Chronic Stress Related to Cancer Incidence, including the Role of Metabolic Syndrome Components
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
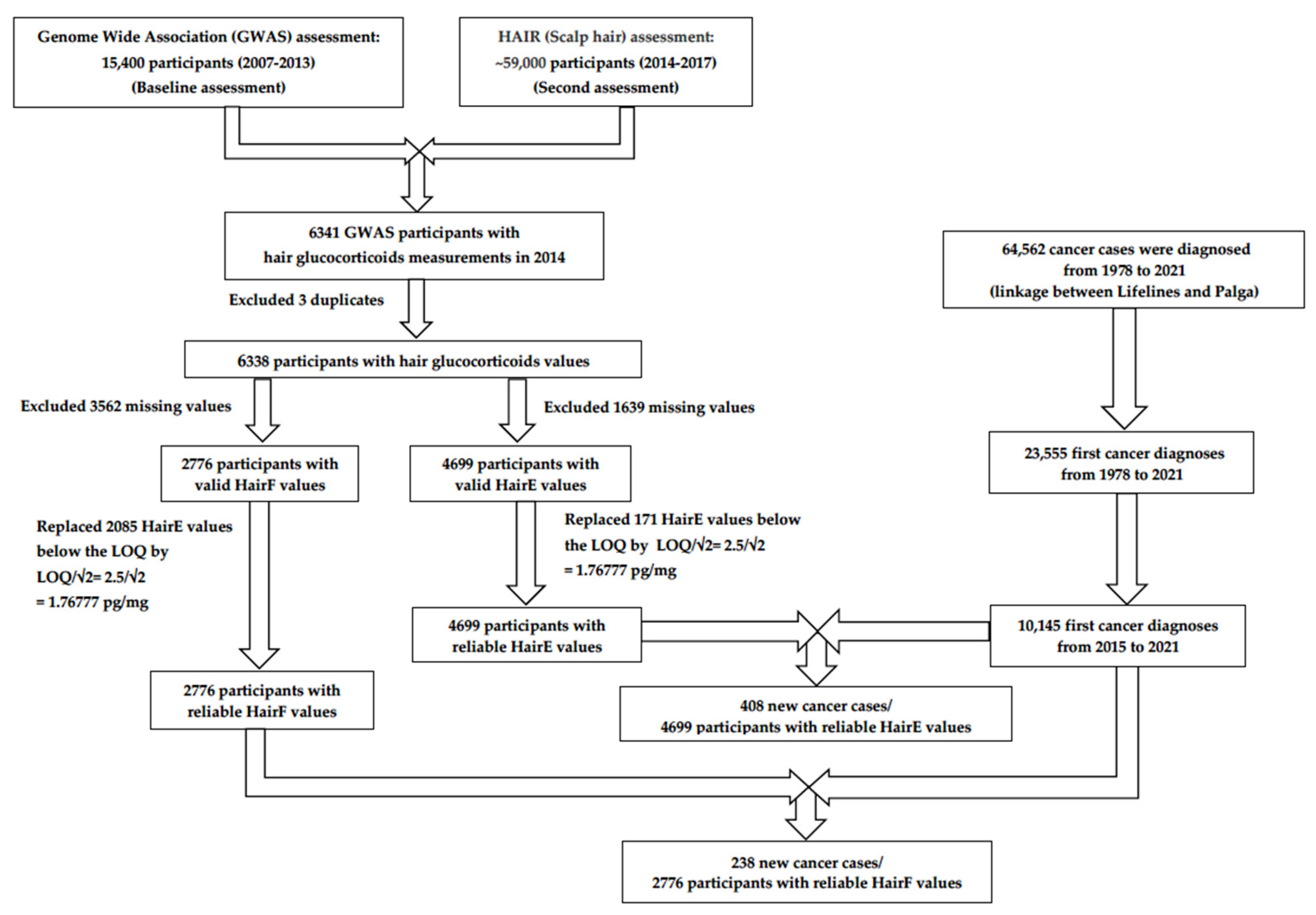
2.1. Study Design
2.2. Study Population
2.3. Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 January 2022).
