Spatial Distribution of Recurrence and Long-Term Toxicity Following Dose Escalation to the Dominant Intra-Prostatic Nodule for Intermediate–High-Risk Prostate Cancer: Insights from a Phase I/II Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiotherapy Planning and Delivery
2.2. Treatment Schema
2.3. Phase I/II Design
2.4. Determination of Recurrences
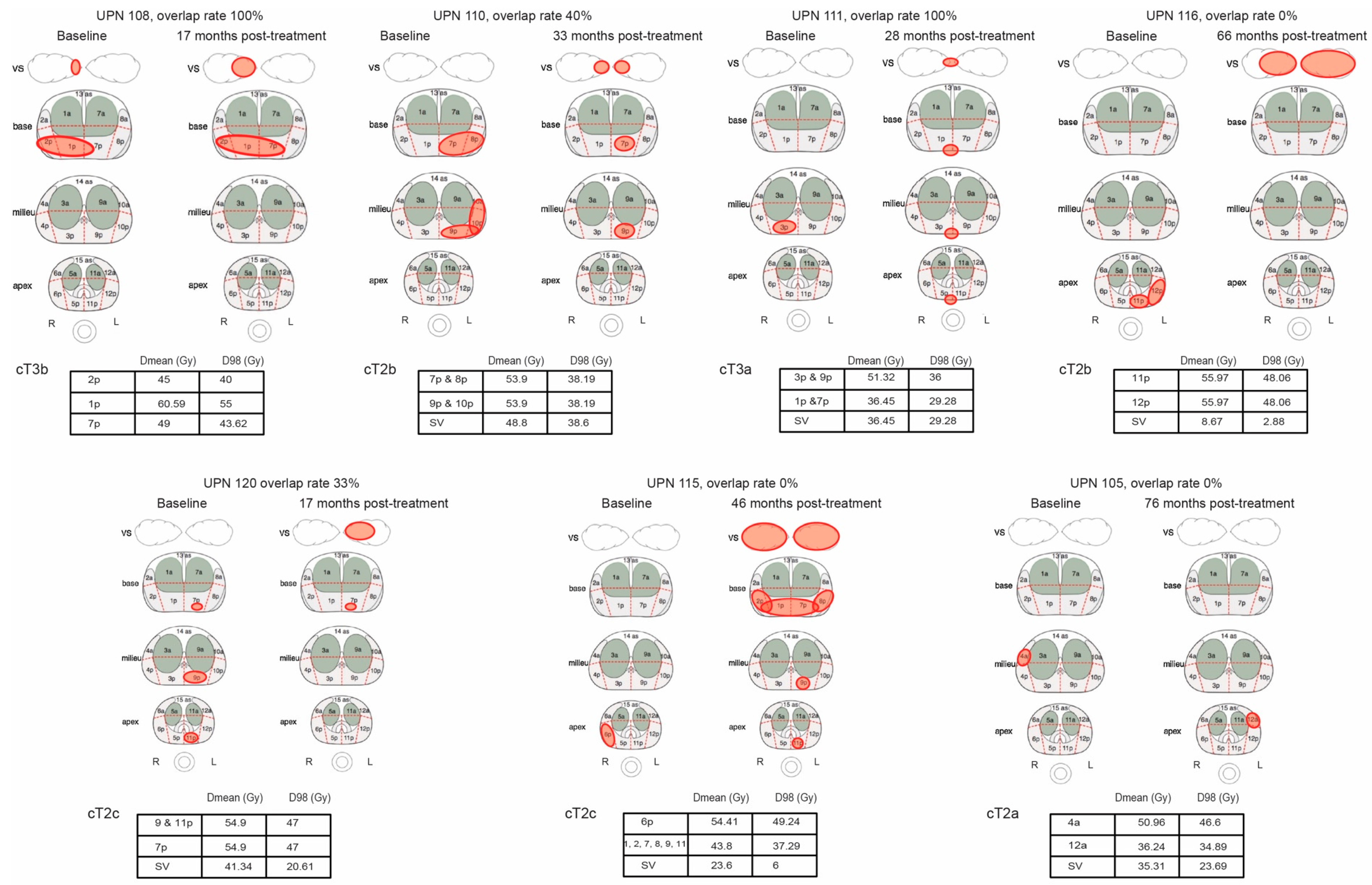
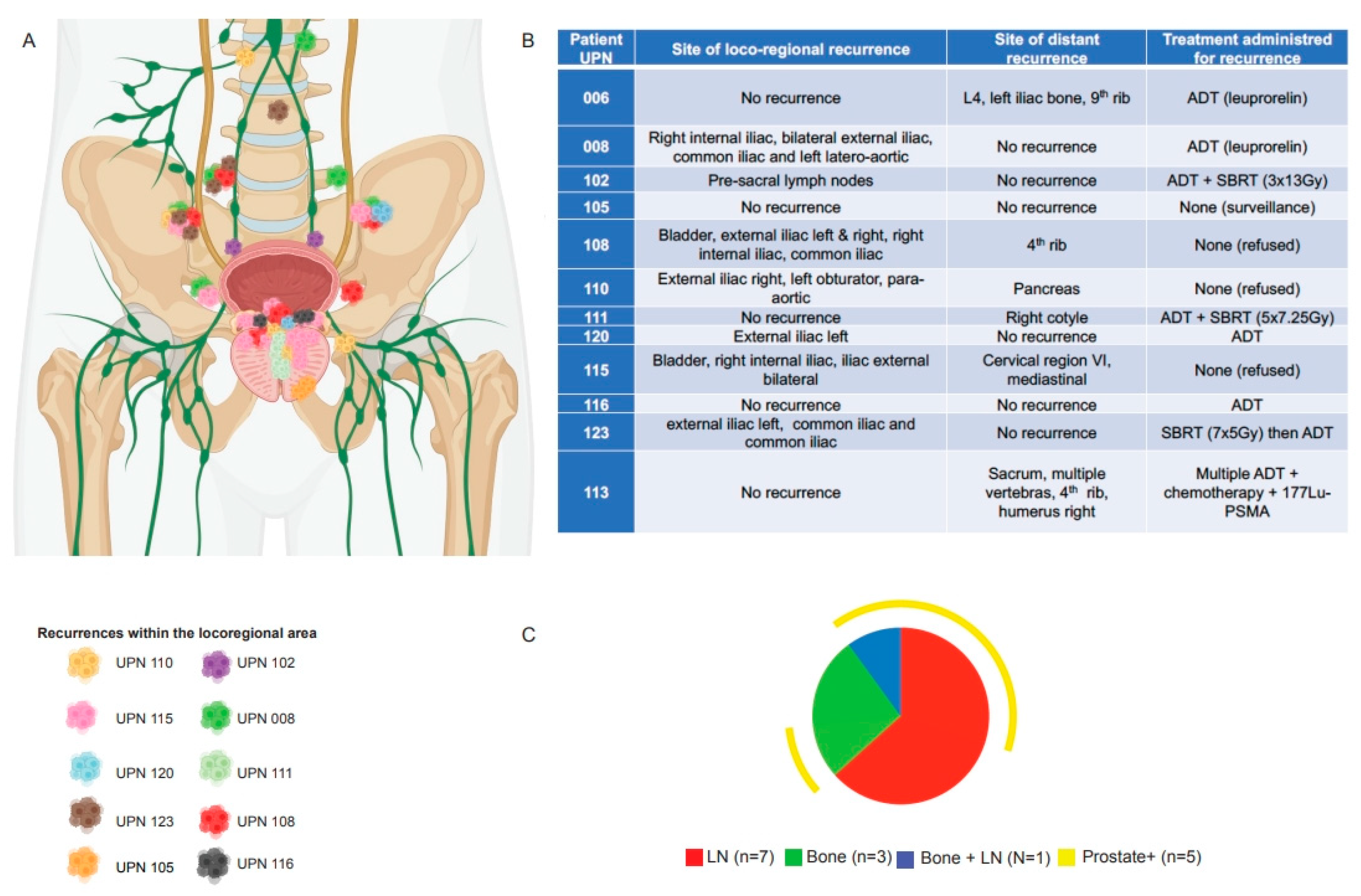
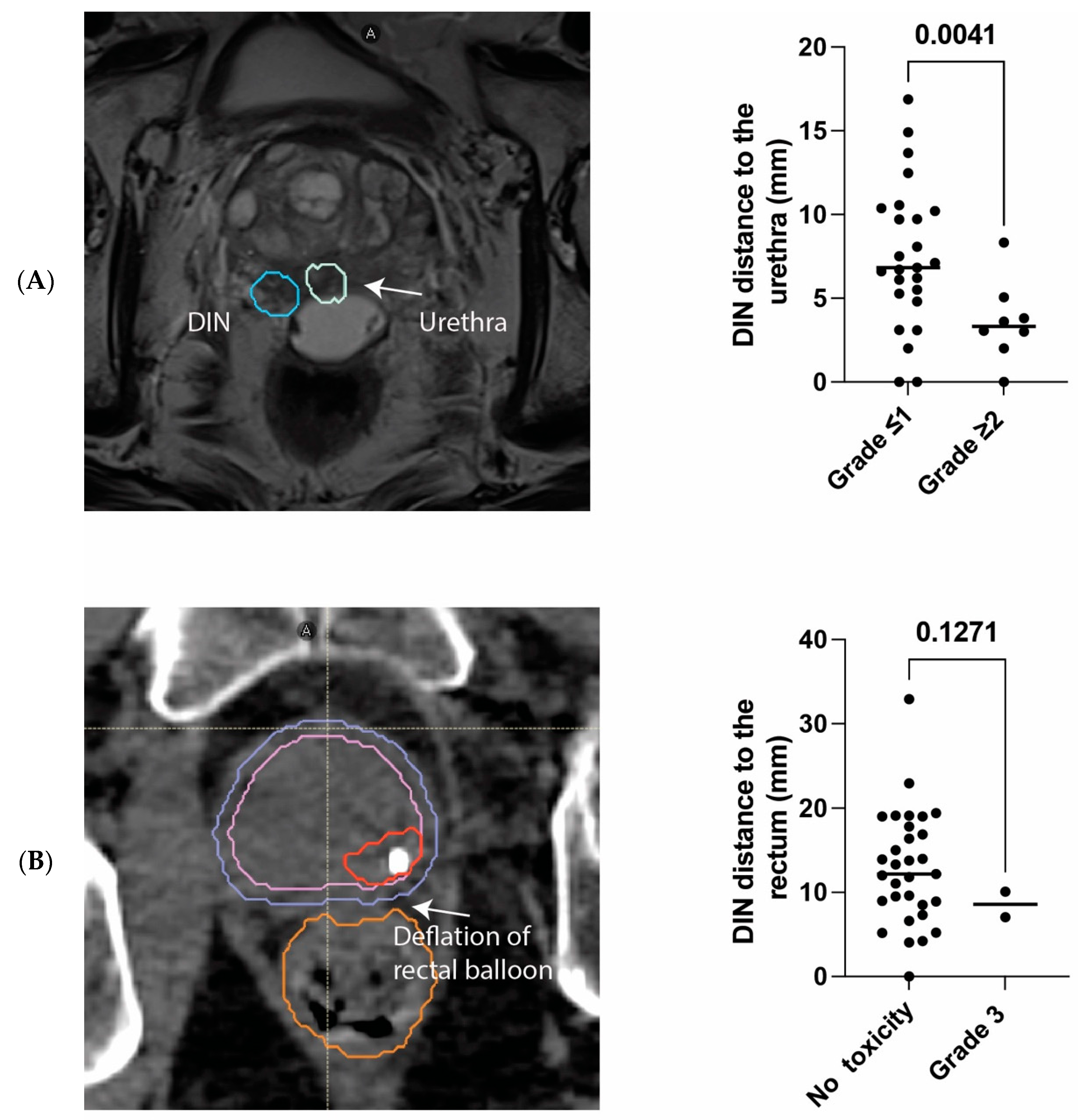
3. Results
3.1. Pattern of Recurrence
3.2. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Widmark, A.; Klepp, O.; Solberg, A.; Damber, J.-E.; Angelsen, A.; Fransson, P.; Lund, J.-Å.; Tasdemir, I.; Hoyer, M.; Wiklund, F.; et al. Endocrine Treatment, with or without Radiotherapy, in Locally Advanced Prostate Cancer (SPCG-7/SFUO-3): An Open Randomised Phase III Trial. Lancet 2009, 373, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Van Tienhoven, G.; Warde, P.; Dubois, J.B.; Mirimanoff, R.-O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Billiet, I.; et al. External Irradiation with or without Long-Term Androgen Suppression for Prostate Cancer with High Metastatic Risk: 10-Year Results of an EORTC Randomised Study. Lancet Oncol. 2010, 11, 1066–1073. [Google Scholar] [CrossRef]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G.; et al. Combined Androgen Deprivation Therapy and Radiation Therapy for Locally Advanced Prostate Cancer: A Randomised, Phase 3 Trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef]
- Peeters, S.T.H.; Heemsbergen, W.D.; Koper, P.C.M.; van Putten, W.L.J.; Slot, A.; Dielwart, M.F.H.; Bonfrer, J.M.G.; Incrocci, L.; Lebesque, J.V. Dose-Response in Radiotherapy for Localized Prostate Cancer: Results of the Dutch Multicenter Randomized Phase III Trial Comparing 68 Gy of Radiotherapy with 78 Gy. J. Clin. Oncol. 2006, 24, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Sydes, M.R.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Cowan, R.A.; Huddart, R.A.; Jose, C.C.; Matthews, J.H.; Millar, J.; et al. Escalated-Dose versus Standard-Dose Conformal Radiotherapy in Prostate Cancer: First Results from the MRC RT01 Randomised Controlled Trial. Lancet Oncol. 