Multi-Cohort Transcriptomic Profiling of Medical Gas Plasma-Treated Cancers Reveals the Role of Immunogenic Cell Death
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Single-Cohort Data Collection and Re-Analysis
2.3. Multi-Cohort Meta-Analysis
2.4. Comparative Functional Enrichment Analysis
3. Results
3.1. Summary of Eligible Cohort Studies
3.2. Single-Cohort Transcriptomic Re-Analysis Revealed Consistently Up-Regulated Genes
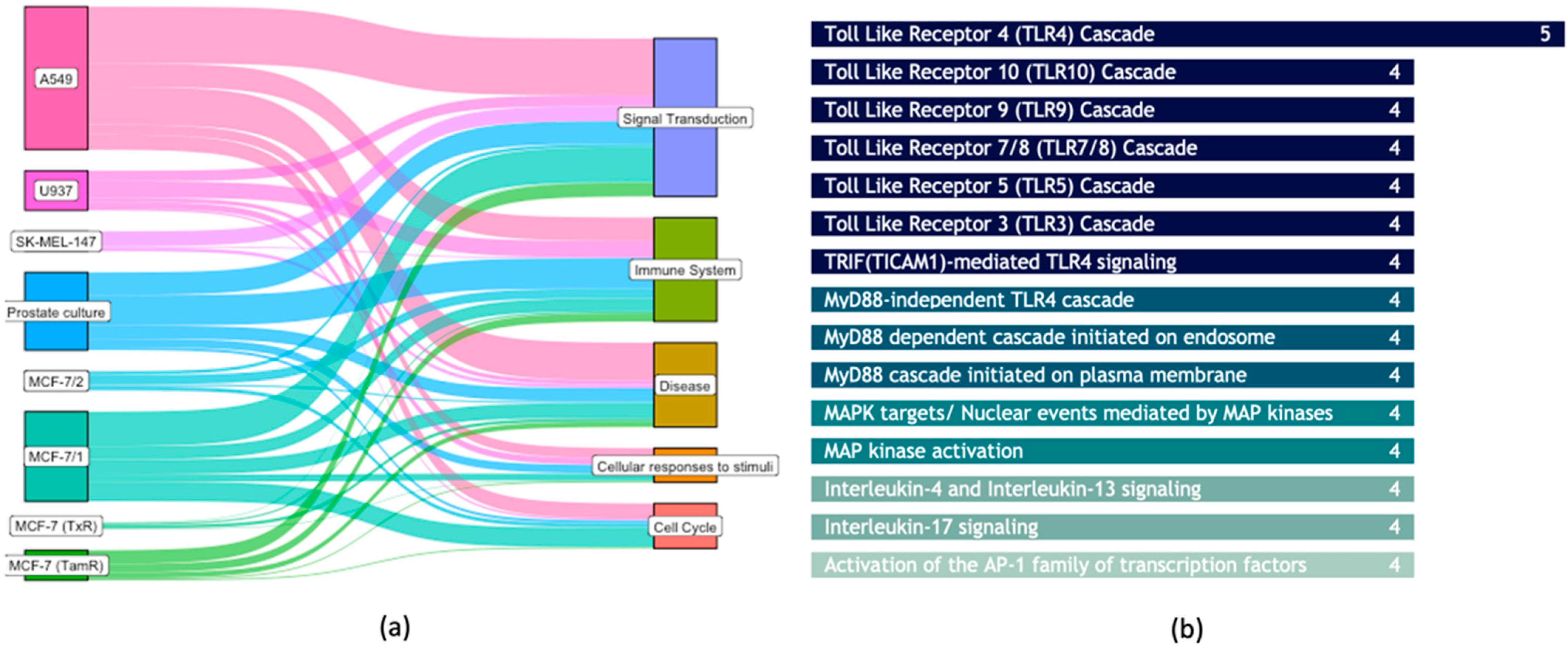
3.3. Common Biological Aberrations Caused by Plasma-Treated Cancer Cells
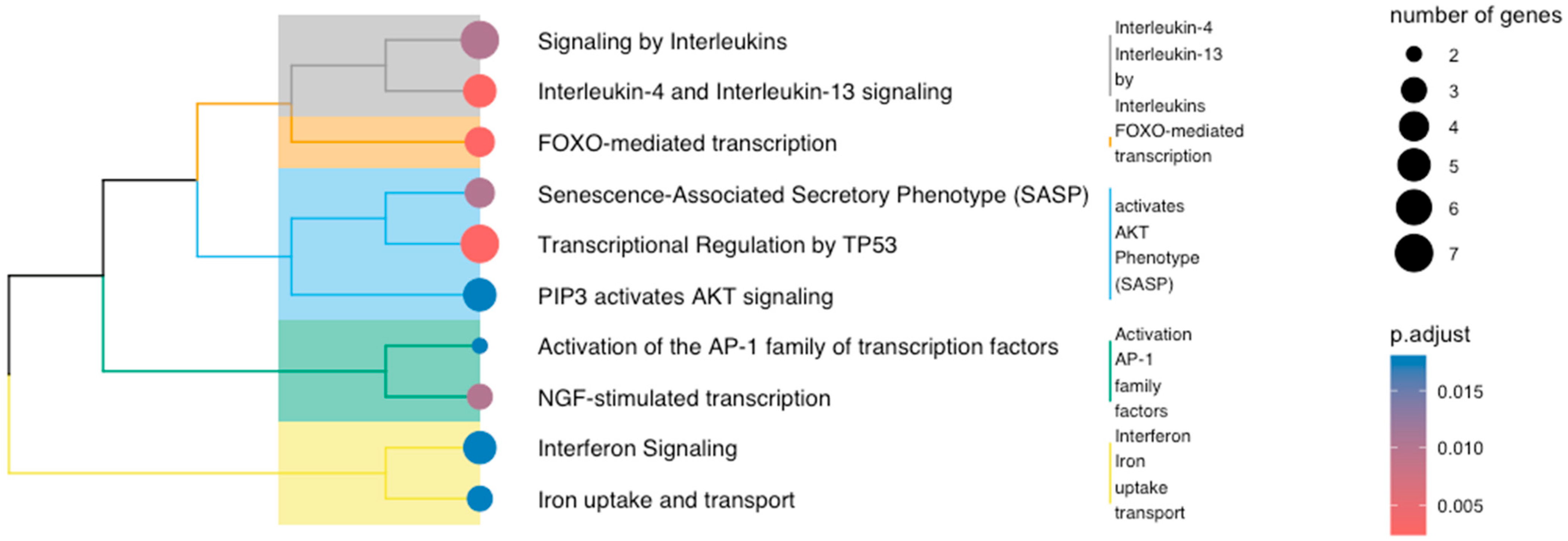
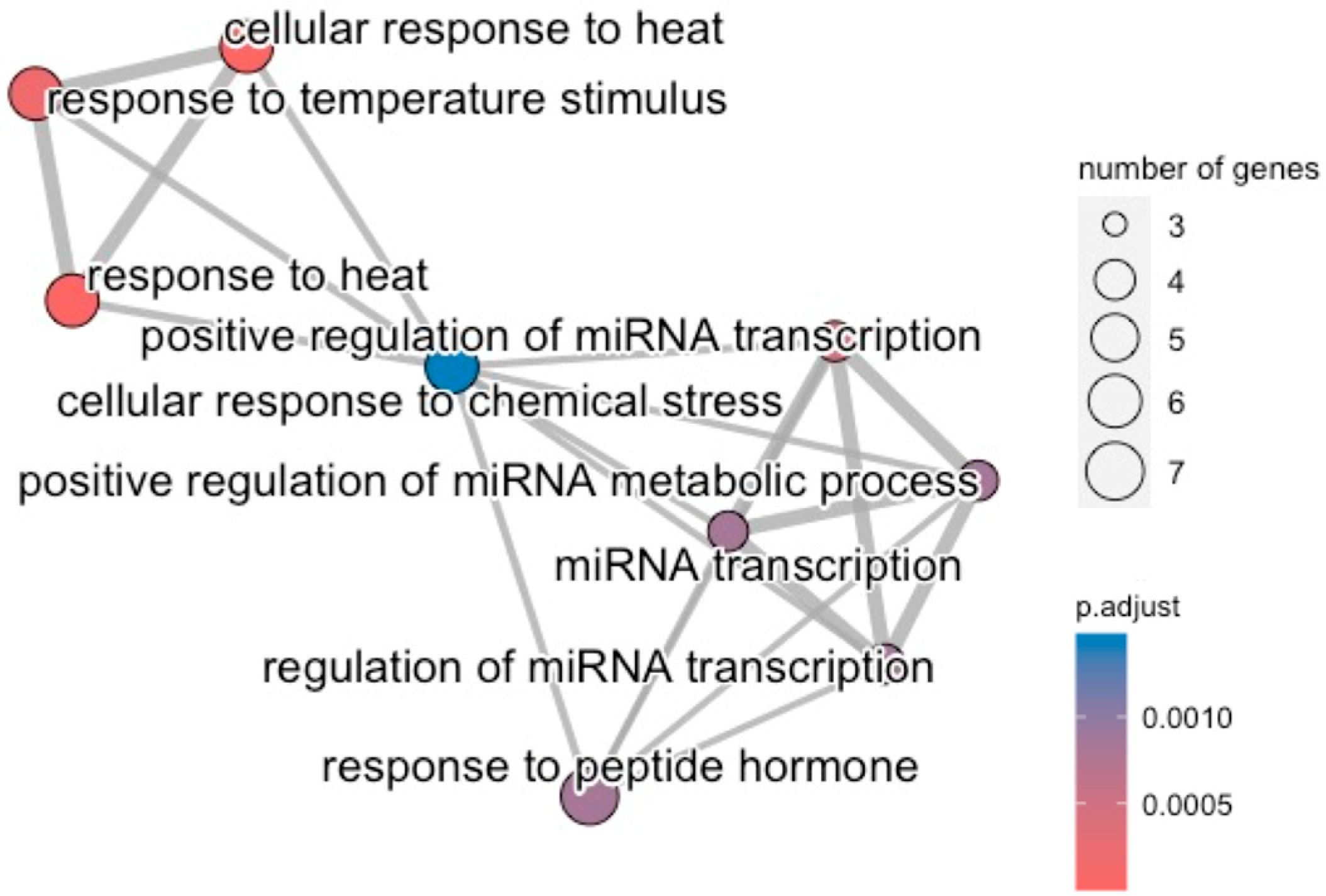
3.4. Multi-Cohort Transcriptomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanikar, R.R.; Bailung, H.; Khanikar, R.R.; Bailung, H. Cold Atmospheric Pressure Plasma Technology for Biomedical Application. In Plasma Science and Technology; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Jiang, J.; Duan, J.W.; Wu, X.J.Z.; Zhang, S.; Duan, X.R.; Song, J.Q.; Chen, H.X. Cold Atmospheric Plasma Applications in Dermatology: A Systematic Review. J. Biophotonics 2021, 14, e2652. [Google Scholar] [CrossRef] [PubMed]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bazaka, K.; Thompson, E.W.; Ostrikov, K. Cold Atmospheric Plasma: A Promising Controller of Cancer Cell States. Cancers 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rui, X.; Li, D.; Wang, Y.; Tan, F. Plasma Oncology: Adjuvant Therapy for Head and Neck Cancer Using Cold Atmospheric Plasma. Front. Oncol. 2022, 12, 994172. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.S.; Choi, E.H.; Chang, B.; Choi, J.H.; Kim, K.S.; Park, H.K. Selective Cytotoxic Effect of Non-Thermal Micro-DBD Plasma. Phys. Biol. 2016, 13, 056001. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward Understanding the Selective Anticancer Capacity of Cold Atmospheric Plasma—A Model Based on Aquaporins (Review). Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Min, T.; Xie, X.; Ren, K.; Sun, T.; Wang, H.; Dang, C.; Zhang, H. Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor. Front. Med. 2022, 9, 884887. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of Lipid Peroxidation on Membrane Permeability of Cancer and Normal Cells Subjected to Oxidative Stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, F.; Zafari, P.; Alimohammadi, M.; Moonesi, M.; Rafiei, A.; Bekeschus, S. Cold Physical Plasma in Cancer Therapy: Mechanisms, Signaling, and Immunity. Oxid. Med. Cell. Longev. 