- Abate, M.; Citro, M.; Caputo, M.; Pisanti, S.; Martinelli, R. Psychological Stress and Cancer: New Evidence of An Increasingly Strong Link. Transl. Med. UniSa 2020, 23, 53–57. [Google Scholar] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Hong, H.; Ji, M.; Lai, D. Chronic Stress Effects on Tumor: Pathway and Mechanism. Front. Oncol. 2021, 11, 738252. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liu, L.; Zhang, C.; Zheng, T.; Wang, J.; Lin, M.; Zhao, Y.; Wang, X.; Levine, A.J.; Hu, W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 7013–7018. [Google Scholar] [CrossRef]
- Ayroldi, E.; Cannarile, L.; Adorisio, S.; Delfino, D.V.; Riccardi, C. Role of Endogenous Glucocorticoids in Cancer in the Elderly. Int. J. Mol. Sci. 2018, 19, 3774. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Aromatase and breast cancer. Front. Biosci. 1998, 3, d922–d933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, X.; Yuan, J.; Sun, Z.; Li, C.; Li, R.; Li, L.; Zhu, C.; Wan, R.; Guo, R.; et al. The role of corticotropin-releasing hormone receptor 1 in the development of colitis-associated cancer in mouse model. Endocr. Relat. Cancer 2014, 21, 639–651. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxid. Med. Cell Longev. 2019, 2019, 1270397. [Google Scholar] [CrossRef] [PubMed]
- Bahri, N.; Fathi Najafi, T.; Homaei Shandiz, F.; Tohidinik, H.R.; Khajavi, A. The relation between stressful life events and breast cancer: A systematic review and meta-analysis of cohort studies. Breast Cancer Res. Treat. 2019, 176, 53–61. [Google Scholar] [CrossRef]
- Song, H.; Saito, E.; Sawada, N.; Abe, S.K.; Hidaka, A.; Shimazu, T.; Yamaji, T.; Goto, A.; Iwasaki, M.; Sasazuki, S.; et al. Perceived stress level and risk of cancer incidence in a Japanese population: The Japan Public Health Center (JPHC)-based Prospective Study. Sci. Rep. 2017, 7, 12964. [Google Scholar] [CrossRef]
- Lillberg, K.; Verkasalo, P.K.; Kaprio, J.; Teppo, L.; Helenius, H.; Koskenvuo, M. Stressful life events and risk of breast cancer in 10,808 women: A cohort study. Am. J. Epidemiol. 2003, 157, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, K.; Nyberg, S.T.; Theorell, T.; Fransson, E.I.; Alfredsson, L.; Bjorner, J.B.; Bonenfant, S.; Borritz, M.; Bouillon, K.; Burr, H.; et al. Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ 2013, 346, f165. [Google Scholar] [CrossRef] [PubMed]
- Butow, P.; Price, M.; Coll, J.; Tucker, K.; Meiser, B.; Milne, R.; Wilson, J.; Heiniger, L.; Baylock, B.; Bullen, T.; et al. Does stress increase risk of breast cancer? A 15-year prospective study. Psychooncology 2018, 27, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.R.; Zhang, Z.F.; Kristensen, T.S.; Netterstrøm, B.; Schnohr, P.; Grønbaek, M. Self reported stress and risk of breast cancer: Prospective cohort study. BMJ 2005, 331, 548. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Wester, V.L.; van Rossum, E.F. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef] [PubMed]
