The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Context
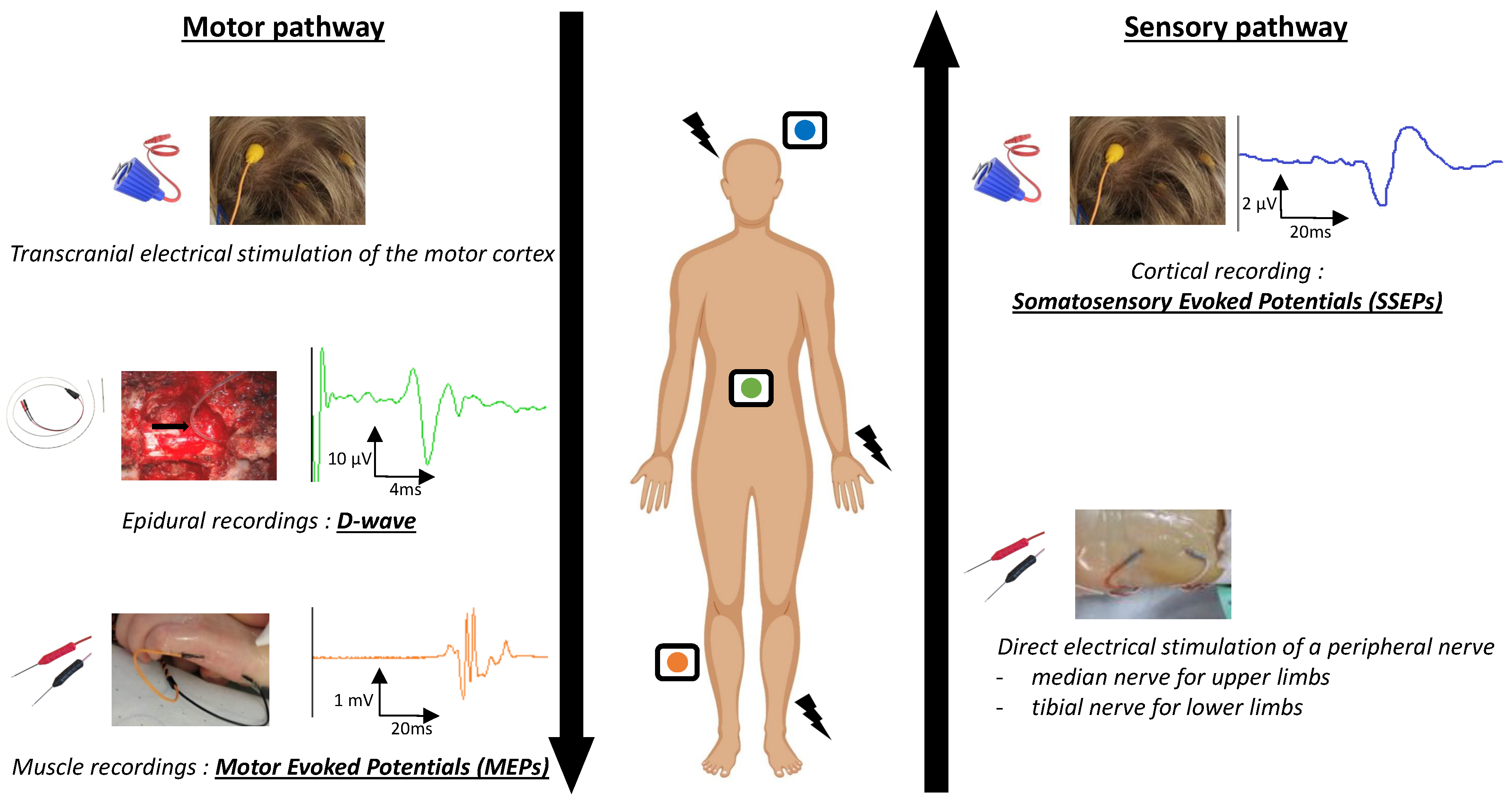
1.2. IONM Techniques
1.2.1. Anesthesia Requirements
1.2.2. Somatosensory Evoked Potentials (SSEPs)
1.2.3. Motor Evoked Potentials (MEPs)
1.2.4. D-Waves (DW)
1.2.5. Multimodal Monitoring
1.3. Objectives of the Study
2. Materials and Methods
2.1. Patients
2.2. Electrophysiological Recordings
2.2.1. Preoperative MEPs and SSEPs
2.2.2. Intraoperative Neurophysiological Monitoring
2.3. Data Extraction
2.3.1. Clinical Characteristics
2.3.2. Anatomopathological Characteristics
2.3.3. Radiological Characteristics
2.3.4. Intraoperative Evoked Potentials
2.4. Statistical Analysis
2.4.1. Primary Outcome
2.4.2. Secondary Outcome
3. Results
3.1. Patient Characteristics
3.2. Overall Results of Electrophysiological Investigations
3.3. Relationship between Patient’s Clinical Outcome Three Months after Surgery and Neurophysiological Investigations
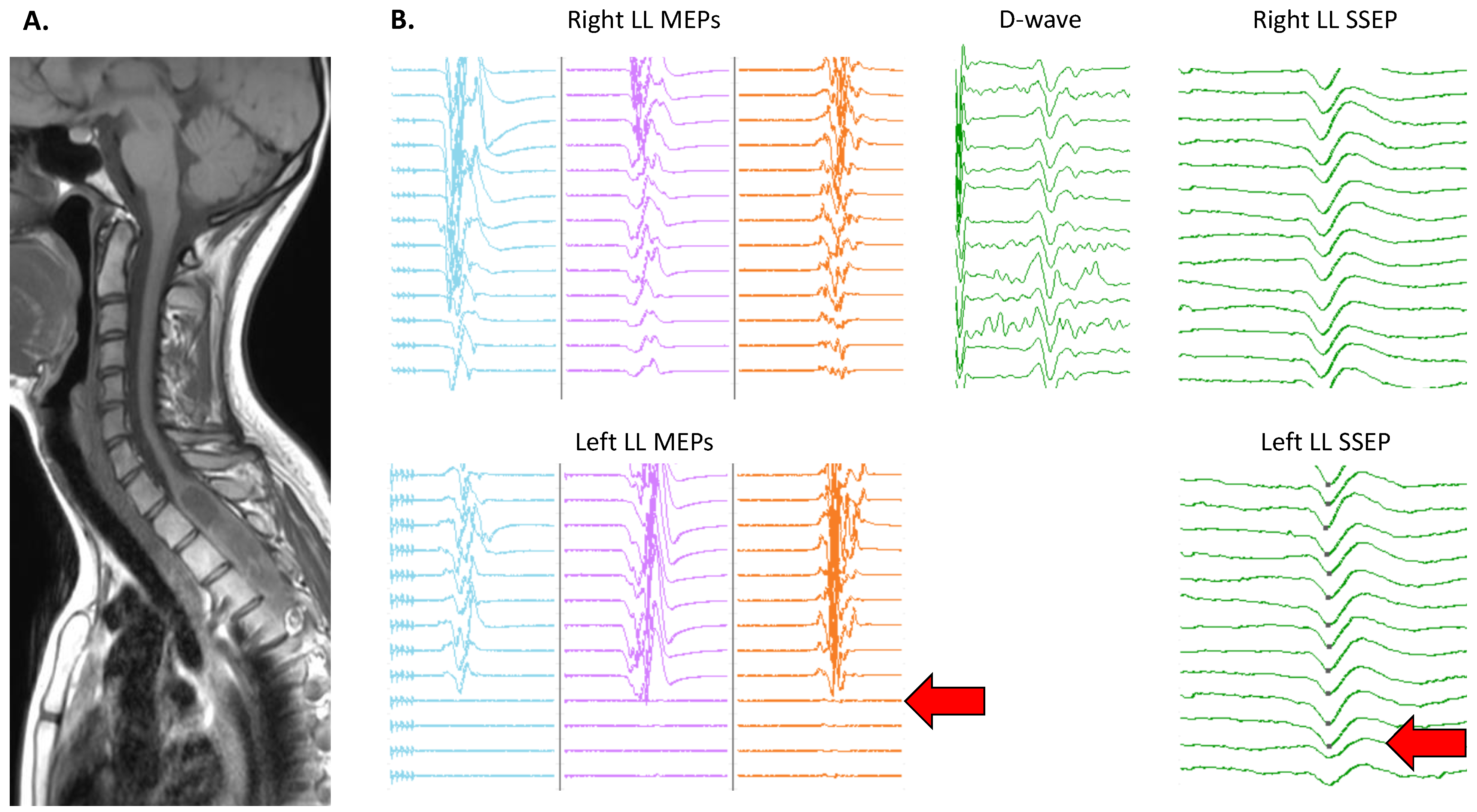
3.4. Topographical Specificity of IONM Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | D-Wave |
| IONM | Intraoperative Neuromonitoring |
| MEPs | Motor Evoked Potentials |
| MMS | Modified McCormick Scale |
| SSEPs | Somatosensory Evoked Potentials |
| SCT | Spinal Cord Tumor |
References
- Duong, L.M.; McCarthy, B.J.; McLendon, R.E.; Dolecek, T.A.; Kruchko, C.; Douglas, L.L.; Ajani, U.A. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer 2012, 118, 4220–4227. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Gillis, C.C.; Shih, P.; O’Toole, J.E.; Fessler, R.G. Intramedullary Spinal Cord Tumors: Part I—Epidemiology, Pathophysiology, and Diagnosis. Glob. Spine J. 2015, 5, 425. [Google Scholar] [CrossRef]
- Hersh, A.M.; Pennington, Z.; Lubelski, D.; Elsamadicy, A.A.; Dea, N.; Desai, A.; Gokaslan, Z.L.; Goodwin, C.R.; Hsu, W.; Jallo, G.I.; et al. Treatment of intramedullary spinal cord tumors: A modified Delphi technique of the North American Spine Society Section of Spine Oncology. J. Neurosurg. Spine 2024, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cristante, L.; Herrmann, H.D. Surgical management of intramedullary spinal cord tumors: Functional outcome and sources of morbidity. Neurosurgery 1994, 35, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.J.; Farmer, J.P.; Freed, D. Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J. Neurosurg. 1993, 79, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Skrap, B.; Tramontano, V.; Faccioli, F.; Meglio, M.; Pinna, G.; Sala, F. Surgery for intramedullary spinal cord ependymomas in the neuromonitoring era: Results from a consecutive series of 100 patients. J. Neurosurg. Spine 2021, 36, 858–868. [Google Scholar] [CrossRef] [PubMed]