2021, 2021, 9916796. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Hou, J.; Ma, J.; Yu, K.N.; Li, W.; Cheng, C.; Bao, L.; Han, W. Non-Thermal Plasma Treatment Altered Gene Expression Profiling in Non-Small-Cell Lung Cancer A549 Cells. BMC Genom. 2015, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of Solid Tumor Cells in DMEM to Reactive Oxygen Species Generated by Non-Thermal Plasma and Chemically Induced ROS Systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold Atmospheric Plasma and Iron Oxide-Based Magnetic Nanoparticles for Synergetic Lung Cancer Therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef]
- Ma, J.; Yu, K.N.; Cheng, C.; Ni, G.; Shen, J.; Han, W. Targeting Nrf2-Mediated Heme Oxygenase-1 Enhances Non-Thermal Plasma-Induced Cell Death in Non-Small-Cell Lung Cancer A549 Cells. Arch. Biochem. Biophys. 2018, 658, 54–65. [Google Scholar] [CrossRef]
- Motaln, H.; Recek, N.; Rogelj, B. Intracellular Responses Triggered by Cold Atmospheric Plasma and Plasma-Activated Media in Cancer Cells. Molecules 2021, 26, 1336. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, L.; Zou, F.; Hu, H.; Liu, K.; Lin, Z. Gene Expression Profiling and Functional Analysis Reveals That P53 Pathway-Related Gene Expression Is Highly Activated in Cancer Cells Treated by Cold Atmospheric Plasma-Activated Medium. PeerJ 2017, 5, e3751. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Bien-Möller, S.; Marx, S.; Bekeschus, S.; Schroeder, H.W.S.; Mustea, A.; Stope, M.B. Devitalization of Glioblastoma Cancer Cells by Non-Invasive Physical Plasma: Modulation of Proliferative Signalling Cascades. Anticancer Res. 2023, 43, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.W.; Kim, H.; Kim, H.W.; Yun, S.H.; Park, J.E.; Choi, E.H.; Kim, S.J. Genome-Wide Comparison of the Target Genes of the Reactive Oxygen Species and Non-Reactive Oxygen Species Constituents of Cold Atmospheric Plasma in Cancer Cells. Cancers 2020, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, Q.; Adhikari, M.; Malyavko, A.; Lin, L.; Zolotukhin, D.B.; Yao, X.; Kirschner, M.; Sherman, J.H.; Keidar, M. A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment. ACS Appl. Mater. 2020, 12, 34548–34563. