Epigenetic Therapies in Triple-Negative Breast Cancer: Concepts, Visions, and Challenges
Abstract
:Simple Summary
Abstract
1. Introduction: Epigenetics—Concepts, Misconceptions, and a Working Definition
2. Epigenetic Phenomena
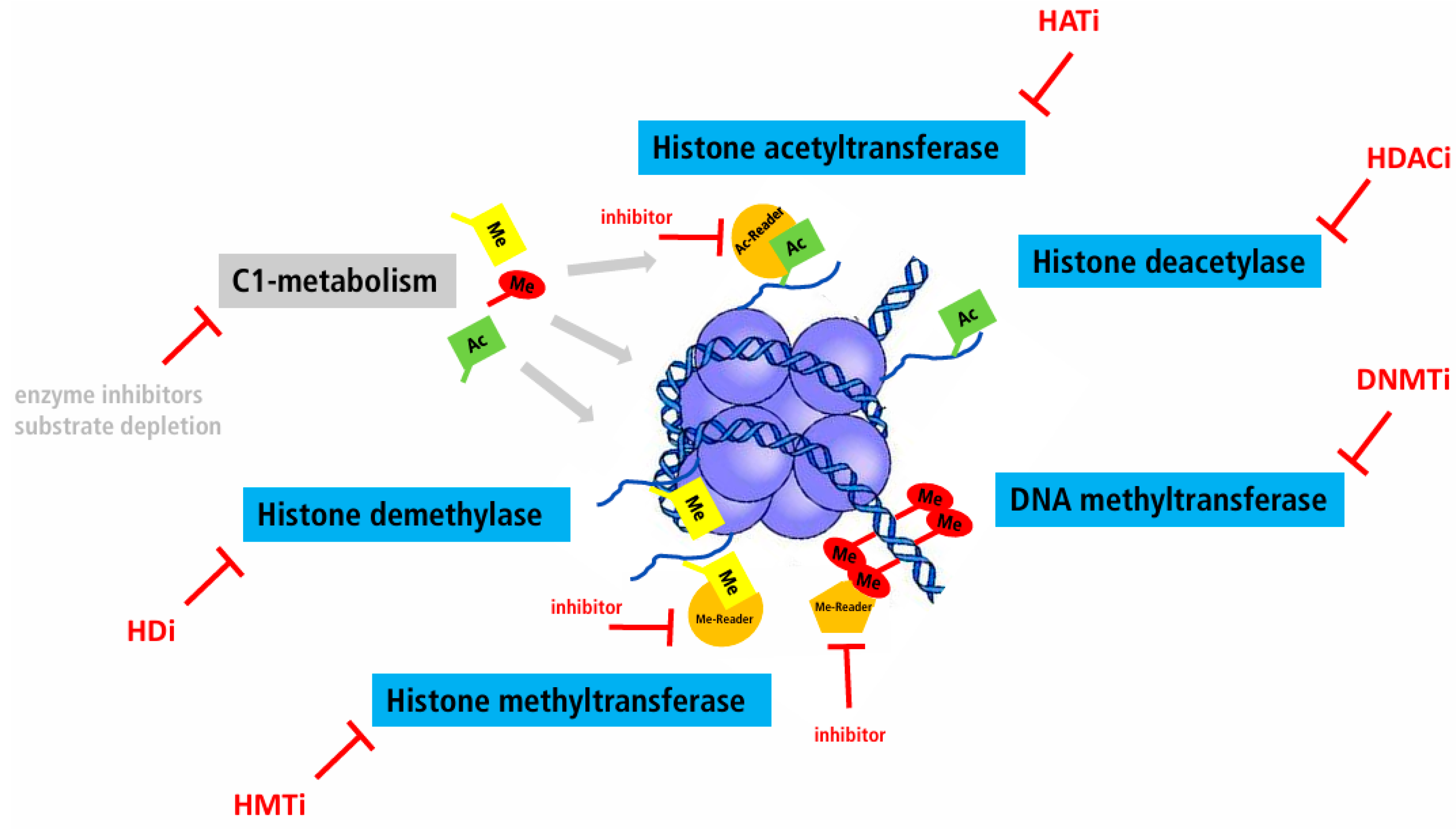
3. Epigenetic Mechanisms
- -
- They are best studied and understood in molecular terms.