- Manenschijn, L.; Koper, J.W.; Lamberts, S.W.; van Rossum, E.F. Evaluation of a method to measure long term cortisol levels. Steroids 2011, 76, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, L.K.; Hinkelmann, K.; Muhtz, C.; Dettenborn, L.; Wingenfeld, K.; Spitzer, C.; Kirschbaum, C.; Wiedemann, K.; Otte, C. Hair cortisol and cortisol awakening response are associated with criteria of the metabolic syndrome in opposite directions. Psychoneuroendocrinology 2015, 51, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Staufenbiel, S.M.; Penninx, B.W.; de Rijke, Y.B.; van den Akker, E.L.; van Rossum, E.F. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology 2015, 60, 182–194. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in hair and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef]
- Mirzaian, M.; van Zundert, S.K.M.; Schilleman, W.F.; Mohseni, M.; Kuckuck, S.; van Rossum, E.F.C.; van Schaik, R.H.N.; van den Berg, S.A.A. Determination of cortisone and cortisol in human scalp hair using an improved LC-MS/MS-based method. Clin. Chem. Lab. Med. 2024, 62, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Vanaelst, B.; Michels, N.; De Vriendt, T.; Huybrechts, I.; Vyncke, K.; Sioen, I.; Bammann, K.; Rivet, N.; Raul, J.S.; Molnar, D.; et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur. J. Pediatr. 2013, 172, 843–846. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tian, Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control 2015, 26, 257–268. [Google Scholar] [CrossRef]
- Radišauskas, R.; Kuzmickienė, I.; Milinavičienė, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8565. [Google Scholar] [CrossRef]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.; Dotinga, A.; Vonk, J.M.; van Dijk, F.; van Zon, S.K.; Wijmenga, C.; Wolffenbuttel, B.H.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef]
- Verjans, R.; Bruggink, A.H.; Kibbelaar, R.; Bart, J.; Debernardi, A.; Schaaij-Visser, T.B.M.; Willems, S.M.; Van Kemenade, F.J. The Dutch National TissueArchive Portal enables efficient, consistent, and transparent procurement of diagnostic tissue samples for scientific use. Cell Tissue Bank. 2021, 22, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Casparie, M.; Tiebosch, A.T.; Burger, G.; Blauwgeers, H.; van de Pol, A.; van Krieken, J.H.; Meijer, G.A. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2019/en#/ (accessed on 11 March 2023).
- Cortés-Ibáñez, F.O.; van Pinxteren, B.; Sijtsma, A.; Bruggink, A.; Sidorenkov, G.; van der Vegt, B.; de Bock, G.H. The validity of self-reported cancer in a population-based cohort compared to that in formally registered sources. Cancer Epidemiol. 2022, 81, 102268. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ibáñez, F.O.; Jaramillo-Calle, D.A.; Vinke, P.C.; Byambasukh, O.; Corpeleijn, E.; Sijtsma, A.; Eulenburg, C.; Vonk, J.M.; de Bock, G.H. Comparison of health behaviours between cancer survivors and the general population: A cross-sectional analysis of the Lifelines cohort. J. Cancer Surviv. Res. Pract. 2020, 14, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Noppe, G.; de Rijke, Y.B.; Dorst, K.; van den Akker, E.L.; van Rossum, E.F. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin. Endocrinol. 2015, 83, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Odijk, R.; Berg, S.; Dorst, K.; Rijke, Y. An optimized LC-MS/MS method for cortisol and cortisone in human hair. Psychoneuroendocrinology 2019, 107, 22–23. [Google Scholar] [CrossRef]
- Harel, O.; Perkins, N.; Schisterman, E.F. The Use of Multiple Imputation for Data Subject to Limits of Detection. Sri Lankan J. Appl. Stat. 2014, 5, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 21 February 2022).
