Song, J.X.; Chen, X.H. ROS-responsive Galactosylated-nanoparticles with Doxorubicin Entrapment for Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2023, 18, 1381. [Google Scholar] [CrossRef]
- Greish, K.; Mathur, A.; Al Zahrani, R.; Elkaissi, S.; Al Jishi, M.; Nazzal, O.; Taha, S.; Pittala, V.; Taurin, S. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018, 291, 184. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Tortorella, S.; d’Argenio, A.; Carbone, C.; Camorani, S.; Locatelli, E.; Auletta, L.; Sorrentino, D.; Fedele, M.; Zannetti, A.; et al. Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J. Exp. Clin. Cancer Res. 2021, 40, 239. [Google Scholar] [CrossRef] [PubMed]
- Babu Varukattu, N.; Lin, W.; Vivek, R.; Rejeeth, C.; Sabarathinam, S.; Yao, Z.; Zhang, H. Targeted and Intrinsic Activity of HA-Functionalized PEI-Nanoceria as a Nano Reactor in Potential Triple-Negative Breast Cancer Treatment. ACS Appl. Bio Mater. 2020, 3, 186. [Google Scholar] [CrossRef] [PubMed]
- Eskiler, G.G.; Cecener, G.; Egeli, U.; Tunca, B. Talazoparib nanoparticles for overcoming multidrug resistance in triple-negative breast cancer. J. Cell Physiol. 2020, 235, 6230. [Google Scholar] [CrossRef] [PubMed]
- Mokhlis, H.A.; Bayraktar, R.; Kabil, N.N.; Caner, A.; Kahraman, N.; Rodriguez-Aguayo, C.; Zambalde, E.P.; Sheng, J.; Karagoz, K.; Kanlikilicer, P.; et al. The Modulatory Role of MicroRNA-873 in the Progression of KRAS-Driven Cancers. Mol. Ther. Nucleic Acids 2019, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. Proc. Natl. Acad. Sci. USA 2019, 116, 18295. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, D.; Lee, S.K.; Yomtoubian, S.; Han, M.S.; Tung, C.H.; Mittal, V. Nanoparticle Delivery of miR-708 Mimetic Impairs Breast Cancer Metastasis. Mol. Cancer Ther. 2019, 18, 579. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Korangath, P.; Mulka, K.; Helenius, M.; Coulter, J.B.; Stewart, J.; Velarde, E.; Crezee, J.; Simons, B.; Stalpers, L.J.A.; et al. Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int. J. Hyperth. 2019, 36, 47. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, B.; Long, Y.; Wang, Z.; Tong, C.; Wang, W.; You, P.; Liu, X. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020, 113, 554. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Lu, L.; Jiang, H.; Wang, F.; Zhang, X. Transcytosable Peptide-Paclitaxel Prodrug Nanoparticle for Targeted Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4646. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Sun, H.; Deng, S.; Yue, L.; Weng, Z.; Yang, J.; Zuo, B.; He, Y.; Zhang, B. (cRGD)2 peptides modified nanoparticles increase tumor-targeting therapeutic effects by co-delivery of albendazole and iodine-131. Anticancer Drugs 2022, 33, 19. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Gu, L.; Wu, K.; Xing, H. Nanoparticles for Chemoimmunotherapy Against Triple-Negative Breast Cancer. Int. J. Nanomed. 2022, 17, 5209. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.; Liu, S.; Liu, F.; Zhou, F. Immunoadjuvant Nanoparticles as Trojan Horses for Enhanced Photo-Immunotherapy in the Treatment of Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 883428. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gouw, A.M.; LaGory, E.L.; Guo, S.; Attarwala, N.; Tang, Y.; Qi, J.; Chen, Y.S.; Gao, Z.; Casey, K.M.; et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol. 2021, 39, 357. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Zhang, M.; Bao, S.; Qiu, G.; Liang, J.; Wang, Q.; Zhu, X.; Qin, G.; Liu, J.; Zhao, C. An Magnetic-Targeting Nano-Diagnosis and Treatment Platform for TNBC. Breast Cancer 2023, 15, 101. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, Z.; Tian, Y.; Zhuang, M.; Piao, S.; Gao, Y.; Tam, A.; Hu, H.; Cheng, W. A Comprehensive Evaluation of ZrC Nanoparticle in Combined Photothermal and Radiation Therapy for Treatment of Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 801352. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. nab-Paclitaxel mechanisms of action and delivery. J. Control. Release 2013, 170, 365. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Pyankov, I.; Maretina, M.; Baranov, V.; Kiselev, A. Peptide Nanoparticle-Mediated Combinatorial Delivery of Cancer-Related siRNAs for Synergistic Anti-Proliferative Activity in Triple Negative Breast Cancer Cells. Pharmaceuticals 2021, 14, 957. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin Nanoparticle of Paclitaxel (Abraxane) Decreases while Taxol Increases Breast Cancer Stem Cells in Treatment of Triple Negative Breast Cancer. Mol. Pharm. 2020, 17, 2275. [Google Scholar] [CrossRef]

| Comb Therapeutics | Cell Line and Model | Stage | Results | Molecular Target | Ref. |
|---|---|---|---|---|---|
| Monoclonal antibody 2D2 and TCR-like CAR-T cell | HEK293T, MDA-MB231-ESO1, PC3-A2-ESO1T2, Mel586, Mel624, Mel1558 cells; MDA-MD231-N4-ESO-1 | In vitro and in vivo | TCR-like antibody derived CAR-T cells were able to inhibit tumor cell growth and overall survival of mouse. | HLA-A2, NY-ESO-1 | [21] |
| Anti-PD1 antibody and CRISPR knockout | 4T1-tumor bearing mice model | In vitro and in vivo | In vivo CRISPR knockout enhanced antitumor immunity and strengthened immune checkpoint blockade | E3 ubiquitin ligase Cop1 | [22] |
| ICIs and PTX | 4T1, EO771; 4T1 and E0771 tumor-bearing mice model | In vitro and in vivo | The combination treatment reduced tumor growth. | PD-1 and CTLA-4 | [23] |
| Dasatinib/radiotracer-attached cetuximab | MDA-MB231 cell and MDA-MB468 PDX tumor model | In vitro and in vivo | The results showed that combination of radiolabeled antibody and dasatinib was able to monitor drug distribution and treatment response in KRAS TNBC. | EGFR | [24] |
| Macitentan/anti-PD-1 antibody | MDA-MB231, 4T1, CT26 and LL/2, EMT6; MDA-MB231, 4T1, and EMT6 tumor-bearing mice | In vitro and in vivo | The combination of MAC and anti-PD-1 antibody showed strong antitumor effect against TNBC, colon and lung cancer. | PD-1, CD8+T endothelin receptor | [25] |
| Radiotherapy/caloric restriction ad libitum diet | 4T1-tumor bearing mice model | In vitro and in vivo | The results revealed that the combination RT and CR enhanced immunotherapy effect against TNBC. | CD+8T cell, TME | [26] |
| 3M-O52 and anti PD1 antibody | E0771, CAL-120 and MDA-MB-231cells; 4T1.2 or E0771 bearing mice | In vitro and in vivo | The results showed the combination treatment reduced tumor growth and metastatic spread to lung | IFN, TME, PD1, Toll-like receptor 7/8 | [27] |
| Cyclophosphamide (Cytoxan) and CSF1R inhibitor or an anti-CSF1R antibody | T11, T12 cell lines; T11, T12 and 215/R tumor-bearing mice model | In vitro and in vivo | The results illustrated the complexity of the tumor immune microenvironment and highlight different immune responses that result from rational immunotherapy combinations. | CSF1R | [28] |
| CAR-T cell and TGF-B inhibitor SA-208 | MDA-MB-231 | In vitro and in vivo | The results showed the combinatorial treatment of CAR-T cell and TGF-B receptor blockade was able to suppress tumor growth. | ROR1, TGF-B receptor | [29] |
| RX-5902 and PD-1 or CTLA-4 combination | 4T1 and MDA-MB231 TNBC tumor bearing mice model | In vitro and in vivo | The combination treatment decreased tumor growth and increased activated T cells | CTLA-4/PD-1 | [30] |