- -
- The analytical tools to identify these modifications and monitor alterations during the course of disease or after therapeutic intervention are well developed.
- -
- Aberrations in DNA methylation and histone modifications are well described in many human diseases, especially in human cancers.
- -
- Targeting DNA methylation and/or histone modifications has been studied in many clinical trials in oncology.
- -
- Drugs targeting DNA methylation and histone modifications are approved and are available for treating human cancer patients.
3.1. DNA Methylation
3.2. Histone Modification
4. Epigenetic Therapy
Definition
- -
- Inhibition of DNA methyltransferases (DNMTi).
- -
- Overexpression or activation of DNA methyltransferases (DNMT).
- -
- Inhibition of histone-modifying enzymes (e.g., histone deacetylase inhibitors, HDACi).
- -
- Overexpression or activation of histone-modifying enzymes.
- -
- Inhibition or activation of DNA methyltransferases or histone-modifying enzymes by substrate depletion.
- -
- Targeted removal or addition of methyl groups.
- -
- Targeted modification of histone proteins.
5. Epigenetics Therapy—The Beginnings
Aza-Cytidine
6. Single Agent versus Combination Therapy
7. Breast Cancer
8. Epigenetic Subtypes in Human Breast Cancer
9. “Directed Epigenetic Therapy” in Triple Negative Breast Cancer
10. Epigenetic Therapy as “Conditioner”
11. Epigenetic Therapy by Genome Editing
12. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Deans, C.; Maggert, K.A. What do you mean, “epigenetic”? Genetics 2015, 199, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, U. Epigenetics: The origins and evolution of a fashionable topic. Dev. Biol. 2016, 416, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The epigenotype. Endeavour 1942, 1, 18–20. [Google Scholar] [CrossRef]
- Hafner, S.J.; Lund, A.H. Great expectations—Epigenetics and the meandering path from bench to bedside. Biomed. J. 2016, 39, 166–176. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Epigenetics and transgenerational transfer: A physiological perspective. J. Exp. Biol. 2010, 213, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R. Epigenetics: An overview. Dev. Genet. 1994, 15, 453–457. [Google Scholar] [CrossRef]
- Holliday, R. Epigenetics: A historical overview. Epigenetics 2006, 1, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, U. Why epigenetics is not a vindication of lamarckism—And why that matters. Stud. Hist. Philos. Biol. Biomed. Sci. 2016, 57, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Colot, V. Plant transgenerational epigenetics. Annu. Rev. Genet. 2016, 50, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.C.; Szukala, A.; Tian, B.; Paun, O. Current research frontiers in plant epigenetics: An introduction to a virtual issue. New Phytol. 2020, 226, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Madhani, H.D. Unbelievable but true: Epigenetics and chromatin in fungi. Trends Genet. 2021, 37, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.J.; Wickramasinghe, V.O. Rna in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, I.; Kouzarides, T. Role of rna modifications in cancer. Nat. Rev. Cancer 2020, 20, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suner, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Patrat, C.; Ouimette, J.F.; Rougeulle, C. X chromosome inactivation in human development. Development 2020, 147, dev183095. [Google Scholar] [CrossRef] [PubMed]