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Faruque, M.O.; Vonk, J.M.; Bültmann, U.; Boezen, H.M. Airborne occupational exposures and inflammatory biomarkers in the Lifelines cohort study. Occup. Environ. Med. 2021, 78, 82. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Klijs, B.; Scholtens, S.; Mandemakers, J.J.; Snieder, H.; Stolk, R.P.; Smidt, N. Representativeness of the LifeLines Cohort Study. PLoS ONE 2015, 10, e0137203. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.E.; Lopez-Duran, N.L.; Sen, S.; Abelson, J.L. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology 2018, 92, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.A.; Guinn, V. Cumulative cortisol exposure increases during the academic term: Links to performance-related and social-evaluative stressors. Psychoneuroendocrinology 2020, 114, 104584. [Google Scholar] [CrossRef] [PubMed]
- Benz, E.J., Jr. The Jeremiah Metzger Lecture Cancer in the Twenty-First Century: An Inside View from an Outsider. Trans. Am. Clin. Clim. Assoc. 2017, 128, 275–297. [Google Scholar]
- Davison, B.; Singh, G.R.; McFarlane, J. Hair cortisol and cortisone as markers of stress in Indigenous and non-Indigenous young adults. Stress. 2019, 22, 210–220. [Google Scholar] [CrossRef]
- Lupien, S.; Lecours, A.R.; Lussier, I.; Schwartz, G.; Nair, N.P.; Meaney, M.J. Basal cortisol levels and cognitive deficits in human aging. J. Neurosci. 1994, 14 Pt 1, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Stephens, M.A.; Mahon, P.B.; McCaul, M.E.; Wand, G.S. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 2016, 66, 47–55. [Google Scholar]
- Uhart, M.; Chong, R.Y.; Oswald, L.; Lin, P.-I.; Wand, G.S. Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology 2006, 31, 642–652. [Google Scholar]
- Feller, S.; Vigl, M.; Bergmann, M.M.; Boeing, H.; Kirschbaum, C.; Stalder, T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology 2014, 39, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Dettenborn, L.; Tietze, A.; Kirschbaum, C.; Stalder, T. The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress 2012, 15, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Manenschijn, L.; Schaap, L.; Van Schoor, N.M.; van der Pas, S.; Peeters, G.M.; Lips, P.T.; Koper, J.W.; Van Rossum, E.F. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J. Clin. Endocrinol. Metab. 2013, 98, 2078–2083. [Google Scholar] [CrossRef]
- Thomson, S.; Koren, G.; Fraser, L.A.; Rieder, M.; Friedman, T.C.; Van Uum, S.H. Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exp. Clin. Endocrinol. Diabetes 2010, 118, 133–138. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, K.M.; Park, J.T.; Seo, E.; An, S.K.; Park, H.Y.; Lee, E. Sex-specific association of hair cortisol concentration with stress-related psychological factors in healthy young adults. Biol. Sex. Differ. 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.Q.; Xie, W.Y.; Tang, Y.J.; Zhang, J.; Liu, J. Genetic variation in the glucocorticoid pathway involved in interindividual differences in the glucocorticoid treatment. Pharmacogenomics 2017, 18, 293–316. [Google Scholar] [CrossRef] [PubMed]
- John, C.D.; Buckingham, J.C. Cytokines: Regulation of the hypothalamo-pituitary-adrenocortical axis. Curr. Opin. Pharmacol. 2003, 3, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.J.; Rutters, F.; Lemmens, S.G.; Born, J.M.; Westerterp-Plantenga, M.S. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol. Behav. 2010, 101, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Van Uum, S.H.; Sauvé, B.; Fraser, L.A.; Morley-Forster, P.; Paul, T.L.; Koren, G. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress 2008, 11, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Minkel, J.; Moreta, M.; Muto, J.; Htaik, O.; Jones, C.; Basner, M.; Dinges, D. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 2014, 33, 1430–1434. [Google Scholar] [CrossRef]
- Blaine, S.K.; Sinha, R. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017, 122, 136–147. [Google Scholar] [CrossRef]
- Birketvedt, G.S.; Geliebter, A.; Florholmen, J.; Gluck, M.E. Neuroendocrine Profile in the Night Eating Syndrome. Curr. Obes. Rep. 2014, 3, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Perogamvros, I.; Keevil, B.G.; Ray, D.W.; Trainer, P.J. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocrinol. Metab. 2010, 95, 4951–4958. [Google Scholar] [CrossRef] [PubMed]
- Raul, J.-S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, E.; Abawi, O.; Mohseni, M.; Abdelmoumen, A.; Wester, V.; van der Voorn, B.; Iyer, A.; van den Akker, E.; Hoeks, S.; van den Berg, S.; et al. Cross-sectional relation of long-term glucocorticoids in hair with anthropometric measurements and their possible determinants: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13376. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef]
- Age and Cancer Risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 12 March 2023).