- André-Obadia, N.; Garassus, P.; Mauguière, F. Exploration of intraspinal tumors using evoked motor potentials (EMP): Correlations with data of evoked somatosensory potentials. Neurophysiol. Clin. 1996, 26, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Dauleac, C.; Boulogne, S.; Barrey, C.Y.; Guyotat, J.; Jouanneau, E.; Mertens, P.; Berhouma, M.; Jung, J.; André-Obadia, N. Predictors of functional outcome after spinal cord surgery: Relevance of intraoperative neurophysiological monitoring combined with preoperative neurophysiological and MRI assessments. Neurophysiol. Clin. 2022, 52, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Dauleac, C.; Boulogne, S.; Cotton, F.; Mertens, P. Spinal cord tractography and neuromonitoring-based surgical strategy for intramedullary ependymoma. Neurosurg. Focus Video 2023, 9, V4. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Skrap, B.; Kothbauer, K.F.; Deletis, V. Intraoperative neurophysiology in intramedullary spinal cord tumor surgery. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 186, pp. 229–244. [Google Scholar] [CrossRef]
- Buhl, L.K.; Bastos, A.B.; Pollard, R.J.; Arle, J.E.; Thomas, G.P.; Song, Y.; Boone, M.D. Neurophysiologic Intraoperative Monitoring for Spine Surgery: A Practical Guide From Past to Present. J. Intensive Care Med. 2021, 36, 1237–1249. [Google Scholar] [CrossRef]
- Nash, C.L.; Lorig, R.A.; Schatzinger, L.A.; Brown, R.H. Spinal cord monitoring during operative treatment of the spine. Clin. Orthop. Relat. Res. 1977, 126, 100–105. [Google Scholar] [CrossRef]
- Sala, F.; Palandri, G.; Basso, E.; Lanteri, P.; Deletis, V.; Faccioli, F.; Bricolo, A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery 2006, 58, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Morota, N.; Deletis, V.; Constantini, S.; Kofler, M.; Cohen, H.; Epstein, F.J. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 1997, 41, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.W.; Beutler, A.S.; Chesnut, R.M.; Patel, P.M.; Kennelly, N.A.; Kalkman, C.J.; Drummond, J.C.; Garfin, S.R. Myogenic Motor-Evoked Potential Monitoring Using Partial Neuromuscular Blockade in Surgery of the Spine. Spine 1996, 21, 1676. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, A.; Imagama, S.; Ito, Z.; Ando, K.; Tauchi, R.; Matsumoto, T.; Nakashima, H.; Matsuyama, Y.; Ishigro, N. The cutoff amplitude of transcranial motor evoked potentials for transient postoperative motor deficits in intramedullary spinal cord tumor surgery. Spine 2014, 39, E1086–E1094. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.T.; Marquardt, G.; Seifert, V.; Szelényi, A. Spinal cord tumor surgery–importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine 2012, 37, E1001–E1008. [Google Scholar] [CrossRef] [PubMed]
- Rijs, K.; Klimek, M.; Scheltens-de Boer, M.; Biesheuvel, K.; Harhangi, B.S. Intraoperative Neuromonitoring in Patients with Intramedullary Spinal Cord Tumor: A Systematic Review, Meta-Analysis, and Case Series. World Neurosurg. 2019, 125, 498–510.e2. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.N.; Shank, C.D.; Rozzelle, C.J.; Walters, B.C. Guidelines for the Use of Electrophysiological Monitoring for Surgery of the Human Spinal Column and Spinal Cord. Neurosurgery 2017, 81, 713. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Brodke, D.S.; Norvell, D.C.; Dettori, J.R. The Evidence for Intraoperative Neurophysiological Monitoring in Spine Surgery: Does It Make a Difference? Spine 2010, 35, S37. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Legatt, A.D.; Emerson, R.G.; Epstein, C.M.; MacDonald, D.B.; Deletis, V.; Bravo, R.J.; López, J.R. ACNS Guideline: Transcranial Electrical Stimulation Motor Evoked Potential Monitoring. J. Clin. Neurophysiol. 2016, 33, 42. [Google Scholar] [CrossRef] [PubMed]
- Syriopoulos, P.K.; Kalampalikis, N.G.; Kotsiantis, S.B.; Vrahatis, M.N. kNN Classification: A review. Ann. Math. Artif. Intell. 2023. [Google Scholar] [CrossRef]
- Klekamp, J. Treatment of intramedullary tumors: Analysis of surgical morbidity and long-term results: Clinical article. J. Neurosurg. Spine 2013, 19, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.; Eggspuehler, A.; Grob, D.; Jeszenszky, D.; Benini, A.; Porchet, F.; Mueller, A.; Dvorak, J. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur. Spine J. 2007, 16 (Suppl. S2), 197–208. [Google Scholar] [CrossRef] [PubMed]
- AlMahdy, R.A.; Wahid, M.; Abdelkader, A.A.; Lotfy, M.; Soliman, M.A.R. The Utility of Multimodal Intraoperative Neuromonitoring in Spine Surgery: Case Series from a Lower-Middle-Income Country Perspective. World Neurosurg. 2021, 152, e220–e226. [Google Scholar] [CrossRef] [PubMed]