| Anti-PD-L1 antibody and sunitinib/Paclitaxel | EMT-6/P, EMT-6/CDDP, REN CA, | In vitro and in vivo | In the EMT-6/CDDP model, combination of anti-PD-L1 with paclitaxel chemotherapy (with or without anti-VEGF) was most effective as a neoadjuvant therapy in breast cancer. | PD-L1/VEGF/VEGFR2 | [31] |
| Pembrolizumab and radiotherapy | 17 patients with TNBC | Phase II trial | Neutral efficacy but encocering? clinical activity | PD-L1 | [32] |
| Pembrolizumab and cyclophosphamide (antineoplastic agent) | 40 patients with TNBC | Phase II | Low outcome for TNBC patients | [33] | |
| Aterolizumab and nabpaclitaxel | 902 patients with TNBC | Phase III | Consistent with the overall IM passion 130 population | PD-L1 | [34] |
| Camrelizumab and Apatinib | 40 patients | Phase II | Objective response rate was much higher than monotherapy | PD-L1 | [35] |
| Durvalumab and nab-paclitaxel, DOX, and Cyclophosphamide | 67 patients with early stage TNBC | Phase I/II | The combination treatment improved survival rate of the patients. | PD-L1 | [36] |
| Nanoparticle | Drug | Cell Line and Model | Stage | Results | Targeting Moiety and Receptor | Ref |
|---|---|---|---|---|---|---|
| Sol-gel polymer nanoparticle | DOX | SUM149PT, HS578T, MDA-MB157 | In vitro and in vivo | DOX-NPs showed higher cell killing activity in comparison to free DOX. | EPR | [58] |
| HA-coated chitosan NPs | Curcumin | 4T1 cell line, 4T1 tumor-bearing mice | In vitro and in vivo | It exhibited higher antitumor efficacy in TNBC-tumor model. | CD44 receptor | [59] |
| Lipid-polymer hybrid nanoparticle | PTX and verterporfin | MDA-MB231 cell line, HCl-002 PDX TNBC mice model | In vitro and in vivo | As compared free drugs, the NPs showed significant suppression for tumor growth. | NFkB, Wnt and VAP pathways, cancer stem cells | [60] |
| mPEG-PLGA-PLL NPs | siRNA CD155 | 4T1 cell line, 4T1 –orthotopic tumor model | In vitro and in vivo | The NPs improved early stage CD8+T cell immunosurveillance. | PD-L1 and CD155 receptor | [61] |
| PLGA Nps | miRNA | MDA-MB231 TNBC cell, MCF-10A normal cells | In vitro and in vivo | The NPs was able to impair TNBC cells | Notch-1 signal, miR-34a downstream | [62] |
| PLGA NPs | IR820 dye | MDA-MB231 cell, Tumor-bearing mice | In vitro and in vivo | The NPs significantly reduced TNBC tumor growth. | EPR | [63] |
| PLGA NPs | ABT-737 (BcL2 inhibitor) | MDA-MB231 cell line, MCF-10A normal cell | In vitro and in vivo | The NPs exhibited high tumor accumulation and strong inhibition of tumor growth in TNBC tumor model. | Notch—1 signal targeting | [64] |
| SMA polymer NPs | Dasatinib (TKI) | MDA-MB231, MCF7, and 4T1 cells; 4T1-bearing tumor model | In vitro and in vivo | The NPs showed 7-fold higher tumor suppression effect than free drug in tumor-bearing mice model. | EPR, ABL kinase Src (Kin2) receptor TKI | [65] |
| TPGS-SMA polymer NPs | CFM-4.16/momelotinib | MDA-MB-231, MDA-MB468; MDA-MB231-bearing mice model | In vitro and in vivo | The NP revealed strong targeting ability to CD44 expressing cell | CD44 receptor | [66] |
| SMA polymer micelle | Taluzamycin-A | MDA-MB-231, MDA-MB468, MCF-7 cells; 4T1-tumor bearing mice | In vitro and in vivo | The NPs were taken up by tumor tissue 4-times greater than free drug. | EPR | [67] |
| Polymer NPs | AL/camptothecin | 4T1-cell line 4T1-tumor bearing mice model | In vitro and in vivo | The combinatorial drug-loaded NPs showed tumor suppression effect against metastatic TNBC. | EPR, light sensitive delivery | [68] |