2007, 8, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Spratt, D.E.; Soni, P.D.; McLaughlin, P.W.; Merrick, G.S.; Stock, R.G.; Blasko, J.C.; Zelefsky, M.J. American Brachytherapy Society Task Group Report: Combination of Brachytherapy and External Beam Radiation for High-Risk Prostate Cancer. Brachytherapy 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised Trial of External-Beam Radiotherapy Alone or with High-Dose-Rate Brachytherapy for Prostate Cancer: Mature 12-Year Results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus Hypofractionated High-Dose Intensity-Modulated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the Randomised, Non-Inferiority, Phase 3 CHHiP Trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Parr, H.; Porta, N.; Tree, A.C.; Dearnaley, D.; Hall, E. A Personalized Clinical Dynamic Prediction Model to Characterize Prognosis for Patients with Localized Prostate Cancer: Analysis of the CHHiP Phase 3 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Catton, C.N.; Lukka, H.; Gu, C.-S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.-P.; Ahmed, S.; Cheung, P.; et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Dignam, J.J.; Amin, M.B.; Bruner, D.W.; Low, D.; Swanson, G.P.; Shah, A.B.; D’Souza, D.P.; Michalski, J.M.; Dayes, I.S.; et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients with Low-Risk Prostate Cancer. J. Clin. Oncol. 2016, 34, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Boike, T.P.; Lotan, Y.; Cho, L.C.; Brindle, J.; DeRose, P.; Xie, X.-J.; Yan, J.; Foster, R.; Pistenmaa, D.; Perkins, A.; et al. Phase I Dose-Escalation Study of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer. J. Clin. Oncol. 