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.P.; Laurencin-Dalicieux, S.; Cousty, S. Use of Cold-Atmospheric Plasma in Oncology: A Concise Systematic Review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S. Medical Gas Plasma Technology: Roadmap on Cancer Treatment and Immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Clemen, R. Plasma, Cancer, Immunity. J. Phys. D Appl. Phys. 2022, 55, 473003. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open Software Development for Computational Biology and Bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Sean, D.; Meltzer, P.S. GEOquery: A Bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Mudunuri, U.; Che, A.; Yi, M.; Stephens, R.M. BioDBnet: The Biological Database Network. Bioinformatics 2009, 25, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping Identifiers for the Integration of Genomic Datasets with the R/Bioconductor Package BiomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Haynes, W.A.; Vallania, F.; Liu, C.; Bongen, E.; Tomczak, A.; Andres-Terrè, M.; Lofgren, S.; Tam, A.; Deisseroth, C.A.; Li, M.D.; et al. Empowering Multi-Cohort Gene Expression Analysis to Increase Reproducibility. In Proceedings of the Pacific Symposium on Biocomputing, Kohala, HI, USA, 3–7 January 2017; pp. 144–153. [Google Scholar] [CrossRef]
- Yu, G.; He, Q.Y. ReactomePA: An R/Bioconductor Package for Reactome Pathway Analysis and Visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Ulgen, E.; Ozisik, O.; Sezerman, O.U. PathfindR: An R Package for Comprehensive Identification of Enriched Pathways in Omics Data Through Active Subnetworks. Front. Genet. 2019, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID Database: A Comprehensive Biomedical Resource of Curated Protein, Genetic, and Chemical Interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Rezwani, M.; Pourfathollah, A.A.; Noorbakhsh, F. Rbioapi: User-Friendly R Interface to Biologic Web Services’ API. Bioinformatics 2022, 38, 2952–2953. [Google Scholar] [CrossRef]
- Packer, J.R.; Hirst, A.M.; Droop, A.P.; Adamson, R.; Simms, M.S.; Mann, V.M.; Frame, F.M.; O’Connell, D.; Maitland, N.J. Notch Signalling Is a Potential Resistance Mechanism of Progenitor Cells within Patient-Derived Prostate Cultures Following ROS-Inducing Treatments. FEBS Lett. 2020, 594, 209–226. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Uchiyama, H.; Zhao, Q.L.; Yunoki, T.; Andocs, G.; Nojima, N.; Takeda, K.; Ishikawa, K.; Hori, M.; Kondo, T. Effects of Nitrogen on the Apoptosis of and Changes in Gene Expression in Human Lymphoma U937 Cells Exposed to Argon-Based Cold Atmospheric Pressure Plasma. Int. J. Mol. Med. 2016, 37, 1706–1714. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Tamoxifen Sensitivity in Resistant MCF-7 Breast Cancer Cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Paclitaxel Sensitivity to Paclitaxel-Resistant Breast Cancer Cells by Reversing Expression of Resistance-Related Genes. Cancers 2019, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Burt, K.G.; Jacobsen, T.; Fernandes, T.D.; Alipui, D.O.; Weber, K.T.; Levine, M.; Chavan, S.S.; Yang, H.; Tracey, K.J.; et al. High Mobility Group Box-1 Induces Pro-Inflammatory Signaling in Human Nucleus Pulposus Cells via Toll-Like Receptor 4-Dependent Pathway. J. Orthop. Res. 2019, 37, 220–231. [Google Scholar] [CrossRef]
- Hadefi, A.; Leprovots, M.; Thulliez, M.; Bastin, O.; Lefort, A.; Libert, F.; Nonclercq, A.; Delchambre, A.; Reniers, F.; Devière, J.; et al. Cold Atmospheric Plasma Differentially Affects Cell Renewal and Differentiation of Stem Cells and APC-Deficient-Derived Tumor Cells in Intestinal Organoids. Cell Death Discov. 2022, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.I.; Boo, Y.C. A Regulatory Role of Kruppel-like Factor 4 in Endothelial Argininosuccinate Synthetase 1 Expression in Response to Laminar Shear Stress. Biochem. Biophys. Res. Commun. 2012, 420, 450–455. [Google Scholar] [CrossRef]
- Krysan, K.; Reckamp, K.L.; Dalwadi, H.; Sharma, S.; Rozengurt, E.; Dohadwala, M.; Dubinett, S.M. Prostaglandin E2 Activates Mitogen-Activated Protein Kinase/Erk Pathway Signaling and Cell Proliferation in Non-Small Cell Lung Cancer Cells in an Epidermal Growth Factor Receptor-Independent Manner. Cancer Res. 2005, 65, 6275–6281. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-N.; Oh, C.; Won Chang, J.; Liu, L.; Ae Lim, M.; Li Jin, Y.; Piao, Y.; Jong Kim, H.; Won, H.-R.; Eun Lee, S.; et al. EGR1/GADD45α Activation by ROS of Non-Thermal Plasma Mediates Cell Death in Thyroid Carcinoma. Cancers 2021, 13, 351. [Google Scholar] [CrossRef]
- Odagiu, L.; May, J.; Boulet, S.; Baldwin, T.A.; Labrecque, N. Role of the Orphan Nuclear Receptor NR4A Family in T-Cell Biology. Front. Endocrinol. 2021, 11, 624122. [Google Scholar] [CrossRef]
