Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Samples
2.2. IHC and Its Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. SSTR2A and HIF-2α Expression with Clinicopathological Profile
3.3. TME with SSTR2A and HIF-2α Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hescot, S.; Curras-Freixes, M.; Deutschbein, T.; van Berkel, A.; Vezzosi, D.; Amar, L.; de la Fouchardière, C.; Valdes, N.; Riccardi, F.; Do Cao, C.; et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 2367–2374. [Google Scholar] [CrossRef]
- Wang, K.; Crona, J.; Beuschlein, F.; Grossman, A.B.; Pacak, K.; Nölting, S. Targeted therapies in pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 2022, 107, 2963–2972. [Google Scholar] [CrossRef]
- Jimenez, C.; Subbiah, V.; Stephen, B.; Ma, J.; Milton, D.; Xu, M.; Zarifa, A.; Akhmedzhanov, F.O.; Tsimberidou, A.; Habra, M.A.; et al. Phase II clinical trial of pembrolizumab in patients with progressive metastatic pheochromocytomas and paragangliomas. Cancers 2020, 12, 2307. [Google Scholar] [CrossRef]
- Esfahani, S.A.; Ferreira, C.D.; Summer, P.; Mahmood, U.; Heidari, P. Addition of peptide receptor radiotherapy to immune checkpoint inhibition therapy improves outcomes in neuroendocrine tumors. J. Nucl. Med. 2023, 64, 1056–1061. [Google Scholar] [CrossRef]
- Kao, T.W.; Bai, G.H.; Wang, T.L.; Shih, I.M.; Chuang, C.M.; Lo, C.L.; Tsai, M.C.; Chiu, L.Y.; Lin, C.C.; Shen, Y.A. Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance. J. Exp. Clin. Cancer Res. 2023, 42, 171. [Google Scholar] [CrossRef]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef]
- Givechian, K.B.; Wnuk, K.; Garner, C.; Benz, S.; Garban, H.; Rabizadeh, S.; Niazi, K.; Soon-Shiong, P. Identification of an immune gene expression signature associated with favorable clinical features in Treg-enriched patient tumor samples. NPJ Genom. Med. 2018, 3, 14. [Google Scholar] [CrossRef]
- Yahaya, M.A.F.; Lila, M.A.M.; Ismail, S.; Zainol, M.; Afizan, N. Tumour-associated macrophages (TAMs) in colon cancer and how to reeducate them. J. Immunol. Res. 2019, 2019, 2368249. [Google Scholar] [CrossRef]