- Simpson, E.R. Biology of aromatase in the mammary gland. J. Mammary Gland. Biol. Neoplasia 2000, 5, 251–258. [Google Scholar] [CrossRef]
| Characteristics | Total | <2.5 pg/mg | ≥2.5 pg/mg | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Total | 2776 | 100 | 2085 | 75.1 | 691 | 24.9 | ||
| Gender | Male | 653 | 23.5 | 462 | 70.8 | 191 | 29.2 | 0.003 a |
| Female | 2123 | 76.5 | 1623 | 76.4 | 500 | 23.6 | ||
| Age (Mean ± SD) (years) | 52.88 ± 10.08 | 53.00 ± 10.02 | 52.53 ± 10.27 | 0.284 b | ||||
| Hypertension | No | 1693 | 61.0 | 1293 | 76.4 | 400 | 23.6 | 0.054 a |
| Yes | 1083 | 39.0 | 792 | 73.1 | 291 | 26.9 | ||
| Diabetes | No | 2634 | 94.9 | 1989 | 75.5 | 645 | 24.5 | 0.034 a |
| Yes | 142 | 5.1 | 96 | 67.6 | 46 | 32.4 | ||
| Dyslipidemia | No | 1799 | 64.8 | 1365 | 75.9 | 434 | 24.1 | 0.204 a |
| Yes | 977 | 35.2 | 720 | 73.7 | 257 | 26.3 | ||
| Body mass index | Normal | 1158 | 41.7 | 857 | 74.0 | 301 | 26.0 | 0.209 a |
| Overweight | 1152 | 41.5 | 885 | 76.8 | 267 | 23.2 | ||
| Obesity | 462 | 16.6 | 340 | 73.6 | 122 | 26.4 | ||
| Smoking | Never-smokers | 1189 | 42.8 | 901 | 75.8 | 288 | 24.2 | 0.610 a |
| Ex-smokers | 1043 | 37.6 | 781 | 74.9 | 262 | 25.1 | ||
| Current smokers | 502 | 18.1 | 369 | 73.5 | 133 | 26.5 | ||
| Alcohol drinking | Non-drinkers | 1315 | 47.4 | 1009 | 76.7 | 306 | 23.3 | 0.061 a |
| Drinkers | 1461 | 52.6 | 1076 | 73.6 | 385 | 26.4 | ||
| Cancer | No | 2538 | 91.4 | 1906 | 75.1 | 632 | 24.9 | 0.970 |
| Yes | 238 | 8.6 | 179 | 75.2 | 59 | 24.8 | ||
| Characteristics | Total | HairE Levels (pg/mg) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | P05 | P25 | Median | P75 | P95 | |||
| Total | 4699 | 100 | 2.69 | 4.19 | 5.52 | 7.62 | 15.00 | ||
| Gender | Male | 1077 | 22.9 | 3.45 | 5.14 | 6.72 | 9.59 | 19.60 | <0.001 a |
| Female | 3622 | 77.1 | 2.61 | 4.02 | 5.20 | 7.10 | 12.95 | ||
| Age (Mean ± SD) (years) | 53.09 ± 10.07 | rs = 0.09 | <0.001 c | ||||||
| Hypertension | No | 2889 | 61.5 | 2.65 | 4.07 | 5.34 | 7.30 | 13.43 | <0.001 a |
| Yes | 1810 | 38.5 | 2.83 | 4.44 | 5.79 | 8.17 | 17.23 | ||
| Diabetes | No | 4455 | 94.8 | 2.68 | 4.17 | 5.48 | 7.55 | 14.72 | <0.001 a |
| Yes | 244 | 5.2 | 3.46 | 4.77 | 6.25 | 9.59 | 23.42 | ||
| Dyslipidemia | No | 3029 | 64.5 | 2.68 | 4.14 | 5.37 | 7.37 | 13.70 | <0.001 a |
| Yes | 1670 | 35.5 | 2.73 | 4.77 | 5.77 | 8.18 | 17.23 | ||