- Grunstein, M.; Gasser, S.M. Epigenetics in saccharomyces cerevisiae. Cold Spring Harb. Perspect. Biol. 2013, 5, a017491. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. In brief: Genomic imprinting and imprinting diseases. J. Pathol. 2014, 232, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T.; Reinberg, D. Overview and concepts of epigenetics. In Epigenetics; Allis, C.D., Jenuwein, T., Reinberg, D., Eds.; Cold Spring Harbor: New York, NY, USA, 2007; pp. 23–62. [Google Scholar]
- Pikaard, C.S.; Mittelsten Scheid, O. Epigenetic regulation in plants. Cold Spring Harb. Perspect. Biol. 2014, 6, a019315. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D. DNA methylation and chromatin—Unraveling the tangled web. Oncogene 2002, 21, 5361–5379. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. The yin and yang of histone marks in transcription. Annu. Rev. Genom. Hum. Genet. 2021, 22, 147–170. [Google Scholar] [CrossRef]
- Millan-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Sarkies, P. Encyclopaedia of eukaryotic DNA methylation: From patterns to mechanisms and functions. Biochem. Soc. Trans. 2022, 50, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N.; Gerber-Huber, S. DNA methylation and cpg suppression. Cell Differ. 1985, 17, 199–205. [Google Scholar] [CrossRef]
- Antequera, F.; Bird, A. Cpg islands: A historical perspective. Methods Mol. Biol. 2018, 1766, 3–13. [Google Scholar] [PubMed]
- Attwood, J.T.; Yung, R.L.; Richardson, B.C. DNA methylation and the regulation of gene transcription. Cell Mol. Life Sci. 2002, 59, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. DNA methylation and cancer. Cancer Res. 1986, 46, 461–466. [Google Scholar]
- McCabe, M.T.; Brandes, J.C.; Vertino, P.M. Cancer DNA methylation: Molecular mechanisms and clinical implications. Clin. Cancer Res. 2009, 15, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Issa, J.P. Epigenetic changes in solid and hematopoietic tumors. Semin. Oncol. 2005, 32, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Witte, T.; Plass, C.; Gerhauser, C. Pan-cancer patterns of DNA methylation. Genome Med. 2014, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Knudsen, K.E. Retinoblastoma tumor suppressor: Where cancer meets the cell cycle. Exp. Biol. Med. 2006, 231, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Greger, V.; Passarge, E.; Hopping, W.; Messmer, E.; Horsthemke, B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum. Genet. 1989, 83, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Glodzik, D.; Bosch, A.; Hartman, J.; Aine, M.; Vallon-Christersson, J.; Reutersward, C.; Karlsson, A.; Mitra, S.; Nimeus, E.; Holm, K.; et al. Comprehensive molecular comparison of brca1 hypermethylated and brca1 mutated triple negative breast cancers. Nat. Commun. 2020, 11, 3747. [Google Scholar] [CrossRef] [PubMed]
- Pruss, D.; Hayes, J.J.; Wolffe, A.P. Nucleosomal anatomy—Where are the histones? Bioessays 1995, 17, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749. [Google Scholar] [CrossRef] [PubMed]
- Sahu, V.; Lu, C. Oncohistones: Hijacking the histone code. Annu. Rev. Cancer Biol. 2022, 6, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Eads, C.A.; Danenberg, K.D.; Kawakami, K.; Saltz, L.B.; Danenberg, P.V.; Laird, P.W. Cpg island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999, 59, 2302–2306. [Google Scholar] [PubMed]