- Calsina, B.; Piñeiro-Yáñez, E.; Martínez-Montes, Á.M.; Caleiras, E.; Fernández-Sanromán, Á.; Monteagudo, M.; Torres-Pérez, R.; Fustero-Torre, C.; Pulgarín-Alfaro, M.; Gil, E.; et al. Genomic and immune landscape of metastatic pheochromocytoma and paraganglioma. Nat. Commun. 2023, 14, 1122. [Google Scholar] [CrossRef]
- Ghosal, S.; Vanova, K.H.; Uher, O.; Das, S.; Patel, M.; Meuter, L.; Huynh, T.T.; Jha, A.; Talvacchio, S.; Knue, M.; et al. Immune signature of pheochromocytoma and paraganglioma in context of neuroendocrine neoplasms associated with prognosis. Endocrine 2023, 79, 171–179. [Google Scholar] [CrossRef]
- Tufton, N.; Hearnden, R.J.; Berney, D.M.; Drake, W.M.; Parvanta, L.; Chapple, J.P.; Akker, S.A. The immune cell infiltrate in the tumour microenvironment of phaeochromocytomas and paragangliomas. Endocr. Relat. Cancer 2022, 29, 589–598. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liu, L.; Wang, J.; Wu, J.; Sun, C. Role of macrophages in tumor progression and therapy (Review). Int. J. Oncol. 2022, 60, 57. [Google Scholar] [CrossRef]
- Yamazaki, T.; Vanpouille-Box, C.; Demaria, S.; Galluzzi, L. Immunogenic cell death driven by radiation-impact on the tumor microenvironment. Cancer Treat. Res. 2020, 180, 281–296. [Google Scholar] [CrossRef]
- Xu, L.; Xie, X.; Luo, Y. The role of macrophage in regulating tumour microenvironment and the strategies for reprogramming tumour-associated macrophages in antitumour therapy. Eur. J. Cell. Biol. 2021, 100, 151153. [Google Scholar] [CrossRef]
- Terakawa, A.; Tanabe, A.; Nakayama, H.; Minamimoto, R. External beam radiotherapy in advanced pheochromocytoma and paraganglioma: An observation of a rare abscopal effect. JCEM Case Rep. 2023, 1, luad111. [Google Scholar] [CrossRef]
