Impact of Institutional Practices and Surgical Complexity on Sarcoma Surgery Costs: Driving Efficiency in Value-Based Healthcare
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
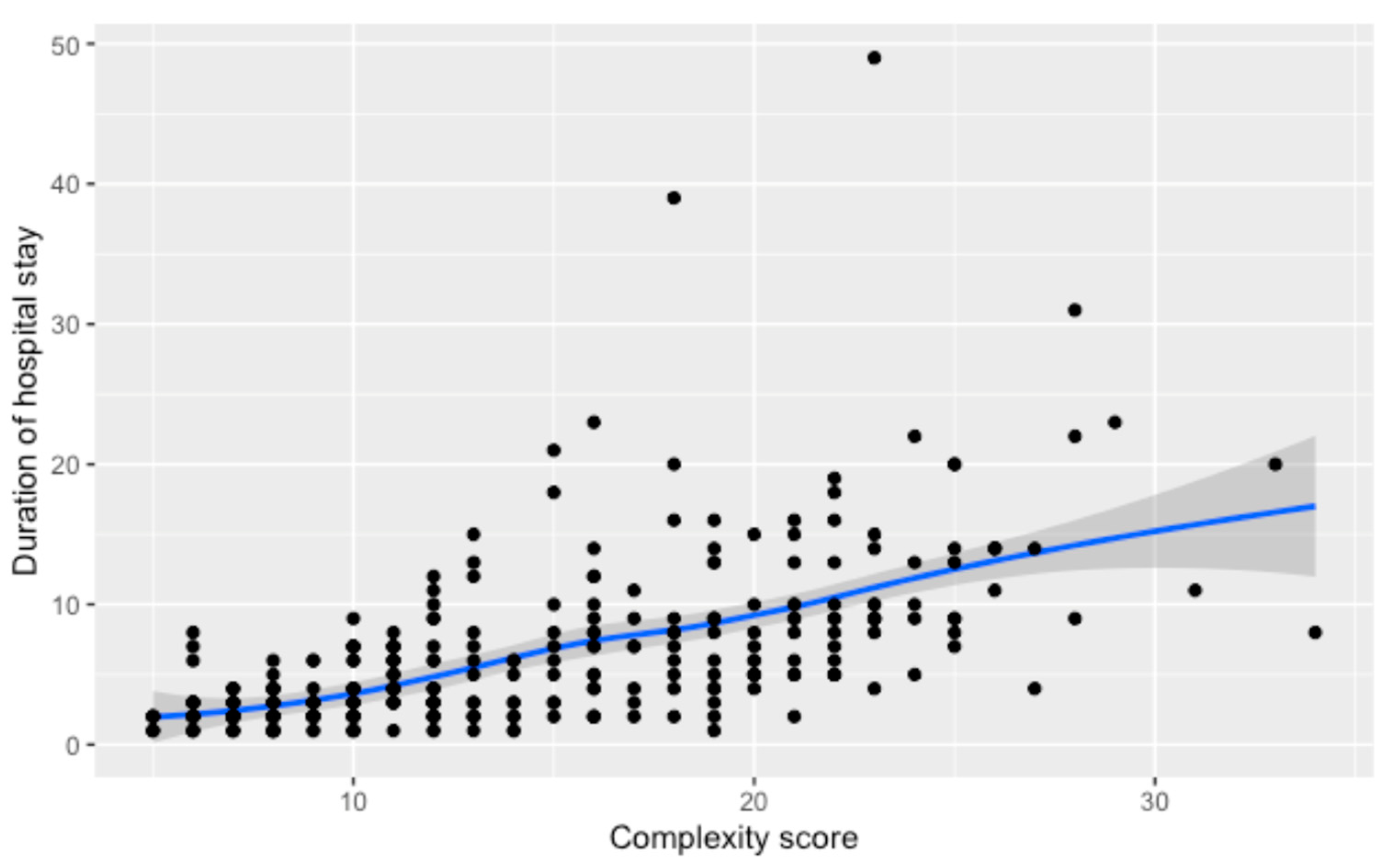
3.1. Duration of Hospital Stay
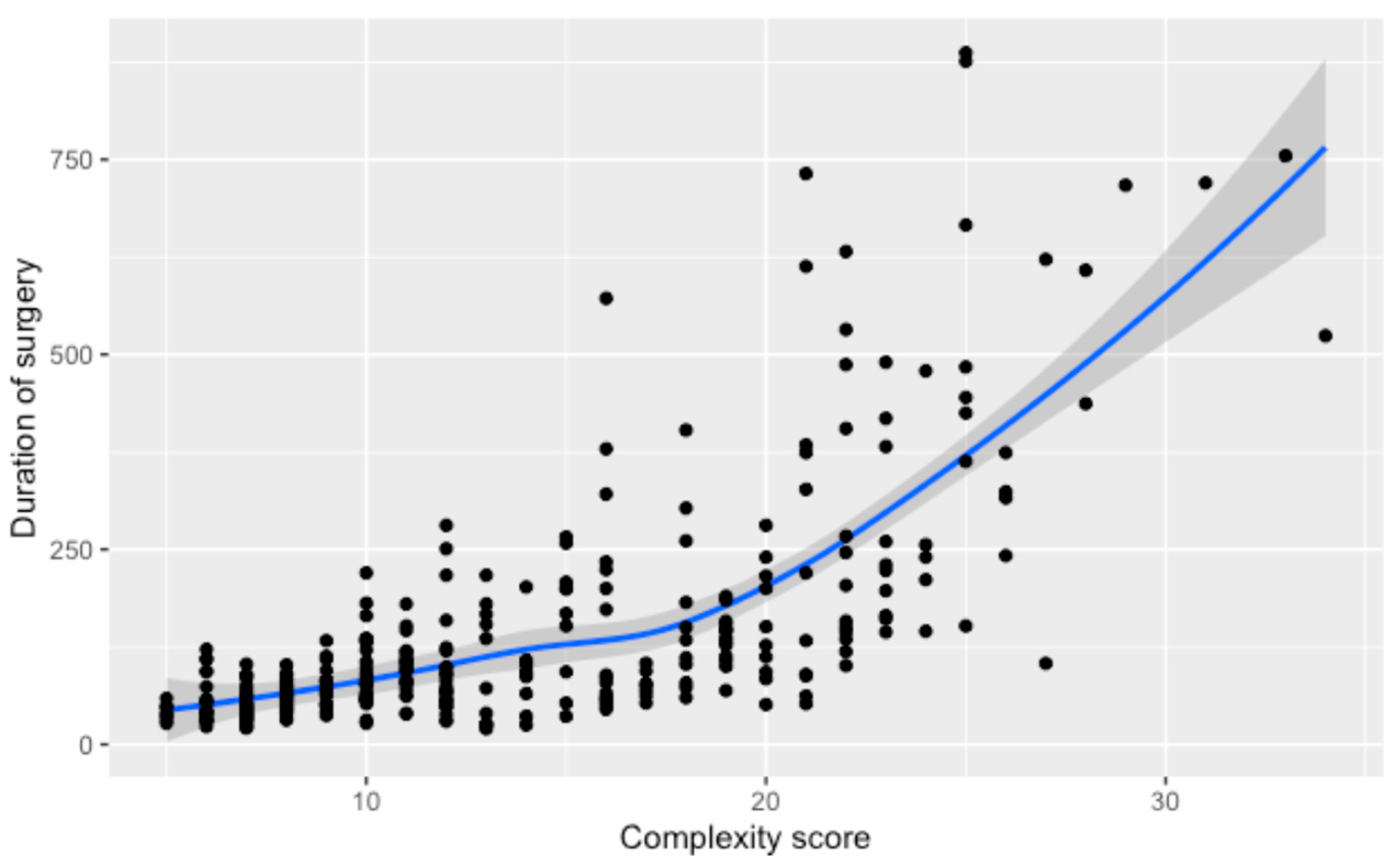
3.2. Duration of Surgery
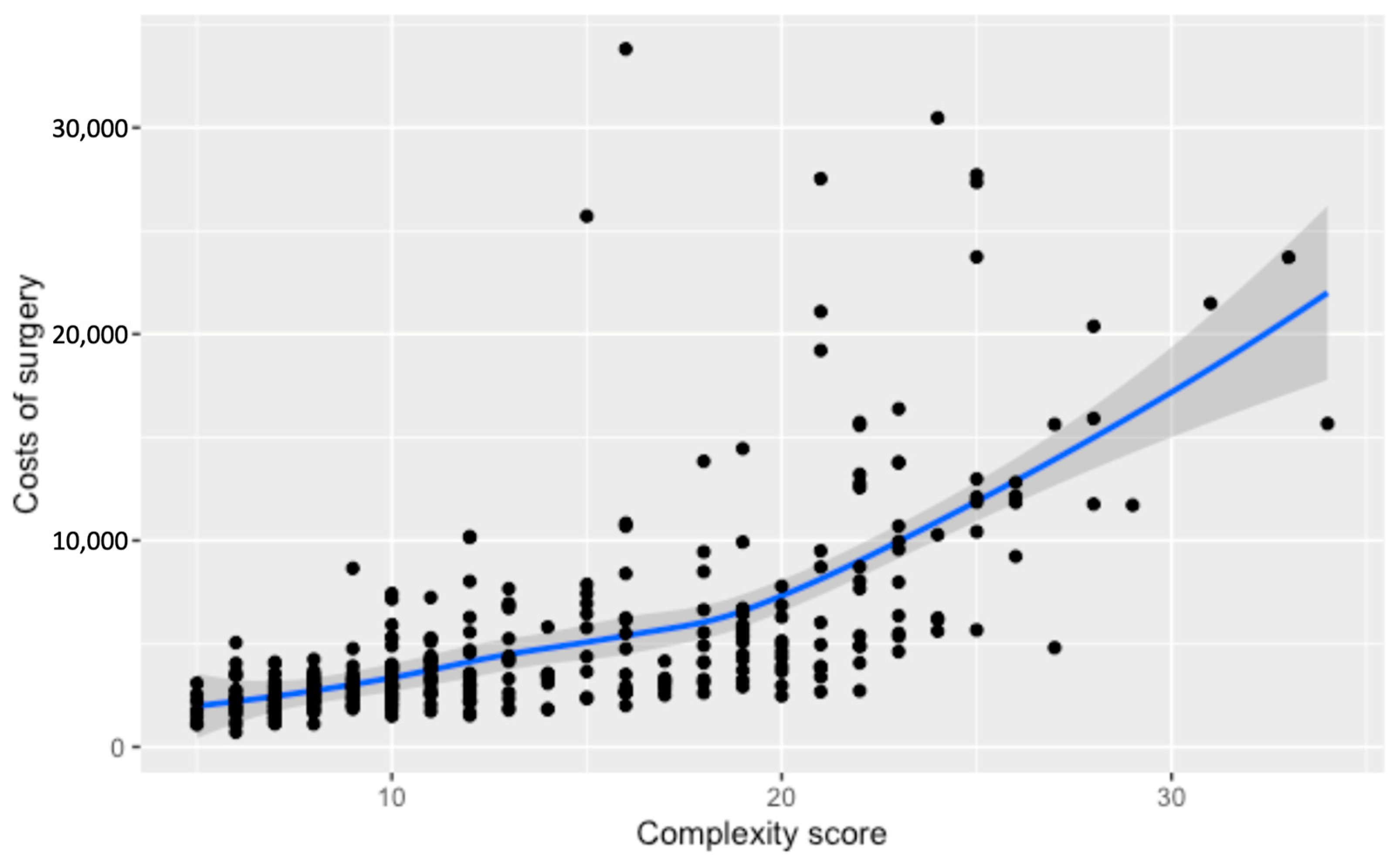
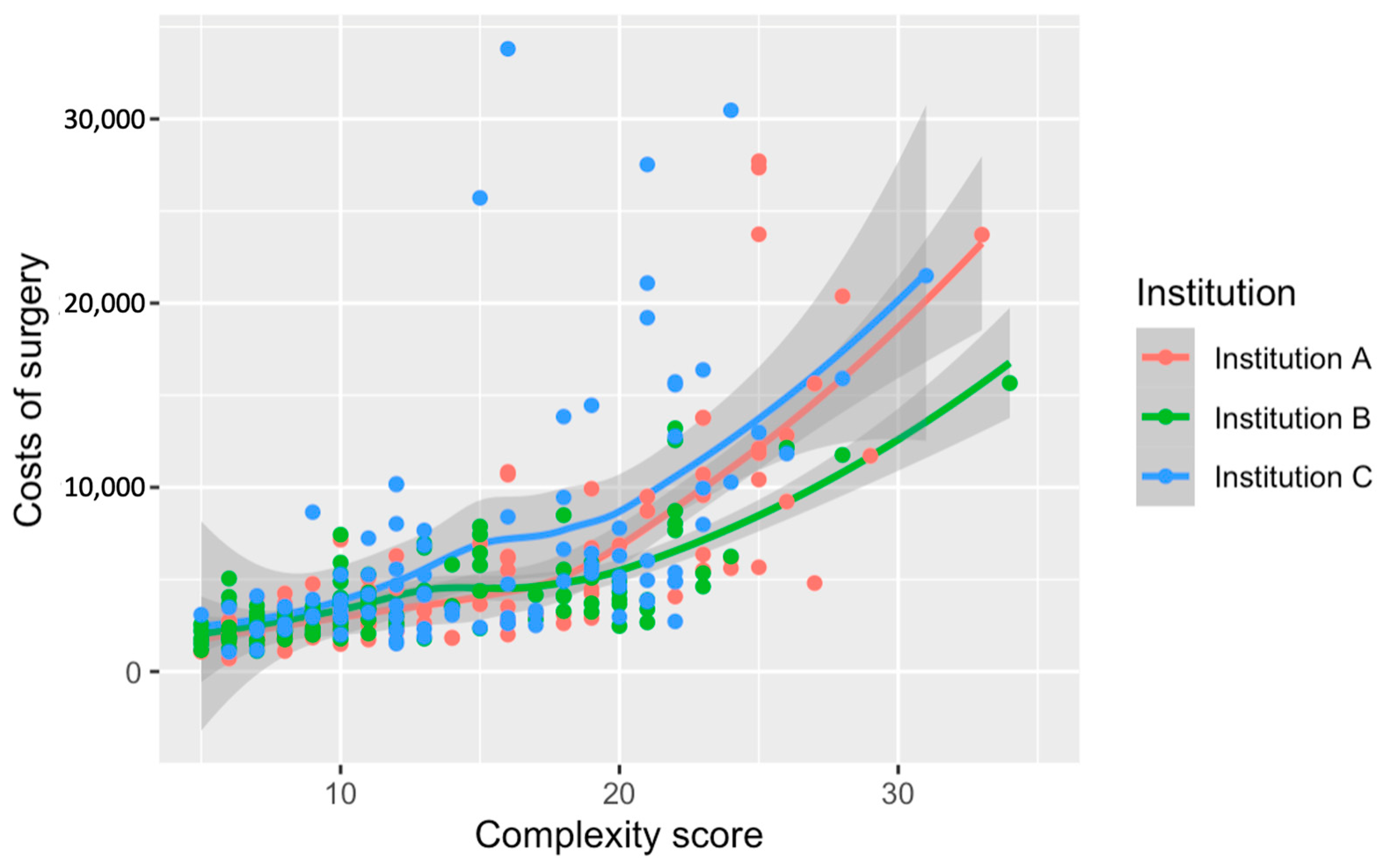
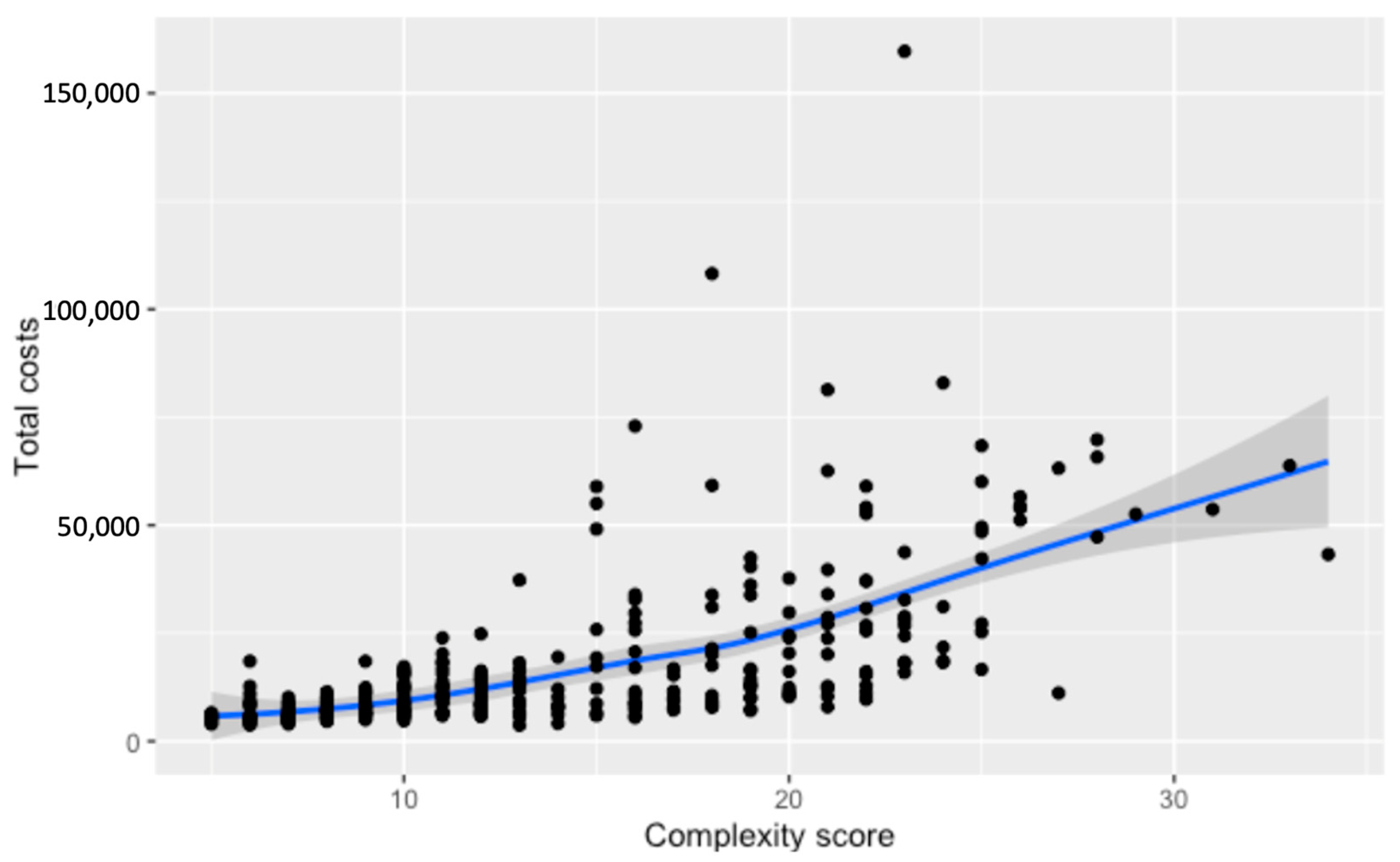
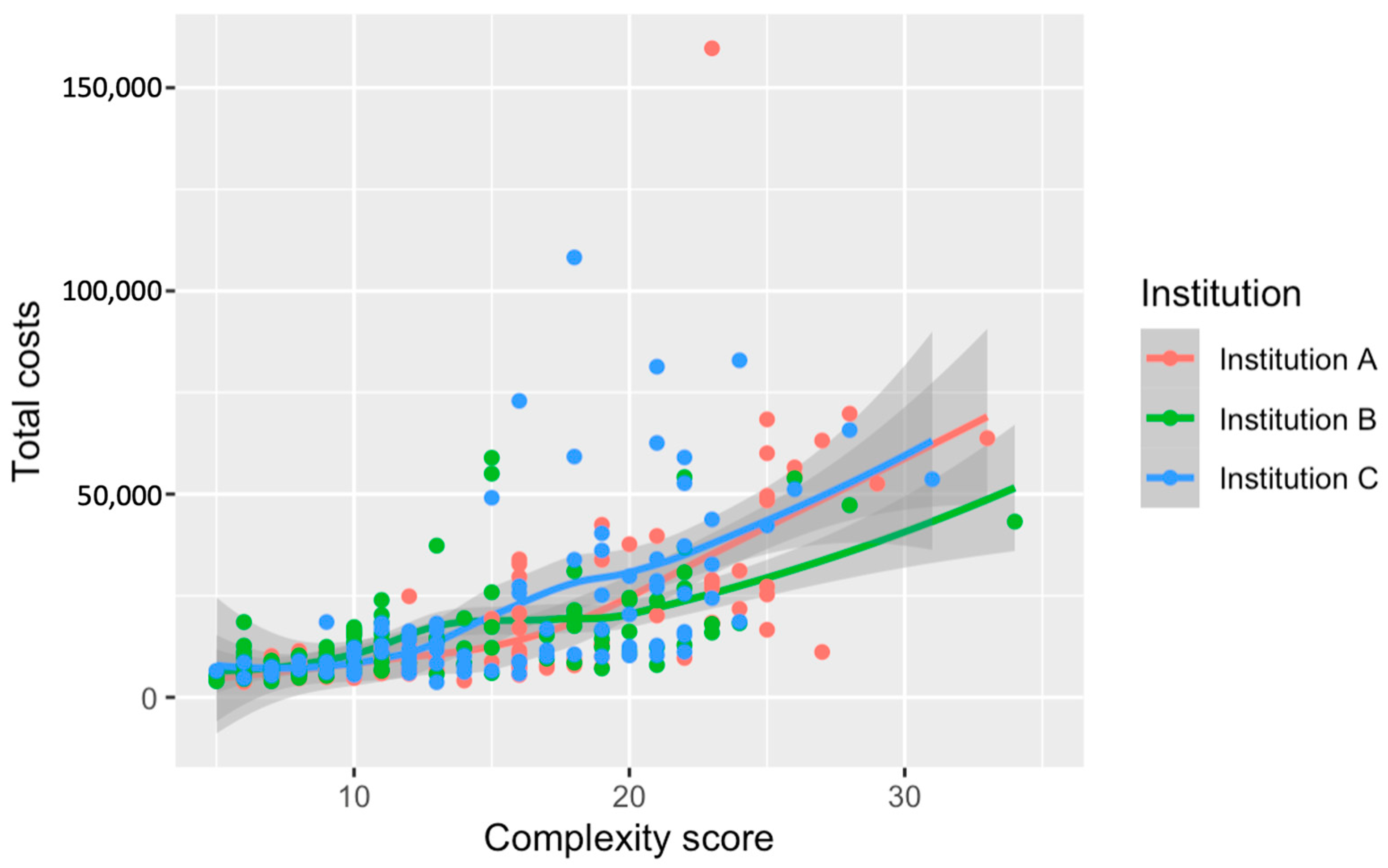
3.3. Costs of Surgery
3.4. Total Costs for Hospitalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blay, J.Y.; Bonvalot, S.; Gouin, F.; Le Cesne, A.; Penel, N. Criteria