2011, 29, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.N.; Cho, L.C.; Straka, C.; Christie, A.; Lotan, Y.; Pistenmaa, D.; Kavanagh, B.D.; Nanda, A.; Kueplian, P.; Brindle, J.; et al. Predictors of Rectal Tolerance Observed in a Dose-Escalated Phase 1-2 Trial of Stereotactic Body Radiation Therapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Arrayeh, E.; Westphalen, A.C.; Kurhanewicz, J.; Roach, M.; Jung, A.J.; Carroll, P.R.; Coakley, F.V. Does Local Recurrence of Prostate Cancer After Radiation Therapy Occur at the Site of Primary Tumor? Results of a Longitudinal MRI and MRSI Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e787–e793. [Google Scholar] [CrossRef] [PubMed]
- Cellini, N.; Morganti, A.G.; Mattiucci, G.C.; Valentini, V.; Leone, M.; Luzi, S.; Manfredi, R.; Dinapoli, N.; Digesu’, C.; Smaniotto, D. Analysis of Intraprostatic Failures in Patients Treated with Hormonal Therapy and Radiotherapy: Implications for Conformal Therapy Planning. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Puech, P.; Sufana Iancu, A.; Renard, B.; Villers, A.; Lemaitre, L. Detecting Prostate Cancer with MRI—Why and How. Diagn. Interv. Imaging 2012, 93, 268–278. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical Outcome after Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Collins, S.; Lei, S.; Piel, N.; Oermann, E.; Chen, V.; Ju, A.; Dahal, K.; Hanscom, H.; Kim, J.; Yu, X.; et al. Six-Dimensional Correction of Intra-Fractional Prostate Motion with CyberKnife Stereotactic Body Radiation Therapy. Front. Oncol. 2011, 1, 48. [Google Scholar]
- Le Tourneau, C.; Lee, J.J.; Siu, L.L. Dose Escalation Methods in Phase I Cancer Clinical Trials. J. Natl. Cancer Inst. 2009, 101, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Simon, R. Optimal Two-Stage Designs for Phase II Clinical Trials. Control. Clin. Trials 1989, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.M. Interpreting Quality of Life Data: Population-Based Reference Data for the EORTC QLQ-C30. Eur. J. Cancer 2001, 37, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the Significance of Changes in Health-Related Quality-of-Life Scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Abghari-Gerst, M.; Armstrong, W.R.; Nguyen, K.; Calais, J.; Czernin, J.; Lin, D.; Jariwala, N.; Rodnick, M.; Hope, T.A.; Hearn, J.; et