- Grass, G.D.; Krishna, N.; Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 2016, 40, 10–24. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Fendler, W.P.; Lueckerath, K.; Berliner, C.; Kurzidem, S.; Hadaschik, E.; Klode, J.; Zimmer, L.; Livingstone, E.; Schadendorf, D.; et al. Response to combined peptide receptor radionuclide therapy and checkpoint immunotherapy with ipilimumab plus nivolumab in metastatic merkel cell carcinoma. J. Nucl. Med. 2022, 63, 396–398. [Google Scholar] [CrossRef]
- Lin, A.L.; Tabar, V.; Young, R.J.; Cohen, M.; Cuaron, J.; Yang, T.J.; Rosenblum, M.; Rudneva, V.A.; Geer, E.B.; Bodei, L. Synergism of checkpoint inhibitors and peptide receptor radionuclide therapy in the treatment of pituitary carcinoma. J. Endocr. Soc. 2021, 5, bvab133. [Google Scholar] [CrossRef]
- Fischer, A.; Kloos, S.; Maccio, U.; Friemel, J.; Remde, H.; Fassnacht, M.; Pamporaki, C.; Eisenhofer, G.; Timmers, H.; Robledo, M.; et al. Metastatic pheochromocytoma and paraganglioma: Somatostatin receptor 2 expression, genetics, and therapeutic responses. J. Clin. Endocrinol. Metab. 2023, 108, 2676–2685. [Google Scholar] [CrossRef]
- Elston, M.S.; Meyer-Rochow, G.Y.; Conaglen, H.M.; Clarkson, A.; Clifton-Bligh, R.J.; Conaglen, J.V.; Gill, A.J. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase-deficient pheochromocytomas and paragangliomas. Hum. Pathol. 2015, 46, 390–396. [Google Scholar] [CrossRef]
- Leijon, H.; Remes, S.; Hagström, J.; Louhimo, J.; Mäenpää, H.; Schalin-Jäntti, C.; Miettinen, M.; Haglund, C.; Arola, J. Variable somatostatin receptor subtype expression in 151 primary pheochromocytomas and paragangliomas. Hum. Pathol. 2019, 86, 66–75. [Google Scholar] [CrossRef]
- Shurin, M.R.; Umansky, V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J. Clin. Investig. 2022, 132, e159473. [Google Scholar] [CrossRef]
- Messai, Y.; Gad, S.; Noman, M.Z.; Le Teuff, G.; Couve, S.; Janji, B.; Kammerer, S.F.; Rioux-Leclerc, N.; Hasmim, M.; Ferlicot, S.; et al. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von Hippel-Lindau gene mutation status. Eur. Urol. 2016, 70, 623–632. [Google Scholar] [CrossRef]
- Ahmed, R.; Ornstein, M.C. Targeting HIF-2 Alpha in renal cell carcinoma. Curr. Treat. Options Oncol. 2023, 24, 1183–1198. [Google Scholar] [CrossRef]