- Li, X.; Egervari, G.; Wang, Y.; Berger, S.L.; Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018, 19, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Issa, J.P.; Garcia-Manero, G.; Giles, F.J.; Mannari, R.; Thomas, D.; Faderl, S.; Bayar, E.; Lyons, J.; Rosenfeld, C.S.; Cortes, J.; et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004, 103, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Lubbert, M.; Daskalakis, M.; Kunzmann, R.; Engelhardt, M.; Guo, Y.; Wijermans, P. Nonclonal neutrophil responses after successful treatment of myelodysplasia with low-dose 5-aza-2′-deoxycytidine (decitabine). Leuk. Res. 2004, 28, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.P.; Gharibyan, V.; Cortes, J.; Jelinek, J.; Morris, G.; Verstovsek, S.; Talpaz, M.; Garcia-Manero, G.; Kantarjian, H.M. Phase ii study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J. Clin. Oncol. 2005, 23, 3948–3956. [Google Scholar] [CrossRef]
- Stomper, J.; Rotondo, J.C.; Greve, G.; Lubbert, M. Hypomethylating agents (hma) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: Mechanisms of resistance and novel hma-based therapies. Leukemia 2021, 35, 1873–1889. [Google Scholar] [CrossRef]
- Garner, I.M.; Brown, R. Is there a role for epigenetic therapies in modulating DNA damage repair pathways to enhance chemotherapy and overcome drug resistance? Cancers 2022, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Bertos, N.R.; Park, M. Breast cancer—One term, many entities? J. Clin. Investig. 2011, 121, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Prat, A.; Ellis, M.J.; Perou, C.M. Practical implications of gene-expression-based assays for breast oncologists. Nat. Rev. Clin. Oncol. 2011, 9, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Szymiczek, A.; Lone, A.; Akbari, M.R. Molecular intrinsic versus clinical subtyping in breast cancer: A comprehensive review. Clin. Genet. 2021, 99, 613–637. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Taurelli Salimbeni, B.; Corvaja, C.; Valenza, C.; Zagami, P.; Curigliano, G. The triple negative breast cancer drugs graveyard: A review of failed clinical trials 2017–2022. Expert Opin. Investig. Drugs 2022, 31, 1203–1226. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Tung, N.; Garber, J.E. Parp inhibition in breast cancer: Progress made and future hopes. NPJ Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Villwock, S.; Habenberger, P.; Choidas, A.; Rose, M.; Klebl, B.M. White paper: Mimetics of class 2 tumor suppressor proteins as novel drug candidates for personalized cancer therapy. Cancers 2022, 14, 4386. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wylie, R.C.; Hansen, N.J.; Andrews, L.G.; Tollefsbol, T.O. Profiling DNA methylation by bisulfite genomic sequencing: Problems and solutions. Methods Mol. Biol. 2004, 287, 169–179. [Google Scholar]
- Bibikova, M.; Lin, Z.; Zhou, L.; Chudin, E.; Garcia, E.W.; Wu, B.; Doucet, D.; Thomas, N.J.; Wang, Y.; Vollmer, E.; et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 2006, 16, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Ammerpohl, O.; Martin-Subero, J.I.; Richter, J.; Vater, I.; Siebert, R. Hunting for the 5th base: Techniques for analyzing DNA methylation. Biochim. Biophys. Acta 2009, 1790, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Tycko, B.; Liu, T.M.; Lin, H.J.; Huang, T.H. Methods in DNA methylation profiling. Epigenomics 2009, 1, 331–345. [Google Scholar] [CrossRef]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the illumina methylationepic beadchip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Barros-Silva, D.; Marques, C.J.; Henrique, R.; Jeronimo, C. Profiling DNA methylation based on next-generation sequencing approaches: New insights and clinical applications. Genes 2018, 9, 429. [Google Scholar] [CrossRef]
- Gouil, Q.; Keniry, A. Latest techniques to study DNA methylation. Essays Biochem. 2019, 63, 639–648. [Google Scholar]