- Verla, T.; Fridley, J.S.; Khan, A.B.; Mayer, R.R.; Omeis, I. Neuromonitoring for Intramedullary Spinal Cord Tumor Surgery. World Neurosurg. 2016, 95, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R. Measuring outcomes for neurophysiological intraoperative monitoring. Clin. Neurophysiol. 2016, 127, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R.; Galloway, G.M.; Nuwer, M.R.; Lopez, J.R.; Zamel, K.M. Intraoperative wake-up test. In Intraoperative Neurophysiologic Monitoring; Cambridge University Press: Cambridge, UK, 2010; pp. 221–224. [Google Scholar] [CrossRef]
- Nambiar, M.; Kavar, B. Clinical presentation and outcome of patients with intradural spinal cord tumours. J. Clin. Neurosci. 2012, 19, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.D.; Simpson, C.; Nicholas, R.S.; Miles, J.; Findlay, G.F.G.; Pigott, T.J.D. Outcome predictors and complications in the management of intradural spinal tumours. Eur. Spine J. 2006, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, Y.E.; Park, M.; Lee, J.; Kim, D.; Park, Y.G. Correlation between preoperative somatosensory evoked potentials and intraoperative neurophysiological monitoring in spinal cord tumors. J. Clin. Monit. Comput. 2021, 35, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Katsos, K.; Johnson, S.E.; Ibrahim, S.; Bydon, M. Current Applications of Machine Learning for Spinal Cord Tumors. Life 2023, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.C.; Ho, A.L.; Feng, A.Y.; Medress, Z.A.; Pendharkar, A.V.; Rezaii, P.; Ratliff, J.K.; Desai, A.M. Prediction of Discharge Status and Readmissions after Resection of Intradural Spinal Tumors. Neurospine 2022, 19, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, M.; Margetis, K. A Machine Learning-Based Online Prediction Tool for Predicting Short-Term Postoperative Outcomes Following Spinal Tumor Resections. Cancers 2023, 15, 812. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, S.; Sivaganesan, A.; Patel, A.J.; Agadi, S.; Schmidt, R.J.; Mani, P.; Jea, A. Practice trends in the utilization of intraoperative neurophysiological monitoring in pediatric neurosurgery as a function of complication rate, and patient-, surgeon-, and procedure-related factors. World Neurosurg. 2014, 81, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Baig Mirza, A.; Vastani, A.; Syrris, C.; Boardman, T.; Ghani, I.; Murphy, C.; Gebreyohanes, A.; Vergani, F.; Mirallave-Pescador, A.; Lavrador, J.P.; et al. Intraoperative Neurophysiological Monitoring for Intradural Extramedullary Spinal Tumours. Glob. Spine J. 2022, 14, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, L.; Putz, M.; Sordyl, R.; Pokora, S.; Mandera, M. Relevance of intraoperative motor evoked potentials and D-wave monitoring for the resection of intramedullary spinal cord tumors in children. Neurosurg. Rev. 2022, 45, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 67) | |
