Immunotherapy of Clear-Cell Renal-Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
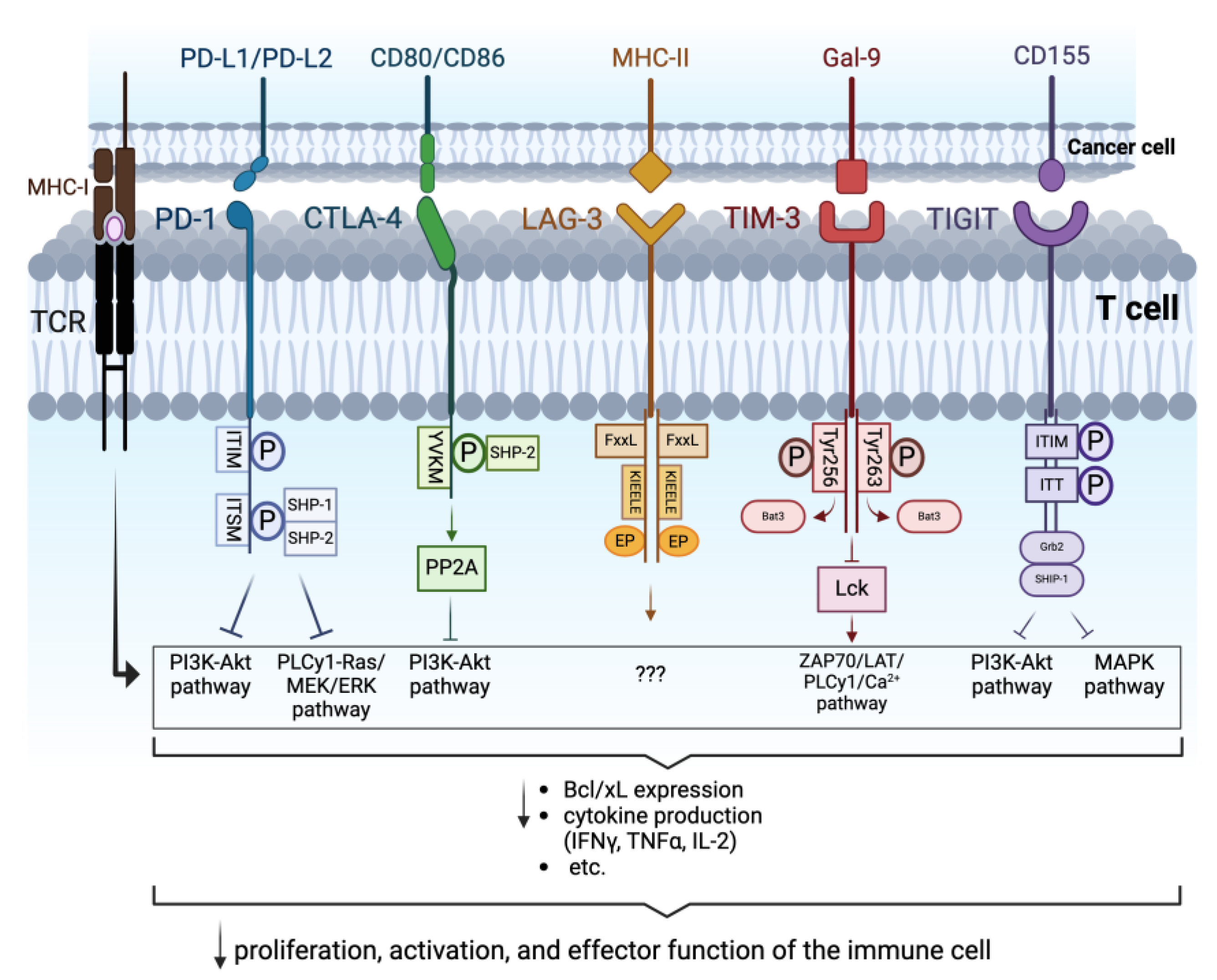
2. Immune Checkpoints
2.1. Programmed Cell Death Protein-1
2.2. Antigen-4 Associated with Cytotoxic T Lymphocytes
2.3. Lymphocyte-Activation Gene 3
2.4. T Cell Immunoglobulin and Mucin Domain 3
2.5. T Cell Immunoreceptor with Immunoglobulin and ITIM Domains
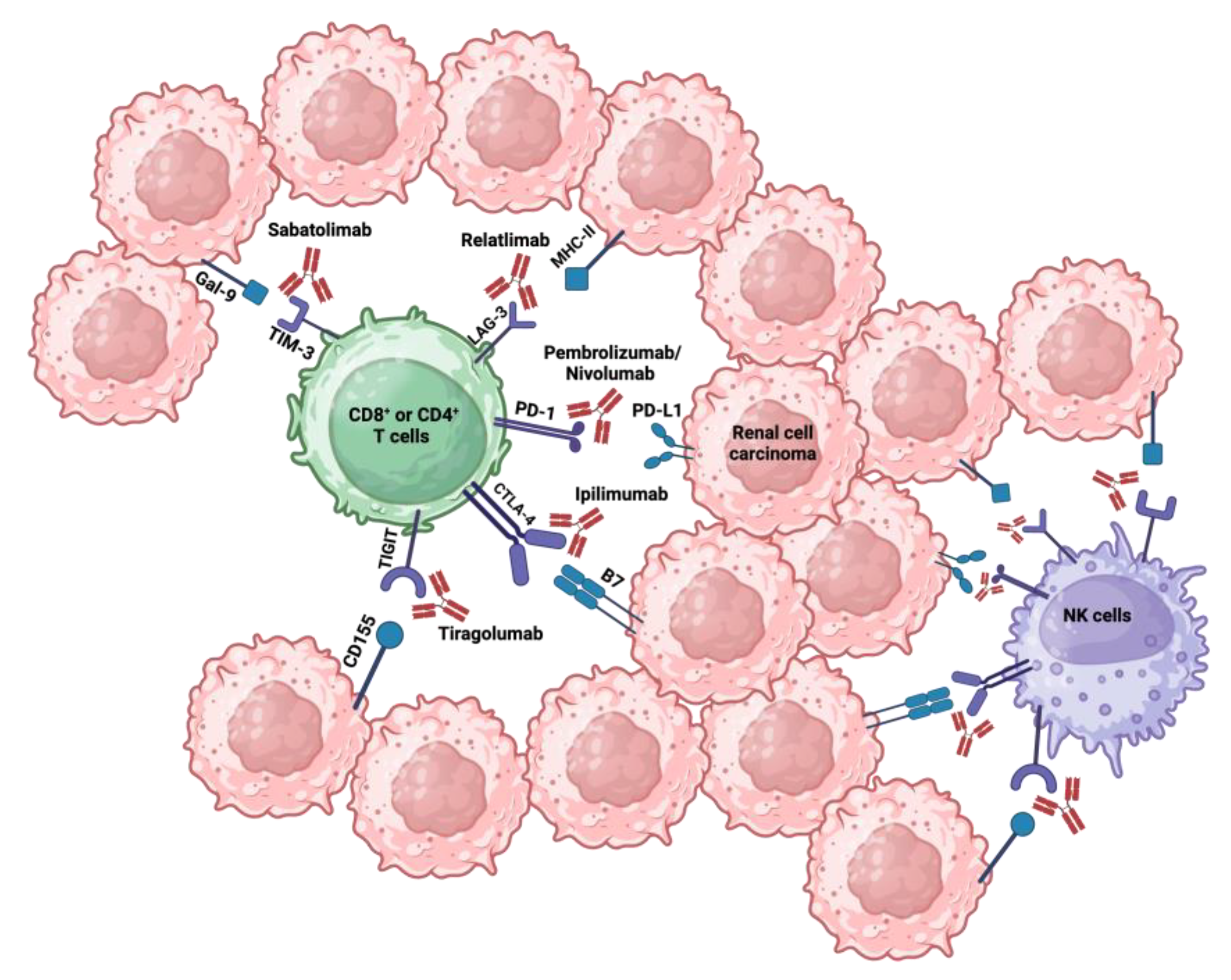
3. Immune Checkpoint Inhibitors
3.1. Programmed Cell Death Protein 1 Inhibitors
3.2. Antigen-4 Associated with Cytotoxic T Lympocytes Inhibitors
3.3. Lymphocyte-Activation Gene 3 Inhibitors
3.4. T Cell Immunoglobulin and Mucin Domain 3 Inhibitors
3.5. T Cell Immunoreceptor with Immunoglobulin and ITIM Domains Inhibitors
3.6. Combining Other Therapeutical Approaches with ICIs
4. Role of the von Hippel Lindau (VHL) Tumor Suppressor Gene
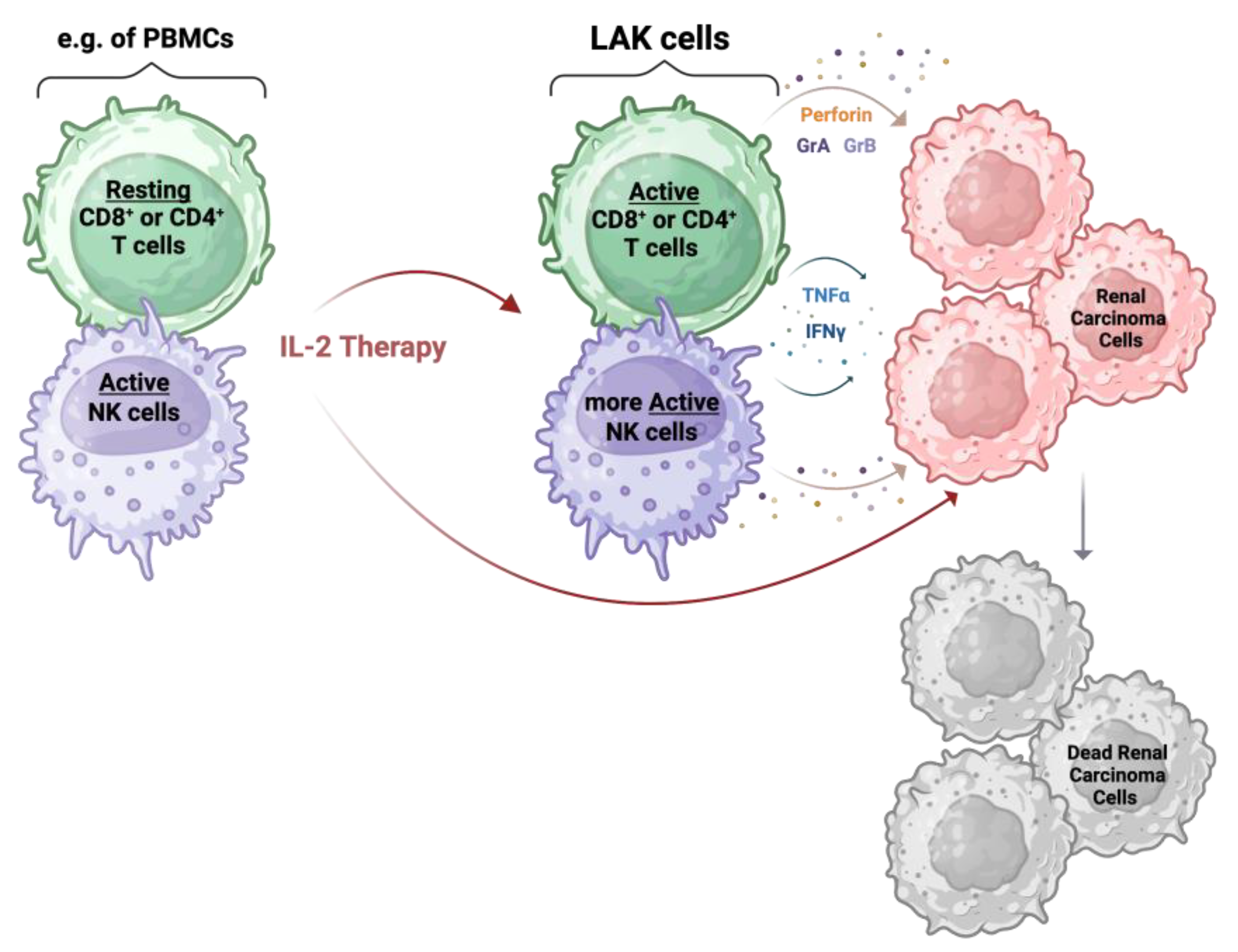
5. Interleukin-2
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Porth, C. Essentials of Pathophysiology: Concepts of Altered Health States; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 638. [Google Scholar]
- Lara, P.N.; Jonasch, E. Kidney Cancer: Principles and Practice; Springer Science & Business Media: New York, NY, USA, 2012; p. 4. [Google Scholar]
- Woolf, N. Pathology: Basic and Systemic; W.B. Saunders: London, UK, 1998; pp. 699–702. [Google Scholar]
- Copstead, L.-E.; Banasik, J. Pathophysiology; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 663–666. [Google Scholar]
- Yang, J.; Wang, K.; Yang, Z. Treatment strategies for clear cell renal cell carcinoma: Past, present and future. Front. Oncol. 2023, 13, 1133832. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Jones, R.J. Immune checkpoint inhibitors in renal cell carcinoma. Clin. Sci. 2017, 131, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Chatzkel, J.; Schell, M.J.; Chahoud, J.; Zhang, J.; Jain, R.; Swank, J.; Ludlow, S.; Lombardi, K.; Lucas, Y.; Croft, C.; et al. Coordinated Pembrolizumab and High Dose IL-2 (5-in-a-Row Schedule) for Therapy of Metastatic Clear Cell Renal Cancer. Clin. Genitourin. Cancer 2022, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Grimm, E.A.; Mazumder, A.; Zhang, H.Z.; Rosenberg, S.A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 1982, 155, 1823–1841. [Google Scholar] [CrossRef] [PubMed]