al. A Comprehensive Assessment of 68Ga-PSMA-11 PET in Biochemically Recurrent Prostate Cancer: Results from a Prospective Multi-Center Study in 2005 Patients. J. Nucl. Med. 2022, 63, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Barrier, A.; Ouzzane, A.; Villers, A. Rôle de l’IRM Prostatique Dans Le Cancer de La Prostate En 2016: Mise Au Point et Perspectives d’avenir. Afr. J. Urol. 2017, 23, 272–277. [Google Scholar] [CrossRef]
- Kishan, A.U.; Dang, A.; Katz, A.J.; Mantz, C.A.; Collins, S.P.; Aghdam, N.; Chu, F.-I.; Kaplan, I.D.; Appelbaum, L.; Fuller, D.B.; et al. Long-Term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw. Open 2019, 2, e188006. [Google Scholar] [CrossRef] [PubMed]
- Groen, V.H.; Haustermans, K.; Pos, F.J.; Draulans, C.; Isebaert, S.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; et al. Patterns of Failure Following External Beam Radiotherapy with or without an Additional Focal Boost in the Randomized Controlled FLAME Trial for Localized Prostate Cancer. Eur. Urol. 2022, 82, 252–257. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Jackson, W.C.; Silva, J.; Hartman, H.E.; Dess, R.T.; Kishan, A.U.; Beeler, W.H.; Gharzai, L.A.; Jaworski, E.M.; Mehra, R.; Hearn, J.W.D.; et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-Hypofractionated versus Conventionally Fractionated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the HYPO-RT-PC Randomised, Non-Inferiority, Phase 3 Trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.M.; Bloch, D.A.; Cotrutz, C.; Beckman, A.C.; Henning, G.T.; Woodhouse, S.A.; Williamson, S.K.; Mohideen, N.; Dombrowski, J.J.; Hong, R.L.; et al. Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer: Survival and Toxicity Endpoints. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Macias, V.A.; Blanco, M.L.; Barrera, I.; Garcia, R. A Phase II Study of Stereotactic Body Radiation Therapy for Low-Intermediate-High-Risk Prostate Cancer Using Helical Tomotherapy: Dose-Volumetric Parameters Predicting Early Toxicity. Front. Oncol. 2014, 4, 336. [Google Scholar] [CrossRef]