|---|---|
| Age (Years) | 50 (14) |
| Gender | |
| Male | 43 (64%) |
| Female | 24 (36%) |
| Lesion size (mm) | 32 (20) |
| Preoperative McCormick Scale | |
| Mild | 44 (64%) |
| Severe | 24 (36%) |
| Tumor level | |
| High | 32 (48%) |
| Low | 35 (52%) |
| Tumor location | |
| Intradural extramedullary | 40 (60%) |
| Intradural intramedullary | 27 (40%) |
| Preop Evoked Potentials | |
| Normal | 42 (63%) |
| Altered | 25 (37%) |
| IONM any alert | |
| No Alert | 29 (43%) |
| Alert | 38 (57%) |
| IONM persistent alert | |
| No alert | 39 (58%) |
| Alert | 28 (42%) |
| Histology | |
| Ependymoma | 24 (35%) |
| Schwannoma | 14 (21%) |
| Meningioma | 17 (25%) |
| Other | 12 (18%) |
| MRI residual lesion | |
| No | 52 (79%) |
| Yes | 14 (21%) |
| IONM Alerts | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| transient alert MEPs-DW | 0 | 0.85 | 0 | 0.91 | 0.79 |
| transient alert SSEPs | 0 | 0.90 | 0 | 0.78 | 0.73 |
| transient alert MEPs-DW & SSEPs | 0 | 0.72 | 0 | 0.96 | 0.70 |
| any alert MEPs-DW | 0.86 | 0.76 | 0.63 | 0.92 | 0.79 |
| any alert SSEPs | 0.6 | 0.75 | 0.54 | 0.79 | 0.70 |
| persistent alert MEPs-DW | 0.86 | 0.89 | 0.79 | 0.93 | 0.88 |
| persistent alert SSEPs | 0.55 | 0.92 | 0.78 | 0.80 | 0.80 |
| any alert MEPs-DW & SSEPs | 0.95 | 0.62 | 0.55 | 0.96 | 0.73 |
| persistent alert MEPs-DW & SSEPs | 0.95 | 0.84 | 0.75 | 0.97 | 0.88 |
| Predictors | Good Outcome (n = 45) | Bad Outcome (n = 22) | Univariate (p-Value) | Multivariate (p-Value) |
|---|---|---|---|---|
| Age (years) | 50 (15) | 49 (12) | NS | |
| Gender | ||||
| Male | 15 (33%) | 9 (41%) | NS | |
| Female | 30 (67%) | 13 (59%) | ||
| Lesion size (mm) | 29 (19) | 39 (19) | 0.01 | NS |
| Preop McCormick Scale | ||||
| Mild | 30 (67%) | 14 (64%) | NS | |
| Severe | 15 (33%) | 8 (36%) | ||
| Tumor level | ||||
| High | 17 (38%) | 15 (68%) | 0.03 | NS |
| Low | 28 (62%) | 7 (32%) | ||
| Tumor location | ||||
| Intradural extramedullary | 30 (67%) | 10 (45%) | NS | |
| Intradural intramedullary | 15 (33%) | 12 (55%) | ||
| Preop evoked potentials | ||||
| Normal | 33 (73%) | 9 (41%) | 0.02 | 0.04 |