- Bechmann, N.; Eisenhofer, G. Hypoxia-inducible factor 2α: A key player in tumorigenesis and metastasis of pheochromocytoma and paraganglioma? Exp. Clin. Endocrinol. Diabetes 2022, 130, 282–289. [Google Scholar] [CrossRef]
- Deeks, E.D. Belzutifan: First approval. Drugs 2021, 81, 1921–1927. [Google Scholar] [CrossRef]
- Pinato, D.J.; Black, J.R.; Trousil, S.; Dina, R.E.; Trivedi, P.; Mauri, F.A.; Sharma, R. Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: Relationship with the hypoxic response, immune evasion and malignant behavior. Oncoimmunology 2017, 6, e1358332. [Google Scholar] [CrossRef]
- Celada, L.; Cubiella, T.; San-Juan-Guardado, J.; Gutiérrez, G.; Beiguela, B.; Rodriguez, R.; Poch, M.; Astudillo, A.; Grijalba, A.; Sánchez-Sobrino, P.; et al. Pseudohypoxia in paraganglioma and pheochromocytoma is associated with an immunosuppressive phenotype. J. Pathol. 2023, 259, 103–114. [Google Scholar] [CrossRef]
- Fischer, A.; Maccio, U.; Wang, K.; Friemel, J.; Broglie Daeppen, M.A.; Vetter, D.; Lehmann, K.; Reul, A.; Robledo, M.; Hantel, C.; et al. PD-L1 and HIF-2α upregulation in head and neck paragangliomas after embolization. Cancers. 2023, 15, 5199. [Google Scholar] [CrossRef]
- Pacak, K.; Nazari, M.A.; Taieb, D. Immune landscape of pheochromocytoma and paraganglioma: A potentially novel avenue for prognostic reclassification? J. Clin. Endocrinol. Metab. 2023, 108, e1456–e1457. [Google Scholar] [CrossRef]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007, 20, 1172–1182. [Google Scholar] [CrossRef]
- López-Jiménez, E.; Gómez-López, G.; Leandro-García, L.J.; Muñoz, I.; Schiavi, F.; Montero-Conde, C.; de Cubas, A.A.; Ramires, R.; Landa, I.; Leskelä, S.; et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol. Endocrinol. 2010, 24, 2382–2391. [Google Scholar] [CrossRef]
- Celada, L.; Cubiella, T.; San-Juan-Guardado, J.; San José Martínez, A.; Valdés, N.; Jiménez-Fonseca, P.; Díaz, I.; Enguita, J.M.; Astudillo, A.; Álvarez-González, E.; et al. Differential HIF2α protein expression in human carotid body and adrenal medulla under physiologic and tumorigenic conditions. Cancers 2022, 14, 2986. [Google Scholar] [CrossRef]