| PLGA polymer and lipid hybrid NPs | siRNA | MDA-MB453, MDA-MB231 cells; MDA-MB453 and MDA-MB231 –bearing mice model | In vitro and in vivo | The results showed that the NPs inhibited POLR2A and significantly reduced POLR2A-positive tumor growth | POLR2A | [69] |
| P-glactose—polymethacrylate NPs | DOX | Human MDA-MB231, 4T1, HUVEC cells; 4T1 tumor-bearing mice | In vitro and in vivo | DOX-loaded NPs revealed higher cellular uptake and tumor accumulation as well as tumor suppression effect | Glactose, EPR | [70] |
| SMA-WIN polymer NPs | DOX and canabinoid | MDA-MB231, 4T1, MCF7; 4T1-bearing mice | In vitro and in vivo | The dual drug-loaded NPs significantly reduced tumor growth as comparison in free drugs. | EPR | [71] |
| PLGA NPs | Cisplatin | MDA-MB231, BT-549, and MDA-231-EGFR-KO cells; MDA-M231 and MDA-MB231-KO-bearing mice | In vitro and in vivo | The cisplatin-PLGA Nps revealed strong tumor suppression efficacy in TNBC mice model | EGFR | [72] |
| HA-CePEI NPs | Cerium oxide (Ceria) | MDA-MB231, and HBL-100 cells | In vitro and in vivo | The NPs showed a strong apoptotic effect in TNBC cells due to its ROS generation and targeting ability | CD44 | [73] |
| SLNPs | PARP inhibitor talaroparib | HCC1937, MCF10A, and HCC1937-RC cells | In vitro and in vivo | The NPs were able to reduce MDR1, BCRP, and MRP1 gene expression, leading efficient therapeutic activity. | EPR | [74] |
| LNPs | microRNA (miR-878) | MCF10A, MDA-MB436, MDA-MB231, MDA-MB453, BT-20, HCC1937, SKBR3, T47D, HEK293 noraml HPDA, PANC1, BxPC3, MiaPaCa-2, Capan-2 | In vitro and in vivo | The NPs inhibited tumor growth in PDAC and TNBC tu tumors by suppressing cell proliferation and inducing apoptosis | EPR | [75] |
| Lipogel tNLGs | 3CRISPR plasmid | MDA-MB231, MDA-MB436, MCF10A; MDA-MB231-tumor-beairng mice | In vitro and in vivo | The NPs suppressed the expression of LCN2 oncogene and inhibited minimal host toxicity | ICAM1 | [76] |
| LbL-coated Gold NPs | miRNA (miR-708) | MDA-MB231, 293T, MDA-MB231-LM2 cells; 4T1-tumor-bearing mice | In vitro and in vivo | miRNA-gold NPs exhibited minimal host toxicity | EPR | [77] |
| Magnetic iron oxide NPs | Immune check point inhibitor | 4T1 cell line and 4T1-tumor bearing mice | In vitro and in vivo | The MIO NPs reduced tumor growth in TNBC tumor model | EPR, PD-1, CTLA-4 | [78] |
| Graphene oxide Qdot NPs | Gamma bufotacin and DOX | MDA-MB231, BGC-823, Hela, NIH-3T3 RAW264.7 | In vitro and in vivo | The dual drug-loaded NPs were taken up 2-fold higher by tumor cells in comparison with naked one and reduced lung metastasis. | TAT, RGD | [79] |
| Peptide-drug conjugate NPs | PTX | 4T1-mcherry-luc cell; 4T1-tumor bearing mice | In vitro and in vivo | The NPs strongly inhibited tumor growth | NRP1 (Neuropilin 1) | [80] |
| RGD-HAS NPs | Aldendarole/iodine-131 | MDA-MB231; 4T1-cells | In vitro and in vivo | RGA-coupled NPs were able to penetrate into tumor and inhibit tumor growth | cRGD and integrin | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battogtokh, G.; Obidiro, O.; Akala, E.O. Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC). Cancers 2024, 16, 2012. https://doi.org/10.3390/cancers16112012
Battogtokh G, Obidiro O, Akala EO. Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC). Cancers. 2024; 16(11):2012. https://doi.org/10.3390/cancers16112012
Chicago/Turabian StyleBattogtokh, Gantumur, Onyinyechi Obidiro, and Emmanuel O. Akala. 2024. "Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC)" Cancers 16, no. 11: 2012. https://doi.org/10.3390/cancers16112012
APA StyleBattogtokh, G., Obidiro, O., & Akala, E. O. (2024). Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC). Cancers, 16(11), 2012. https://doi.org/10.3390/cancers16112012