for reference centers for sarcomas: Volume but also long-term multidisciplinary organisation. Ann. Oncol. 2019, 30, 2008–2009. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Penel, N.; Gouin, F.; Le Cesne, A.; Toulmonde, M. Improving at a nationwide level the management of patients with sarcomas with an expert network. Ann. Oncol. 2022, 33, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Penel, N.; Valentin, T.; Anract, P.; Duffaud, F.; Dufresne, A.; Verret, B.; Cordoba, A.; Italiano, A.; Brahmi, M.; et al. Improved nationwide survival of sarcoma patients with a network of reference centers. Ann. Oncol. 2024, 35, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Hindi, N.; Bollard, J.; Aguiar, S.; Angel, M.; Araya, B.; Badilla, R.; Bernabeu, D.; Campos, F.; Caro-Sánchez, C.H.S.; et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat. Rev. 2022, 102, 102312. [Google Scholar] [CrossRef] [PubMed]
- Kuek, T.; Schilling, C.G.; Choong, P.F. The impact of cost on quality of surgical management in non-metastatic extremity sarcoma: A cross-country narrative literature review with a systematic approach. J. Orthop. Surg. 2023, 31, 10225536231168989. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Buja, A.; Tropea, S.; Girardi, G.; Franzese, L.C.; Cozzolino, C.; Zorzi, M.; Vecchiato, A.; Del