- Safe, S.; Karki, K. The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer. Mol. Cancer Res. 2021, 19, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.; Le Corre, L.; Odagiu, L.; Labrecque, N. Role of NR4A Family Members in Myeloid Cells and Leukemia. Curr. Res. Immunol. 2022, 3, 23. [Google Scholar] [CrossRef]
- Deng, S.; Chen, B.; Huo, J.; Liu, X. Therapeutic Potential of NR4A1 in Cancer: Focus on Metabolism. Front. Oncol. 2022, 12, 972984. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Li, M.; Chen, Z.; Tang, W.; Cheng, X. NR4A1 as a Potential Therapeutic Target in Colon Adenocarcinoma: A Computational Analysis of Immune Infiltration and Drug Response. Front. Genet. 2023, 14, 1181320. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bian, Y.; Liu, L.; Liu, L.; Liu, X.; Ma, S. Molecular Pathways Associated with Oxidative Stress and Their Potential Applications in Radiotherapy (Review). Int. J. Mol. Med. 2022, 49, 65. [Google Scholar] [CrossRef]
- Aaron Hobbs, G.; Zhou, B.; Cox, A.D.; Campbell, S.L. Rho GTPases, Oxidation, and Cell Redox Control. Small GTPases 2014, 5, e28579. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Regulation of the Insulin Signalling Pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Vashishta, M.; Dwarakanath, B.S. Oxidative Stress and Notch Signaling: Implications in Cancer. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Singapore, 2022; Volume 2. [Google Scholar]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-Activated Medium Triggers Cell Death and the Presentation of Immune Activating Danger Signals in Melanoma and Pancreatic Cancer Cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Bekeschus, S.; Mueller, A.; Miller, V.; Gaipl, U.; Weltmann, K.D. Physical Plasma Elicits Immunogenic Cancer Cell Death and Mitochondrial Singlet Oxygen. Proc. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 138–146. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2016, 17, 97–111. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic Cell Death in Cancer Therapy: Present and Emerging Inducers. J. Cell. Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, S.; Yang, H. Roles of Toll-Like Receptor 3 in Human Tumors. Front. Immunol. 2021, 12, 667454. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Li, W.; Liu, Y.; Xu, D.; Liu, Z.; Huang, C. Aberrant Expressional Profiling of Small RNA by Cold Atmospheric Plasma Treatment in Human Chronic Myeloid Leukemia Cells. Front. Genet. 2022, 12, 809658. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Liebelt, G.; Menz, J.; Berner, J.; Sagwal, S.K.; Wende, K.; Weltmann, K.D.; Boeckmann, L.; von Woedtke, T.; Metelmann, H.R.; et al. Tumor Cell Metabolism Correlates with Resistance to Gas Plasma Treatment: The Evaluation of Three Dogmas. Free Radic. Biol. Med. 2021, 167, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, J.; Chen, Y.; Ostrikov, K. When Onco-Immunotherapy Meets Cold Atmospheric Plasma: Implications on CAR-T Therapies. Front. Oncol. 2022, 12, 837995. [Google Scholar] [CrossRef] [PubMed]
- Basílio, J.; Hochreiter, B.; Hoesel, B.; Sheshori, E.; Mussbacher, M.; Hanel, R.; Schmid, J.A. Antagonistic Functions of Androgen Receptor and NF-ΚB in Prostate Cancer—Experimental and Computational Analyses. Cancers 2022, 14, 6164. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Brody, J.D.; Rajakumaraswamy, N.; Kuhne, M.; Trowe, T.; Dauki, A.M.; Pai, S.; Han, L.; Lin, K.-W.; Petrarca, M.; et al. Phase I Study of GS-3583, a FLT3 Agonist Fc Fusion Protein, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2024, OF1–OF10. [Google Scholar] [CrossRef] [PubMed]
- D’Anniballe, V.M.; Huang, M.N.; Lueck, B.D.; Nicholson, L.T.; McFatridge, I.; Gunn, M.D. Antigen-Loaded Monocyte Administration and Flt3 Ligand Augment the Antitumor Efficacy of Immune Checkpoint Blockade in a Murine Melanoma Model. J. Immunother. 2023, 46, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, L.; Zitvogel, L.; Kepp, O.; Kroemer, G. Immunogenic Cell Death (ICD) Enhancers-Drugs That Enhance the Perception of ICD by Dendritic Cells. Immunol. Rev. 2024, 321, 7–19. [Google Scholar] [CrossRef]
- Sotudian, S.; Paschalidis, I.C. Machine Learning for Pharmacogenomics and Personalized Medicine: A Ranking Model for Drug Sensitivity Prediction. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2324–2333. [Google Scholar] [CrossRef]
- Kardamiliotis, K.; Karanatsiou, E.; Aslanidou, I.; Stergiou, E.; Vizirianakis, I.S.; Malousi, A. Unraveling Drug Response from Pharmacogenomic Data to Advance Systems Pharmacology Decisions in Tumor Therapeutics. Future Pharmacol. 2022, 2, 31–44. [Google Scholar] [CrossRef]


| GEO ID | Samples | Cancer Cells | Cancer Type | Device | Carrier Gas | Treatment | Platform | Citation |
|---|---|---|---|---|---|---|---|---|
| GSE119052 | 12 | Patient epithelial prostate | Prostate cancer | Dielectric Barrier Discharge | Helium (0.3% mol. oxygen) | Direct | Affymetrix Human Clariom D Assay | [42] * |