- Castelblanco, E.; Santacana, M.; Valls, J.; de Cubas, A.; Cascón, A.; Robledo, M.; Matias-Guiu, X. Usefulness of negative and weak-diffuse pattern of SDHB immunostaining in assessment of SDH mutations in paragangliomas and pheochromocytomas. Endocr. Pathol. 2013, 24, 199–205. [Google Scholar] [CrossRef]
- Yu, A.; Xu, X.; Pang, Y.; Li, M.; Luo, J.; Wang, J.; Liu, L. PD-L1 expression is linked to tumor-infiltrating T-cell exhaustion and adverse pathological behavior in pheochromocytoma/paraganglioma. Lab. Investig. 2023, 103, 100210. [Google Scholar] [CrossRef]
- Gao, X.; Yamazaki, Y.; Pecori, A.; Tezuka, Y.; Ono, Y.; Omata, K.; Morimoto, R.; Nakamura, Y.; Satoh, F.; Sasano, H. Histopathological analysis of tumor microenvironment and angiogenesis in pheochromocytoma. Front. Endocrinol. 2020, 11, 587779. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Sierra, M.L.; Agazzi, A.; Bodei, L.; Pacifici, M.; Aricò, D.; De Cicco, C.; Quarna, J.; Sansovini, M.; De Simone, M.; Paganelli, G. Lymphocytic toxicity in patients after peptide-receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE and 90Y-DOTATOC. Cancer Biother. Radiopharm. 2009, 24, 659–665. [Google Scholar] [CrossRef]
- Adant, S.; Shah, G.M.; Beauregard, J.M. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 907–921. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, Z.; Cheng, X.; Fang, Z.; Jiang, C.; Su, J.; Zhou, Z.; Xu, Z.; Holmberg, A.; Nilsson, S.; et al. Somatostatin derivate (smsDX) attenuates the TAM-stimulated proliferation, migration and invasion of prostate cancer via NF-κB regulation. PLoS ONE 2015, 10, e0124292. [Google Scholar] [CrossRef]
- Armani, C.; Catalani, E.; Balbarini, A.; Bagnoli, P.; Cervia, D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J. Leukoc. Biol. 2007, 81, 845–855. [Google Scholar] [CrossRef]