- West, E.J.; Scott, K.J.; Jennings, V.A.; Melcher, A.A. Immune activation by combination human lymphokine-activated killer and dendritic cell therapy. Br. J. Cancer 2011, 105, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Revvity, Inc. From CAR-T to CAR-NK Cell Therapy, the Promise of a Next Generation of Cancer Immunotherapy; White Paper; Revvity, Inc.: Waltham, MA, USA, 2023. [Google Scholar]
- Cramer, D.V.; Long, G.S. Lymphokine-activated killer (LAK) cell purging of bone marrow. Prog. Clin. Biol. Res. 1990, 333, 125–137. [Google Scholar] [PubMed]
- Moreira, M.; Pobel, C.; Epaillard, N.; Simonaggio, A.; Oudard, S.; Vano, Y.A. Resistance to cancer immunotherapy in metastatic renal cell carcinoma. Cancer Drug Resist. 2020, 3, 454–471. [Google Scholar] [PubMed]
- Beksac, A.T.; Paulucci, D.J.; Blum, K.A.; Yadav, S.S.; Sfakianos, J.P.; Badani, K.K. Heterogeneity in renal cell carcinoma. Urol. Oncol. 2017, 35, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, E.; Manfredi, F.; Nuzzo, A.; Ferrari, M.; Bonato, A.; Salfi, A.; Serafin, D.; Zatteri, L.; Antonuzzo, A.; Galli, L. Immune Checkpoint Inhibitor Rechallenge in Renal Cell Carcinoma: Current Evidence and Future Directions. Cancers 2023, 15, 3172. [Google Scholar] [CrossRef]
- Schneider, F.; Kaczorowski, A.; Jurcic, C.; Kirchner, M.; Schwab, C.; Schütz, V.; Görtz, M.; Zschäbitz, S.; Jäger, D.; Stenzinger, A.; et al. Digital Spatial Profiling Identifies the Tumor Periphery as a Highly Active Biological Niche in Clear Cell Renal Cell Carcinoma. Cancers 2023, 15, 5050. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Prado-Garcia, H.; Romero-Garcia, S.; Puerto-Aquino, A.; Rumbo-Nava, U. The PD-L1/PD-1 pathway promotes dysfunction, but not “exhaustion”, in tumor-responding T cells from pleural effusions in lung cancer patients. Cancer Immunol. Immunother. 2017, 66, 765–776. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C. The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy. Front. Immunol. 2019, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Donini, C.; D’Ambrosio, L.; Grignani, G.; Aglietta, M.; Sangiolo, D. Next generation immune-checkpoints for cancer therapy. J. Thorac. Dis. 2018, 10 (Suppl. S13), S1581–S1601. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Sasidharan Nair, V.; Decock, J.; Elkord, E. Immune checkpoints in the tumor microenvironment. Semin. Cancer Biol. 2020, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2022, 4, 579–597. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Wolf, A.M.; Iwai, Y. Development of Cancer Immunotherapy Targeting the PD-1 Pathway. J. Nippon Med. Sch. Nippon Ika Daigaku Zasshi 2019, 86, 10–14. [Google Scholar] [CrossRef]
- Fuertes Marraco, S.A.; Neubert, N.J.; Verdeil, G.; Speiser, D.E. Inhibitory Receptors Beyond T Cell Exhaustion. Front. Immunol. 2015, 6, 310. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef]
- Nunes-Xavier, C.E.; Angulo, J.C.; Pulido, R.; López, J.I. A Critical Insight into the Clinical Translation of PD-1/PD-L1 Blockade Therapy in Clear Cell Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 1. [Google Scholar] [CrossRef]
- Kruk, L.; Mamtimin, M.; Braun, A.; Anders, H.J.; Andrassy, J.; Gudermann, T.; Mammadova-Bach, E. Inflammatory Networks in Renal Cell Carcinoma. Cancers 2023, 15, 2212. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Shen, L.; Du, L.; Xue, H.; Wu, B.; OuYang, B. Mechanistic Insights into the Inhibition of a Common CTLA-4 Gene Mutation in the Cytoplasmic Domain. Molecules 2024, 29, 1330. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Chen, T.; Li, W.; Wang, X.; Yang, Q. Targeting RNA N6-methyladenosine to synergize with immune checkpoint therapy. Mol. Cancer 2023, 22, 36. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Kisielow, M.; Kisielow, J.; Capoferri-Sollami, G.; Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Y.; Guo, Y.; Chen, Y.; Liu, X.; Liu, M. Lymphocyte activation gene 3 negatively regulates the function of intrahepatic hepatitis C virus-specific CD8+ T cells. J. Gastroenterol. Hepatol. 2015, 30, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, S.; Zhao, Y.; Yan, H.; Zhang, Q.; Li, X. Blockade of PD-1 and LAG-3 expression on CD8+ T cells promotes the tumoricidal effects of CD8+ T cells. Front. Immunol. 2023, 14, 1265255. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Lee, C.H.; Jung, S.J.; Seo, W.I.; Chung, J.I.; Lee, D.S.; Jeong, D.H.; Jeon, Y.; Choi, I. Coexpression of lymphocyte-activation gene 3 and programmed death ligand-1 in tumor infiltrating immune cells predicts worse outcome in renal cell carcinoma. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221125588. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Zang, X.; Ramagopal, U.A.; Mukhopadhaya, A.; Fedorov, A.; Fedorov, E.; Zencheck, W.D.; Lary, J.W.; Cole, J.L.; Deng, H.; et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity 2007, 26, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter? Cancers 2021, 13, 6365. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, S.; Fan, L.; Zhang, B.; Xu, S. TIM-3: An update on immunotherapy. Int. Immunopharmacol. 2021, 99, 107933. [Google Scholar] [CrossRef]
- Cong, Y.; Liu, J.; Chen, G.; Qiao, G. The Emerging Role of T-Cell Immunoglobulin Mucin-3 in Breast Cancer: A Promising Target for Immunotherapy. Front. Oncol. 2021, 11, 723238. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, L.; Wang, Y.; Fu, R.; Wang, H.; Shao, Z. Increased TIM3+CD8+T cells in Myelodysplastic Syndrome patients displayed less perforin and granzyme B secretion and higher CD95 expression. Leuk. Res. 2016, 51, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Yu, M.; Xu, H.; Zhong, Z.; Li, Z.; Guo, Y.; Zhang, T.; Zeng, Z.; Jin, F.; He, X. Discovery of TIGIT inhibitors based on DEL and machine learning. Front. Chem. 2022, 10, 982539. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; You, X.; Han, S.; Sun, Y.; Zhang, J.; Zhang, Y. CD155/TIGIT, a novel immune checkpoint in human cancers (Review). Oncol. Rep. 2021, 45, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xu, J.; Gu, X.; Wu, F.; Deng, J.; Cai, X.; Wang, G.; Li, G.; Chen, Z. Immune checkpoint targeting TIGIT in hepatocellular carcinoma. Am. J. Transl. Res. 2020, 12, 3212–3224. [Google Scholar]