| Body mass index | Normal | 1997 | 42.5 | 2.66 | 4.14 | 5.49 | 7.45 | 13.43 | 0.073 b |
| Overweight | 1883 | 40.1 | 2.77 | 4.27 | 5.60 | 7.68 | 14.48 | ||
| Obesity | 814 | 17.3 | 2.67 | 4.18 | 5.47 | 8.11 | 20.33 | ||
| Smoking | Never-smokers | 1973 | 42.0 | 2.75 | 4.17 | 5.45 | 7.43 | 13.90 | <0.001 b,* |
| Ex-smokers | 1788 | 38.1 | 2.67 | 4.18 | 5.44 | 7.54 | 14.23 | ||
| Current smokers | 877 | 18.7 | 2.60 | 4.36 | 5.92 | 8.28 | 19.00 | ||
| Alcohol drinking | Non-drinkers | 2246 | 47.8 | 2.73 | 4.11 | 5.30 | 7.29 | 13.55 | <0.001 a |
| Drinkers | 2453 | 52.2 | 2.67 | 4.30 | 5.75 | 7.96 | 16.03 | ||
| Variables | Univariate Model | |
|---|---|---|
| HR | 95%CI | |
| HairF level | 0.993 | 0.740–1.333 |
| Variables | Univariate Model | Multivariable Model * | ||
|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |
| LogHairE | 1.113 | 0.738–1.678 | 6.403 | 1.110–36.92 |
| Age by 10 years | 1.468 | 1.337–1.611 | ||
| Gender | 2.748 | 1.144–6.604 | ||
| LogHairE*Gender | 0.343 | 0.130–0.908 | ||
| Variables | Males | Females | ||
|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |
| LogHairE | 2.051 | 0.874–4.813 | 0.791 | 0.484–1.292 |
| Age by 10 years | 1.962 | 1.623–2.373 | 1.339 | 1.202–1.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, A.T.; van Dijk, B.A.C.; van der Valk, E.S.; van der Vegt, B.; van Rossum, E.F.C.; de Bock, G.H. Chronic Stress Related to Cancer Incidence, including the Role of Metabolic Syndrome Components. Cancers 2024, 16, 2044. https://doi.org/10.3390/cancers16112044
Pham AT, van Dijk BAC, van der Valk ES, van der Vegt B, van Rossum EFC, de Bock GH. Chronic Stress Related to Cancer Incidence, including the Role of Metabolic Syndrome Components. Cancers. 2024; 16(11):2044. https://doi.org/10.3390/cancers16112044
Chicago/Turabian StylePham, An Thanh, Boukje A. C. van Dijk, Eline S. van der Valk, Bert van der Vegt, Elisabeth F. C. van Rossum, and Geertruida H. de Bock. 2024. "Chronic Stress Related to Cancer Incidence, including the Role of Metabolic Syndrome Components" Cancers 16, no. 11: 2044. https://doi.org/10.3390/cancers16112044
APA StylePham, A. T., van Dijk, B. A. C., van der Valk, E. S., van der Vegt, B., van Rossum, E. F. C., & de Bock, G. H. (2024). Chronic Stress Related to Cancer Incidence, including the Role of Metabolic Syndrome Components. Cancers, 16(11), 2044. https://doi.org/10.3390/cancers16112044