- Cui, Z.; Ruan, Z.; Li, M.; Ren, R.; Ma, Y.; Zeng, J.; Sun, J.; Ye, W.; Xu, W.; Guo, X.; et al. Intermittent hypoxia inhibits anti-tumor immune response via regulating PD-L1 expression in lung cancer cells and tumor-associated macrophages. Int. Immunopharmacol. 2023, 122, 110652. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017, 31, 181–193. [Google Scholar] [CrossRef]
- Crona, J.; Lamarca, A.; Ghosal, S.; Welin, S.; Skogseid, B.; Pacak, K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: A systematic review and individual patient meta-analysis. Endocr. Relat. Cancer 2019, 26, 539–550. [Google Scholar] [CrossRef]
- Taïeb, D.; Nölting, S.; Perrier, N.D.; Fassnacht, M.; Carrasquillo, J.A.; Grossman, A.B.; Clifton-Bligh, R.; Wanna, G.B.; Schwam, Z.G.; Amar, L.; et al. Management of phaeochromocytoma and paraganglioma in patients with germline SDHB pathogenic variants: An international expert Consensus statement. Nat. Rev. Endocrinol. 2024, 20, 168–184. [Google Scholar] [CrossRef]
- Gill, A.J.; Benn, D.E.; Chou, A.; Clarkson, A.; Muljono, A.; Meyer-Rochow, G.Y.; Richardson, A.L.; Sidhu, S.B.; Robinson, B.G.; Clifton-Bligh, R.J. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum. Pathol. 2010, 41, 805–814. [Google Scholar] [CrossRef]
- Papathomas, T.G.; Oudijk, L.; Persu, A.; Gill, A.J.; van Nederveen, F.; Tischler, A.S.; Tissier, F.; Volante, M.; Matias-Guiu, X.; Smid, M.; et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: A multicenter interobserver variation analysis using virtual microscopy: A multinational study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod. Pathol. 2015, 28, 807–821. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Sänger, J.; Arsenic, R.; D’Haese, J.G.; Neumann, J.; Schmitt-Graeff, A.; Wirtz, R.M.; Schulz, S.; Lupp, A. Evaluation of Somatostatin, CXCR4 Chemokine and Endothelin A Receptor Expression in a Large Set of Paragangliomas. Oncotarget 2017, 8, 89958–89969. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.S. Current Status of Functional Imaging in Neuroblastoma, Pheochromocytoma, and Paraganglioma Disease. Wien. Med. Wochenschr. 2019, 169, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Kroiss, A.; Kumar, R.; Pacak, K.; Taieb, D. Imaging and Imaging-Based Treatment of Pheochromocytoma and Paraganglioma. Endocr. Relat. Cancer 2015, 22, T135–T145. [Google Scholar] [CrossRef]