- Zhou, X.; Ren, T.; Zan, H.; Hua, C.; Guo, X. Novel Immune Checkpoints in Esophageal Cancer: From Biomarkers to Therapeutic Targets. Front. Immunol. 2022, 13, 864202. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Bajwa, P. Immune Checkpoint Inhibitors in the Treatment of Renal Cell Carcinoma. Semin. Nephrol. 2020, 40, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Liu, Y.; Shabto, J.M.; Martini, D.; Ravindranathan, D.; Hitron, E.E.; Russler, G.A.; Caulfield, S.; Yantorni, L.; Joshi, S.S.; et al. Modified Glasgow Prognostic Score associated with survival in metastatic renal cell carcinoma treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002851. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Deutsch, J.S.; Lipson, E.J.; Danilova, L.; Topalian, S.L.; Jedrych, J.; Baraban, E.; Ged, Y.; Singla, N.; Choueiri, T.K.; Gupta, S.; et al. Combinatorial biomarker for predicting outcomes to anti-PD-1 therapy in patients with metastatic clear cell renal cell carcinoma. Cell Rep. Med. 2023, 4, 100947. [Google Scholar] [CrossRef]
- Xu, J.X.; Maher, V.E.; Zhang, L.; Tang, S.; Sridhara, R.; Ibrahim, A.; Kim, G.; Pazdur, R. FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis. Oncologist 2017, 22, 311–317. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Q.; Sun, J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J. Immunother. Cancer 2018, 6, 124. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, N.; Rinne, M.L.; Sun, H.; Stein, A.M. Sabatolimab (MBG453) model-informed drug development for dose selection in patients with myelodysplastic syndrome/acute myeloid leukemia and solid tumors. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1653–1665. [Google Scholar] [CrossRef]
- Schwartz, S.; Patel, N.; Longmire, T.; Jayaraman, P.; Jiang, X.; Lu, H.; Baker, L.; Velez, J.; Ramesh, R.; Wavreille, A.S.; et al. Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor. Immunother. Adv. 2022, 2, ltac019. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Longhitano, E.; Muscolino, P.; Lo Re, C.; Ferrara, S.A.; Cernaro, V.; Gembillo, G.; Tessitore, D.; Speranza, D.; Figura, F.; Santarpia, M.; et al. Immune Checkpoint Inhibitors and the Kidney: A Focus on Diagnosis and Management for Personalised Medicine. Cancers 2023, 15, 1891. [Google Scholar] [CrossRef]
- Shapiro, D.D.; Dolan, B.; Laklouk, I.A.; Rassi, S.; Lozar, T.; Emamekhoo, H.; Wentland, A.L.; Lubner, M.G.; Abel, E.J. Understanding the Tumor Immune Microenvironment in Renal Cell Carcinoma. Cancers 2023, 15, 2500. [Google Scholar] [CrossRef]