| GSE59997 | 15 | A549 | Lung cancer | Dielectric Barrier Discharge | Helium | Direct | Affymetrix Human Gene Expression Array | [18] |
| GSE76022 | 6 | U937 | Lymphoma | Plasma Jet | Argon ± Nitrogen | Direct | Affymetrix Human Genome U133 Plus 2.0 | [43] * |
| GSE65972 | 32 | SK-Mel-147 | Melanoma | Plasma Jet kinpen09 | Argon | Indirect | Agilent-062647 INP_039494_Human_GE_v2 | [44] * |
| GSE95208 | 3 | MCF-7 (+MCF-7/TamR) | Breast Ca (Tamoxifen-resistant) | Dielectric Barrier Discharge | Argon | Direct | Illumina HumanHT-12 V4.0 beadchip | [45] |
| GSE110117 ^ | 2 | MCF-7/1 | Breast cancer | Dielectric Barrier Discharge | Argon | Direct | Illumina HumanHT-12 V4.0 beadchip | [45] ** |
| GSE117491 ^ | 2 | MCF-7/2 | Breast Ca | NA | NA | NA | Agilent-072363 SurePrint G3 Human GE v3 | NA |
| GSE131480 | 7 | MCF-7 (+MCF-7/TxR) | Breast Ca (Paclitaxel-resistant) | Dielectric Barrier Discharge | Argon | Direct | Agilent-072363 SurePrint G3 Human GE v3 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantaras, A.; Kotzamanidis, C.; Kyriakidis, K.; Farmaki, E.; Makedou, K.; Tzimagiorgis, G.; Bekeschus, S.; Malousi, A. Multi-Cohort Transcriptomic Profiling of Medical Gas Plasma-Treated Cancers Reveals the Role of Immunogenic Cell Death. Cancers 2024, 16, 2186. https://doi.org/10.3390/cancers16122186
Gkantaras A, Kotzamanidis C, Kyriakidis K, Farmaki E, Makedou K, Tzimagiorgis G, Bekeschus S, Malousi A. Multi-Cohort Transcriptomic Profiling of Medical Gas Plasma-Treated Cancers Reveals the Role of Immunogenic Cell Death. Cancers. 2024; 16(12):2186. https://doi.org/10.3390/cancers16122186
Chicago/Turabian StyleGkantaras, Antonios, Charalampos Kotzamanidis, Konstantinos Kyriakidis, Evangelia Farmaki, Kali Makedou, Georgios Tzimagiorgis, Sander Bekeschus, and Andigoni Malousi. 2024. "Multi-Cohort Transcriptomic Profiling of Medical Gas Plasma-Treated Cancers Reveals the Role of Immunogenic Cell Death" Cancers 16, no. 12: 2186. https://doi.org/10.3390/cancers16122186
APA StyleGkantaras, A., Kotzamanidis, C., Kyriakidis, K., Farmaki, E., Makedou, K., Tzimagiorgis, G., Bekeschus, S., & Malousi, A. (2024). Multi-Cohort Transcriptomic Profiling of Medical Gas Plasma-Treated Cancers Reveals the Role of Immunogenic Cell Death. Cancers, 16(12), 2186. https://doi.org/10.3390/cancers16122186