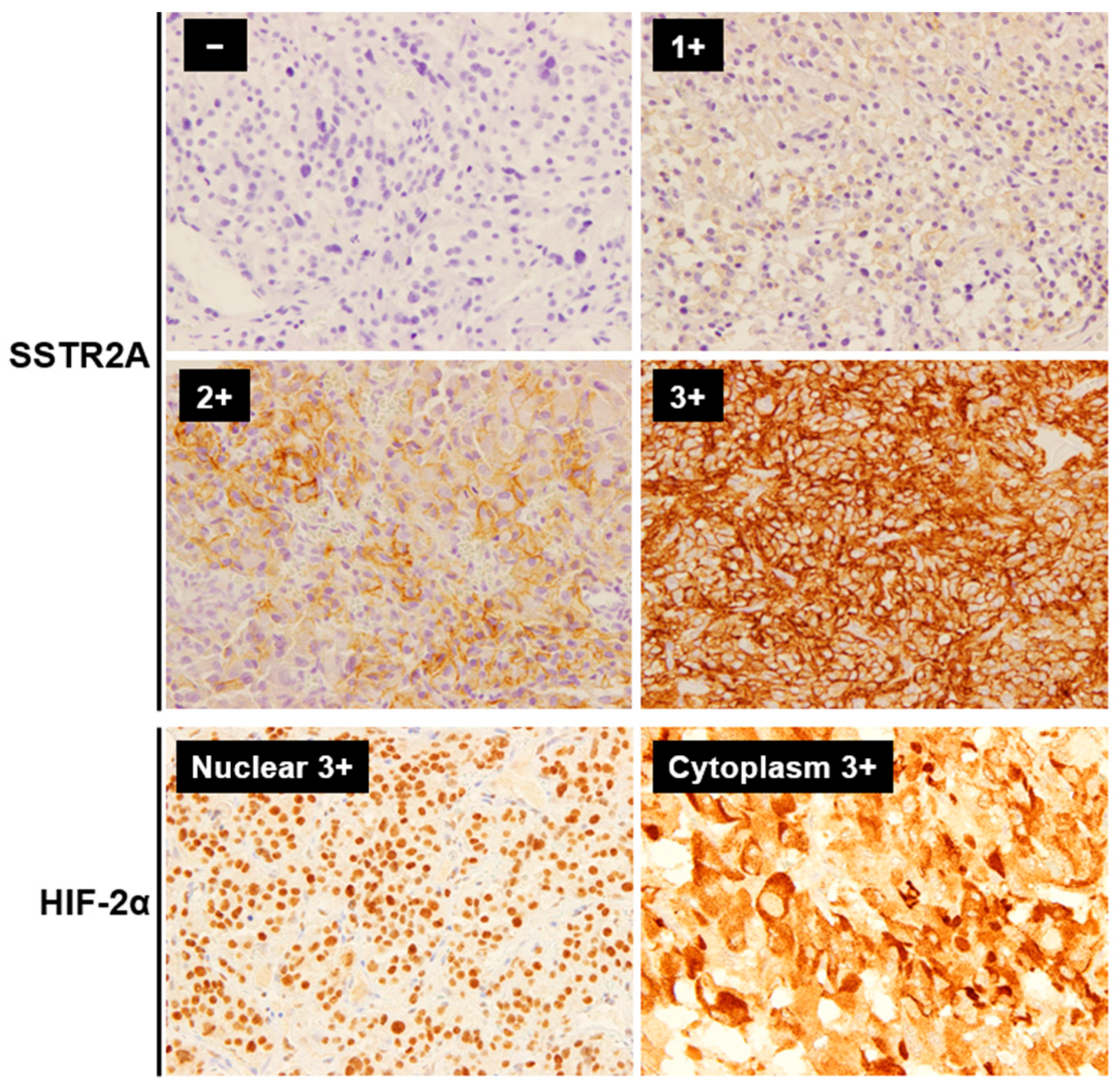
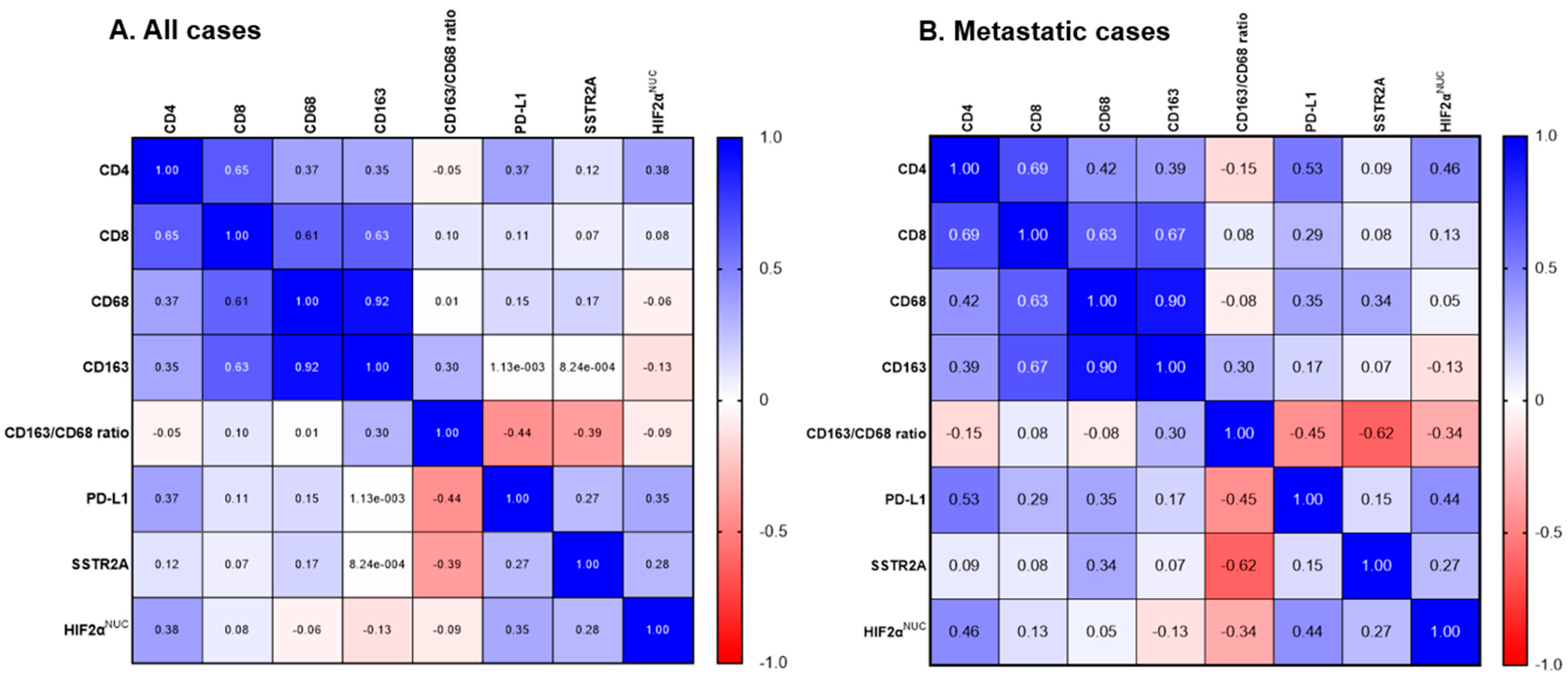
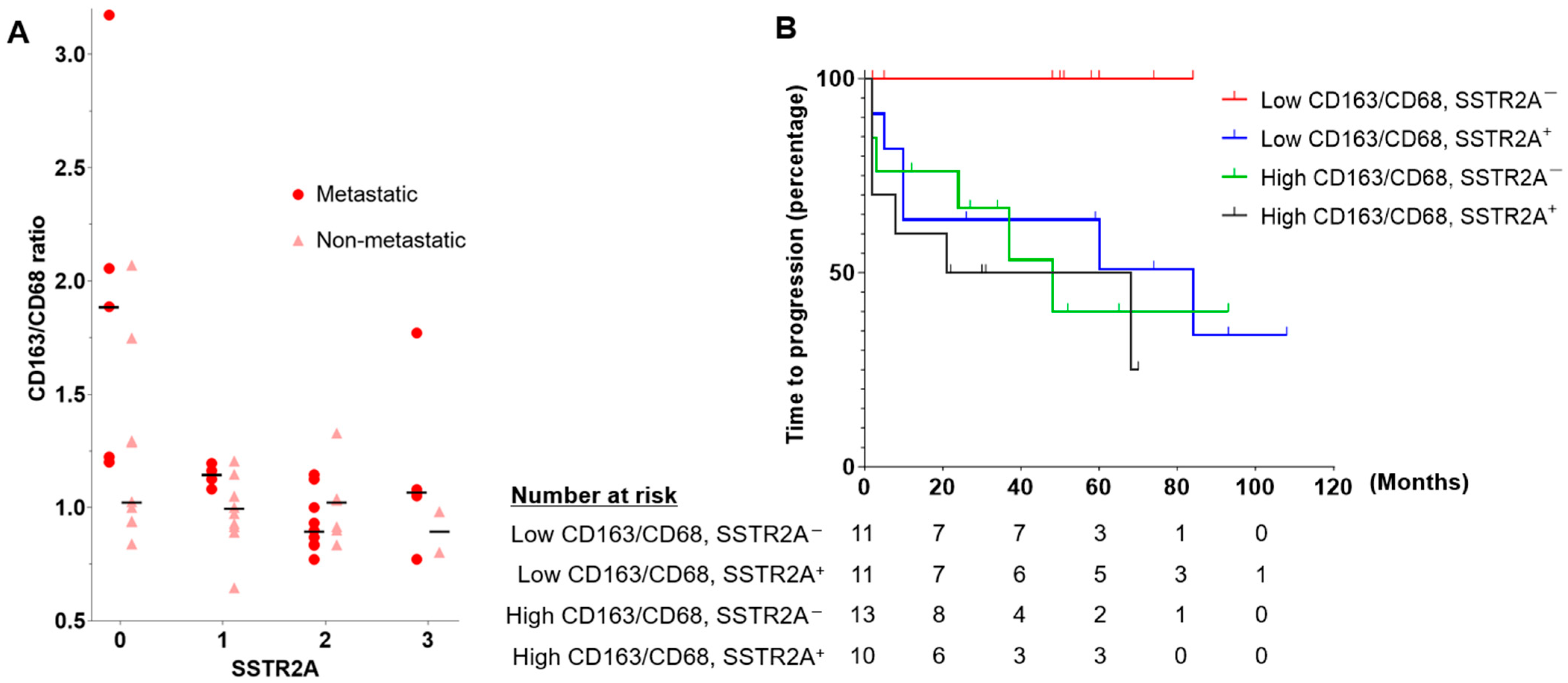
| Metastatic PPGL | Non-Metastatic PPGL | p | |
|---|---|---|---|
| (n = 18) | (n = 27) | ||
| Age at Initial Diagnosis (median, range) | 39 (18–65) | 58 (17–80) | 0.01 |
| Sex | |||
| Female | 10 | 18 | 0.537 |
| Male | 8 | 9 | |
| Primary Tumor Location | |||
| Adrenal | 3 | 15 | 0.012 |
| Extra-adrenal | 15 | 12 | |
| Abdominal | 10 | 10 | |
| Head and neck | 2 | 0 | |
| Bladder | 3 | 2 | |
| Functional Status | |||
| Adrenergic | 1 | 12 | 0.012 |
| Noradrenergic | 8 | 4 | |
| Silent or Dopaminergic | 7 | 9 | |
| Not available | 2 | 2 | |
| Diabetes Mellitus | 7 | 5 | 0.175 |
| Hypertension | 10 | 15 | 1 |
| Ki-67 LI | |||
| ≥3% | 14 | 9 | 0.006 |
| <3% | 4 | 18 | |
| Tumor Size | |||
| ≥50 mm | 14 | 13 | 0.065 |
| <50 mm | 4 | 14 | |
| 123I-MIBG Uptake | |||
| Positive | 9 | 24 | 0.007 |
| Negative | 6 | 1 | |
| Not available | 3 | 2 | |
| SDHB Staining | |||
| Positive | 6 | 26 | <0.001 |
| Negative | 12 | 1 |
| SSTR2A | p | HIF-2αNUC | p | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| (n = 21) | (n = 24) | (n = 14) | (n = 31) | |||
| Age at Initial Diagnosis (median, range) | 40 (17–70) | 59 (18–80) | 0.005 | 41 (17–70) | 50 (18–80) | 0.333 |
| Sex | ||||||
| Female | 15 | 13 | 0.356 | 7 | 21 | 0.326 |
| Male | 6 | 11 | 7 | 10 | ||
| Primary Tumor Location | ||||||
| Adrenal | 7 | 11 | 0.599 | 0 | 18 | <0.001 |
| Extra-adrenal | 14 | 13 | 14 | 13 | ||
| Abdominal | 10 | 10 | 11 | 9 | ||
| Head and neck | 2 | 0 | 1 | 1 | ||
| Bladder | 2 | 3 | 2 | 3 | ||
| Tumor Site | ||||||
| Adrenal | 7 | 10 | 0.704 | 0 | 17 | 0.001 |
| Abdominal | 10 | 9 | 10 | 9 | ||
| Liver | 0 | 1 | 1 | 0 | ||
| Head and neck | 2 | 1 | 1 | 2 | ||
| Bladder | 2 | 3 | 2 | 3 | ||