| Altered | 12 (27%) | 13 (59%) | ||
| IONM any alert | ||||
| No alert | 28 (62%) | 1 (4%) | <0.001 | <0.001 |
| Alert | 17 (38%) | 21 (95%) | ||
| IONM persistent alert | ||||
| No alert | 38 (84%) | 1 (5%) | <0.001 | <0.001 |
| Alert | 7 (16%) | 21 (95%) | ||
| Histology | ||||
| Ependymoma | 10 (22%) | 14 (64%) | ||
| Schwannoma | 11 (24%) | 3 (14%) | 0.001 | NS |
| Meningioma | 14 (31%) | 3 (14%) | ||
| Other | 10 (22%) | 2 (9%) | ||
| MRI Residual lesion | ||||
| No | 37 (82%) | 15 (71%) | NS | |
| Yes | 8 (18%) | 6 (29%) |
| IONM Alerts | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| any alert SSEPs | 0.42 | 0.83 | 0.42 | 0.83 | 0.73 |
| any alert MEPs-DW | 0.78 | 0.82 | 0.58 | 0.92 | 0.81 |
| any alert MEPs-DW & SSEPs | 0.81 | 0.72 | 0.48 | 0.92 | 0.74 |
| Predictors | Stable (n = 152) | Worsening (n = 48) | Univariate (p-Value) | Multivariate (p-Value) |
|---|---|---|---|---|
| Lesion size (mm) | 32 (21) | 40 (18) | <0.001 | 0.02 |
| Tumor level | ||||
| High | 88 (58%) | 38 (79%) | 0.01 | NS |
| Low | 64 (42%) | 10 (21%) | ||
| Preop evoked potentials | NS | NS | ||
| Normal | 55 (36%) | 23 (48%) | ||
| Altered | 97 (64%) | 25 (52%) | ||
| IONM alert | ||||
| No alert | 100 (72%) | 9 (19%) | <0.001 | <0.001 |
| Alert | 42 (28%) | 39 (81%) | ||
| Histology | ||||
| Ependymoma | 50 (33%) | 34 (71%) | ||
| Schwannoma | 30 (20%) | 4 (8.3%) | <0.001 | NS |
| Meningioma | 43 (28%) | 5 (10%) | ||
| Other | 29 (19%) | 5 (10%) | ||
| MRI Residual lesion | ||||
| No | 127 (84%) | 31 (70%) | NS | NS |
| Yes | 25 (16%) | 13 (30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilhan, F.; Boulogne, S.; Morgado, A.; Dauleac, C.; André-Obadia, N.; Jung, J. The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery. Cancers 2024, 16, 2192. https://doi.org/10.3390/cancers16122192
Ilhan F, Boulogne S, Morgado A, Dauleac C, André-Obadia N, Jung J. The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery. Cancers. 2024; 16(12):2192. https://doi.org/10.3390/cancers16122192
Chicago/Turabian StyleIlhan, Furkan, Sébastien Boulogne, Alexis Morgado, Corentin Dauleac, Nathalie André-Obadia, and Julien Jung. 2024. "The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery" Cancers 16, no. 12: 2192. https://doi.org/10.3390/cancers16122192
APA StyleIlhan, F., Boulogne, S., Morgado, A., Dauleac, C., André-Obadia, N., & Jung, J. (2024). The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery. Cancers, 16(12), 2192. https://doi.org/10.3390/cancers16122192