- Hoy, S.M. Tazemetostat: First approval. Drugs 2020, 80, 513–521. [Google Scholar] [CrossRef]
- Liu, S.; Cadoux-Hudson, T.; Schofield, C.J. Isocitrate dehydrogenase variants in cancer—Cellular consequences and therapeutic opportunities. Curr. Opin. Chem. Biol. 2020, 57, 122–134. [Google Scholar] [CrossRef]
- Stone, A.; Zotenko, E.; Locke, W.J.; Korbie, D.; Millar, E.K.; Pidsley, R.; Stirzaker, C.; Graham, P.; Trau, M.; Musgrove, E.A.; et al. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nat. Commun. 2015, 6, 7758. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Tran, I.; Yang, Y.; Shen, G.; Miah, P.; Cotzia, P.; Roses, D.; Schnabel, F.; Darvishian, F.; Snuderl, M. DNA methylation identifies epigenetic subtypes of triple-negative breast cancers with distinct clinicopathologic and molecular features. Mod. Pathol. 2023, 36, 100306. [Google Scholar] [CrossRef] [PubMed]
- Zolota, V.; Tzelepi, V.; Piperigkou, Z.; Kourea, H.; Papakonstantinou, E.; Argentou Mu, I.; Karamanos, N.K. Epigenetic alterations in triple-negative breast cancer-the critical role of extracellular matrix. Cancers 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Idrissou, M.; Sanchez, A.; Penault-Llorca, F.; Bignon, Y.J.; Bernard-Gallon, D. Epi-drugs as triple-negative breast cancer treatment. Epigenomics 2020, 12, 725–742. [Google Scholar] [CrossRef]
- Feng, S.; De Carvalho, D.D. Clinical advances in targeting epigenetics for cancer therapy. FEBS J. 2022, 289, 1214–1239. [Google Scholar] [CrossRef]
- Kim, A.; Mo, K.; Kwon, H.; Choe, S.; Park, M.; Kwak, W.; Yoon, H. Epigenetic regulation in breast cancer: Insights on epidrugs. Epigenomes 2023, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Nin, D.S.; Deng, L.W. Biology of cancer-testis antigens and their therapeutic implications in cancer. Cells 2023, 12, 926. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA methylation causes an interferon response in cancer via dsrna including endogenous retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef]
- Loo Yau, H.; Ettayebi, I.; De Carvalho, D.D. The cancer epigenome: Exploiting its vulnerabilities for immunotherapy. Trends Cell Biol. 2019, 29, 31–43. [Google Scholar] [CrossRef]
- Qin, Y.; Vasilatos, S.N.; Chen, L.; Wu, H.; Cao, Z.; Fu, Y.; Huang, M.; Vlad, A.M.; Lu, B.; Oesterreich, S.; et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene 2019, 38, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Loo Yau, H.; Bell, E.; Ettayebi, I.; de Almeida, F.C.; Boukhaled, G.M.; Shen, S.Y.; Allard, D.; Morancho, B.; Marhon, S.A.; Ishak, C.A.; et al. DNA hypomethylating agents increase activation and cytolytic activity of cd8(+) t cells. Mol. Cell 2021, 81, 1469–1483.e1468. [Google Scholar] [CrossRef] [PubMed]
- Micevic, G.; Bosenberg, M.W.; Yan, Q. The crossroads of cancer epigenetics and immune checkpoint therapy. Clin. Cancer Res. 2023, 29, 1173–1182. [Google Scholar] [CrossRef]
- To, K.K.W.; Xing, E.; Larue, R.C.; Li, P.K. Bet bromodomain inhibitors: Novel design strategies and therapeutic applications. Molecules 2023, 28, 3043. [Google Scholar] [CrossRef] [PubMed]