Fiore, P.; Brunello, A.; et al. Direct Costs of Care for Adults with Soft Tissue Sarcomas: A Population-Based Study. Cancers 2022, 14, 3109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Sulieman, L.M.; VanHouten, J.P.; Halpern, J.L.; Schwartz, H.S.; Devin, C.J.; Holt, G.E. Cost-utility of osteoarticular allograft versus endoprosthetic reconstruction for primary bone sarcoma of the knee: A markov analysis. J. Surg. Oncol. 2017, 115, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mundinger, G.S.; Prucz, R.B.; Frassica, F.J.; Deune, E.G. Concomitant upper extremity soft tissue sarcoma limb-sparing resection and functional reconstruction: Assessment of outcomes and costs of surgery. Hand 2014, 9, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.A.; Frassica, F.J.; Gordon, T.A.; Deune, E.G. Cost analysis of functional restoration surgery for extremity soft-tissue sarcoma. Plast. Reconstr. Surg. 2006, 117, 277–283. [Google Scholar] [CrossRef]
- Barrientos-Ruiz, I.; Serrano-Montilla, J.; Ortiz-Cruz, E.J. Cost analysis of the diagnosis and treatment of soft tissue sarcomas in reference centres. Rev. Esp. Cir. Ortop. Traumatol. 2012, 56, 374–377. [Google Scholar] [CrossRef]