- Bolzicco, G.; Favretto, M.S.; Satariano, N.; Scremin, E.; Tambone, C.; Tasca, A. A Single-Center Study of 100 Consecutive Patients with Localized Prostate Cancer Treated with Stereotactic Body Radiotherapy. BMC Urol. 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Ostler, P.; Hoskin, P.; Dankulchai, P.; Nariyangadu, P.; Hughes, R.J.; Wells, E.; Taylor, H.; Khoo, V.S.; van As, N.J. Prostate Stereotactic Body Radiotherapy—First UK Experience. Clin. Oncol. 2014, 26, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-Y.; Chao, H.-L.; Huang, W.-Y.; Lin, C.-S.; Chen, C.-M.; Lo, C.-H. Stereotactic Ablative Radiotherapy with CyberKnife in the Treatment of Locally Advanced Prostate Cancer: Preliminary Results. Tumori J. 2015, 101, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Djemil, T.; Tendulkar, R.D.; Reddy, C.A.; Thousand, R.A.; Vassil, A.; Stovsky, M.; Berglund, R.K.; Klein, E.A.; Stephans, K.L. Dose-Escalated Stereotactic Body Radiation Therapy for Patients with Intermediate- and High-Risk Prostate Cancer: Initial Dosimetry Analysis and Patient Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 960–964. [Google Scholar] [CrossRef]
- King, C.R.; Freeman, D.; Kaplan, I.; Fuller, D.; Bolzicco, G.; Collins, S.; Meier, R.; Wang, J.; Kupelian, P.; Steinberg, M.; et al. Stereotactic Body Radiotherapy for Localized Prostate Cancer: Pooled Analysis from a Multi-Institutional Consortium of Prospective Phase II Trials. Radiother. Oncol. 2013, 109, 217–221. [Google Scholar] [CrossRef]
- Katz, A.; Kang, J. Stereotactic Body Radiotherapy with or without External Beam Radiation as Treatment for Organ Confined High-Risk Prostate Carcinoma: A Six Year Study. Radiat. Oncol. 2014, 9, 1. [Google Scholar] [CrossRef]