| Functional Status | ||||||
| Adrenergic | 6 | 7 | 0.743 | 1 | 12 | 0.060 |
| Noradrenergic | 7 | 5 | 3 | 9 | ||
| Silent or Dopaminergic | 7 | 9 | 8 | 8 | ||
| Not available | 1 | 3 | 2 | 2 | ||
| Metastatic PPGL | ||||||
| Yes | 12 | 6 | 0.037 | 9 | 9 | 0.047 |
| No | 9 | 18 | 5 | 22 | ||
| Ki-67 LI | ||||||
| ≥3% | 15 | 8 | 0.017 | 13 | 10 | <0.001 |
| <3% | 6 | 16 | 1 | 21 | ||
| GAPP Score | ||||||
| ≥7 | 7 | 5 | 0.274 | 5 | 7 | 0.658 |
| 3–7 | 13 | 14 | 7 | 20 | ||
| <3 | 1 | 5 | 2 | 4 | ||
| 123I-MIBG Uptake | ||||||
| Positive | 14 | 19 | 0.226 | 7 | 26 | 0.003 |
| Negative | 5 | 2 | 6 | 1 | ||
| Not available | 2 | 3 | 1 | 4 | ||
| SDHB Staining | ||||||
| Positive | 12 | 20 | 0.098 | 4 | 28 | <0.001 |
| Negative | 9 | 4 | 10 | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchihara, M.; Tanabe, A.; Kojima, Y.; Shimoi, T.; Maeshima, A.M.; Umamoto, K.; Shimomura, A.; Shimizu, C.; Yamazaki, Y.; Nakamura, E.; et al. Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma. Cancers 2024, 16, 2191. https://doi.org/10.3390/cancers16122191
Uchihara M, Tanabe A, Kojima Y, Shimoi T, Maeshima AM, Umamoto K, Shimomura A, Shimizu C, Yamazaki Y, Nakamura E, et al. Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma. Cancers. 2024; 16(12):2191. https://doi.org/10.3390/cancers16122191
Chicago/Turabian StyleUchihara, Masaki, Akiyo Tanabe, Yuki Kojima, Tatsunori Shimoi, Akiko Miyagi Maeshima, Kotaro Umamoto, Akihiko Shimomura, Chikako Shimizu, Yuto Yamazaki, Eijiro Nakamura, and et al. 2024. "Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma" Cancers 16, no. 12: 2191. https://doi.org/10.3390/cancers16122191
APA StyleUchihara, M., Tanabe, A., Kojima, Y., Shimoi, T., Maeshima, A. M., Umamoto, K., Shimomura, A., Shimizu, C., Yamazaki, Y., Nakamura, E., Matsui, Y., Takemura, N., Miyazaki, H., Sudo, K., Yonemori, K., & Kajio, H. (2024). Immunohistochemical Profiling of SSTR2 and HIF-2α with the Tumor Microenvironment in Pheochromocytoma and Paraganglioma. Cancers, 16(12), 2191. https://doi.org/10.3390/cancers16122191