- Aleckovic, M.; Li, Z.; Zhou, N.; Qiu, X.; Lulseged, B.; Foidart, P.; Huang, X.Y.; Garza, K.; Shu, S.; Kesten, N.; et al. Combination therapies to improve the efficacy of immunotherapy in triple-negative breast cancer. Mol. Cancer Ther. 2023, 22, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Llinas-Arias, P.; Iniguez-Munoz, S.; McCann, K.; Voorwerk, L.; Orozco, J.I.J.; Ensenyat-Mendez, M.; Sese, B.; DiNome, M.L.; Marzese, D.M. Epigenetic regulation of immunotherapy response in triple-negative breast cancer. Cancers 2021, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Stomper, J.; Lubbert, M. Can we predict responsiveness to hypomethylating agents in aml? Semin. Hematol. 2019, 56, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Clifton, S.; Locke, W.; Luu, P.L.; Du, Q.; Lam, D.; Armstrong, N.J.; Kumar, B.; Deng, N.; Harvey, K.; et al. Identification of DNA methylation biomarkers with potential to predict response to neoadjuvant chemotherapy in triple-negative breast cancer. Clin. Epigenetics 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Schambach, A.; Buchholz, C.J.; Torres-Ruiz, R.; Cichutek, K.; Morgan, M.; Trapani, I.; Buning, H. A new age of precision gene therapy. Lancet 2023, 403, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Gjaltema, R.A.F.; Rots, M.G. Advances of epigenetic editing. Curr. Opin. Chem. Biol. 2020, 57, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Yamazaki, T.; Funakoshi, H. Toward the development of epigenome editing-based therapeutics: Potentials and challenges. Int. J. Mol. Sci. 2023, 24, 4778. [Google Scholar] [CrossRef]
- Zeps, N.; Lysaght, T.; Chadwick, R.; Erler, A.; Foo, R.; Giordano, S.; San Lai, P.; Schaefer, G.O.; Xafis, V.; Chew, W.L.; et al. Ethics and regulatory considerations for the clinical translation of somatic cell human epigenetic editing. Stem Cell Rep. 2021, 16, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Alex, K.; Winkler, E.C. Comparative ethical evaluation of epigenome editing and genome editing in medicine: First steps and future directions. J. Med. Ethics 2023, 50, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Qiao, Z.; Yang, Y.; Deng, Y.; Zhang, Z.; Yu, X.; Guo, X. Unveiling epigenetic vulnerabilities in triple-negative breast cancer through 3d organoid drug screening. Pharmaceuticals 2024, 17, 225. [Google Scholar] [CrossRef] [PubMed]
| Reference | |
|---|---|
| Phenotypical variability of identical twins | [16] |
| X-chromosome inactivation | [17] |
| Mating types in S. cerevisiae | [18] |
| Imprinting | [19] |
| Functional and phenotypical variability of cells in higher organisms | [20] |
| Epiphenotypes in Arabidopsis flowers | [21] |
| Phenotypical variability of cloned animals | [20] |
| Reference | |
|---|---|
| Birabresib (BETi) | NCT02259114 |
| Entinostat (HDACi) + Azacytidin (DNMTi) | NCT01349959 |
| Decitabine (DNMTi) + Carboplatin | NCT03295552 |
| Chidamide (HDACi) + Cisplatin | NCT04192903 |
| Entinostat (HADCi) + other drugs | NCT04296902 |
| Abbreviations: BETi: bromodomain and extra-terminal motif inhibitor, DNMTi: DNA methylatransferase inhibitor, HDACi: Histone deacetylase inhibitor |
| Reference | |
|---|---|
| Panobinostat (HDACi)/Everolimus/LCL161 + PRD001 (anti-PD-1) | NCT02890069 |
| Entinostat (HDACi) + Atezolizumab (anti-PD-L1) | NCT02708680 |
| RO6870810 (BETi) + Atezolizumab (anti-PD-L1) | NCT03292172 |
| Abbreviations: BETi: bromodomain and extra-terminal motif inhibitor, HDACi: Histone deacetylase inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, U. Epigenetic Therapies in Triple-Negative Breast Cancer: Concepts, Visions, and Challenges. Cancers 2024, 16, 2164. https://doi.org/10.3390/cancers16122164
Lehmann U. Epigenetic Therapies in Triple-Negative Breast Cancer: Concepts, Visions, and Challenges. Cancers. 2024; 16(12):2164. https://doi.org/10.3390/cancers16122164
Chicago/Turabian StyleLehmann, Ulrich. 2024. "Epigenetic Therapies in Triple-Negative Breast Cancer: Concepts, Visions, and Challenges" Cancers 16, no. 12: 2164. https://doi.org/10.3390/cancers16122164