- Spratt, D.E.; Pei, X.; Yamada, J.; Kollmeier, M.A.; Cox, B.; Zelefsky, M.J. Long-Term Survival and Toxicity in Patients Treated with High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Ricco, A.; Hanlon, A.; Lanciano, R. Propensity Score Matched Comparison of Intensity Modulated Radiation Therapy vs Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Survival Analysis from the National Cancer Database. Front. Oncol. 2017, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- van Dams, R.; Jiang, N.Y.; Fuller, D.B.; Loblaw, A.; Jiang, T.; Katz, A.J.; Collins, S.P.; Aghdam, N.; Suy, S.; Stephans, K.L.; et al. Stereotactic Body Radiotherapy for High-Risk Localized Carcinoma of the Prostate (SHARP) Consortium: Analysis of 344 Prospectively Treated Patients. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Menkarios, C.; Vigneault, É.; Brochet, N.; Nguyen, D.H.; Bahary, J.-P.; Jolicoeur, M.; Beauchemin, M.-C.; Villeneuve, H.; Van Nguyen, T.; Fortin, B.; et al. Toxicity Report of Once Weekly Radiation Therapy for Low-Risk Prostate Adenocarcinoma: Preliminary Results of a Phase I/II Trial. Radiat. Oncol. 2011, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.; Tumati, V.; Xie, X.-J.; Cho, L.C.; Kavanagh, B.D.; Brindle, J.; Raben, D.; Nanda, A.; Cooley, S.; Kim, D.W.N.; et al. Stereotactic Body Radiation Therapy for Low and Intermediate Risk Prostate Cancer—Results from a Multi-Institutional Clinical Trial. Eur. J. Cancer 2016, 59, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gannavarapu, B.S.; Desai, N.B.; Folkert, M.R.; Dohopolski, M.; Gao, A.; Ahn, C.; Cadeddu, J.; Bagrodia, A.; Woldu, S.; et al. Dose-Intensified Stereotactic Ablative Radiation for Localized Prostate Cancer. Front. Oncol. 2022, 12, 779182. [Google Scholar] [CrossRef]
- Folkert, M.R.; Zelefsky, M.J.; Hannan, R.; Desai, N.B.; Lotan, Y.; Laine, A.M.; Kim, D.W.N.; Neufeld, S.H.; Hornberger, B.; Kollmeier, M.A.; et al. A Multi-Institutional Phase 2 Trial of High-Dose SAbR for Prostate Cancer Using Rectal Spacer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 101–109. [Google Scholar] [CrossRef]
| Characteristics | N (Total = 33) | % |
|---|---|---|
| T stage | ||
| T1c | 2 | 6.1 |
| T2a | 10 | 30.3 |
| T2b | 9 | 27.3 |
| T2c | 8 | 24.2 |
| T3a | 3 | 9.1 |
| T3b | 1 | 3 |
| Gleason Score | ||
| 3 + 3 | 3 | 9.1 |
| 3 + 4 | 17 | 51.5 |
| 4 + 3 | 5 | 15.2 |