- Masson, C.; Thouvenin, J.; Boudier, P.; Maillet, D.; Kuchler-Bopp, S.; Barthélémy, P.; Massfelder, T. Biological Biomarkers of Response and Resistance to Immune Checkpoint Inhibitors in Renal Cell Carcinoma. Cancers 2023, 15, 3159. [Google Scholar] [CrossRef] [PubMed]
- Khetani, V.V.; Portal, D.E.; Shah, M.R.; Mayer, T.; Singer, E.A. Combination drug regimens for metastatic clear cell renal cell carcinoma. World J. Clin. Oncol. 2020, 11, 541–562. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Albiges, L.; Fan, L.; Perini, R.F.; Zojwalla, N.J.; Powles, T.; Rini, B.I. Phase III study of the hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 versus everolimus in previously treated patients with advanced clear cell renal cell carcinoma (ccRCC). J. Clin. Oncol. 2020, 38, TPS5094. [Google Scholar] [CrossRef]
- Hayakawa, M. Lymphokine-activated killer (LAK) therapy for advanced renal cell carcinoma: Clinical study on arterial LAK therapy and experimental study on LAK cell activity. Hinyokika Kiyo. Acta Urol. Jpn. 1992, 38, 1311–1318. [Google Scholar]
- Clement, J.M.; McDermott, D.F. The high-dose aldesleukin (IL-2) “select” trial: A trial designed to prospectively validate predictive models of response to high-dose IL-2 treatment in patients with metastatic renal cell carcinoma. Clin. Genitourin. Cancer 2009, 7, E7–E9. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, M.; Irani, K.; Faraday, N.; Lowenstein, C.J. Nitric oxide inhibits exocytosis of cytolytic granules from lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11689–11694. [Google Scholar] [CrossRef] [PubMed]
- Tucker, Z.C.; Laguna, B.A.; Moon, E.; Singhai, S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer Treat Rev. 2012, 38, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S.; Aleksijevic, A.; Scuderi, P.; Hersh, E.M.; Grimes, W.J. Phenotypic and functional analysis of lymphokine-activated killer (LAK) cell clones. Ability of CD3+, LAK cell clones to produce interferon-gamma and tumor necrosis factor upon stimulation with tumor targets. Cancer Immunol. Immunother. 1989, 29, 270–278. [Google Scholar] [PubMed]