| 4 + 4 | 4 | 12.1 |
| 3 + 5 | 1 | 3 |
| 4 + 5 | 3 | 9.1 |
| Low risk | ||
| (GS < 6 and PSA < 10 and T1c/T2a) | 1 | 3 |
| Intermediate risk | ||
| (GS = 7 or PSA > 10 or ≤cT2a) | 14 | 42.4 |
| High risk | ||
| (GS ≥ 8 or ≥cT2c or PSA > 20) | 18 | 54.6 |
| Hormonal therapy | ||
| Yes | 1 | 3 |
| No | 32 | 97 |
| Radiotherapy delivery | ||
| CyberKnife | 27 | 81.8 |
| Tomotherapy | 3 | 9.1 |
| Synergy | 3 | 9.1 |
| UPN | Grade 2 | Grade 3 |
|---|---|---|
| 105 | Nycturia | NR |
| 106 | Urinary urgency and pollakiuria | NR |
| 107 | Pollakiuria and urinary incontinence | NR |
| 110 | Pollakiuria, urinary urgency and incontinence | NR |
| 111 | Pollakiuria | NR |
| 113 | NR | Urinary obstruction |
| 114 | Urinary tract infection | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cloître, M.; Benkhaled, S.; Boughdad, S.; Schaefer, N.; Prior, J.O.; Zeverino, M.; Berthold, D.; Tawadros, T.; Meuwly, J.-Y.; Martel, P.; et al. Spatial Distribution of Recurrence and Long-Term Toxicity Following Dose Escalation to the Dominant Intra-Prostatic Nodule for Intermediate–High-Risk Prostate Cancer: Insights from a Phase I/II Study. Cancers 2024, 16, 2097. https://doi.org/10.3390/cancers16112097
Cloître M, Benkhaled S, Boughdad S, Schaefer N, Prior JO, Zeverino M, Berthold D, Tawadros T, Meuwly J-Y, Martel P, et al. Spatial Distribution of Recurrence and Long-Term Toxicity Following Dose Escalation to the Dominant Intra-Prostatic Nodule for Intermediate–High-Risk Prostate Cancer: Insights from a Phase I/II Study. Cancers. 2024; 16(11):2097. https://doi.org/10.3390/cancers16112097
Chicago/Turabian StyleCloître, Minna, Sofian Benkhaled, Sarah Boughdad, Niklaus Schaefer, John O. Prior, Michele Zeverino, Dominik Berthold, Thomas Tawadros, Jean-Yves Meuwly, Paul Martel, and et al. 2024. "Spatial Distribution of Recurrence and Long-Term Toxicity Following Dose Escalation to the Dominant Intra-Prostatic Nodule for Intermediate–High-Risk Prostate Cancer: Insights from a Phase I/II Study" Cancers 16, no. 11: 2097. https://doi.org/10.3390/cancers16112097