- Giron-Michel, J.; Azzi, S.; Khawam, K.; Mortier, E.; Caignard, A.; Devocelle, A.; Ferrini, S.; Croce, M.; François, H.; Lecru, L.; et al. Interleukin-15 plays a central role in human kidney physiology and cancer through the γc signaling pathway. PLoS ONE 2012, 7, e31624. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.P.; Schwartzentruber, D.J.; Kaufman, H.L.; Agarwala, S.S.; Tarhini, A.A.; Lowder, J.N.; Atkins, M.B. High dose interleukin-2 (Aldesleukin)—Expert consensus on best management practices-2014. J. Immunother. Cancer 2014, 2, 26. [Google Scholar] [CrossRef]
- García-Tuñón, I.; Ricote, M.; Ruiz, A.; Fraile, B.; Paniagua, R.; Royuela, M. Interleukin-2 and its receptor complex (alpha, beta and gamma chains) in in situ and infiltrative human breast cancer: An immunohistochemical comparative study. Breast Cancer Res. 2004, 6, R1–R7. [Google Scholar] [CrossRef]
| ICI Drugs | Target Protein | Clinical Trial | Phase | Response Rate |
|---|---|---|---|---|
| Pembrolizumab | PD-1 |
NCT03142334 (KEYNOTE-564) (type: secondary) | Phase 3 | OS 72 months |
| Nivolumab | PD-1 | NCT01668784 (CheckMate 025) (type: secondary) | Phase 3 | ORR 25.9% PFS 4.21 months |
| Nivolumab + Ipilimumab | PD-1 + CTLA-4 | NCT02231749 (CheckMate 214) (type: primary) | Phase 3 | ORR 41.6% PFS 11.56 months |
| Relatlimab + Nivolumab | LAG-3 + PD-1 + | NCT02996110 (FRACTION-RCC) (arm 2) (type: primary) | Phase 2 | mDOR 32.57 weeks |
| Sabatolimab | TIM-3 | NCT02608268 (type: secondary) | Phase 1–2 |
PFS 1.8 months
OS 4.1 months |
| Tiragolumab + Tobemstomig + Pembrolizumab + Axitinib | TIGIT + PD-1 + LAG-3 + PD-1 + VEGFR-1 | NCT05805501 | Phase 2 | No Study Results Posted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigolo, S.; Filgueira, L. Immunotherapy of Clear-Cell Renal-Cell Carcinoma. Cancers 2024, 16, 2092. https://doi.org/10.3390/cancers16112092
Grigolo S, Filgueira L. Immunotherapy of Clear-Cell Renal-Cell Carcinoma. Cancers. 2024; 16(11):2092. https://doi.org/10.3390/cancers16112092
Chicago/Turabian StyleGrigolo, Sophie, and Luis Filgueira. 2024. "Immunotherapy of Clear-Cell Renal-Cell Carcinoma" Cancers 16, no. 11: 2092. https://doi.org/10.3390/cancers16112092
APA StyleGrigolo, S., & Filgueira, L. (2024). Immunotherapy of Clear-Cell Renal-Cell Carcinoma. Cancers, 16(11), 2092. https://doi.org/10.3390/cancers16112092