- Campbell, S.R.; Wooley, J.R.; Nystrom, L.M. Modern Multidisciplinary Management of Soft Tissue Sarcoma of the Extremity and Trunk. JCO Oncol. Pr. 2024, OP2300050. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Bode, B.; Heesen, P.; Kopf, B.; Michelitsch, C.; Odermatt, M.; Giovanoli, P.; Breitenstein, S.; Schneider, P.; Schupfer, G.; et al. Transdisciplinary sarcoma care: A model for sustainable healthcare transformation. Swiss Med. Wkly. 2024, 154, 3473. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Schelling, G.; Elyes, M.; Studer, G.; Bode-Lesniewska, B.; Scaglioni, M.F.; Giovanoli, P.; Heesen, P. Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes. Cancers 2023, 15, 4395. [Google Scholar] [CrossRef] [PubMed]
- Heesen, P.; Studer, G.; Bode, B.; Windegger, H.; Staeheli, B.; Aliu, P.; Martin-Broto, J.; Gronchi, A.; Blay, J.Y.; Le Cesne, A.; et al. Quality of Sarcoma Care: Longitudinal Real-Time Assessment and Evidence Analytics of Quality Indicators. Cancers 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Morattel, B.; Mustaki, L.; Montemurro, M.; Letovanec, I.; Durham, A.D.; Becce, F.; Omoumi, P.; di Summa, P.G.; Matter, M.; Rudiger, H.A.; et al. Oncological outcome, functional results and costs after unplanned excision of musculoskeletal soft tissue sarcoma. Eur. J. Surg. Oncol. 2020, 46, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Alamanda, V.K.; Delisca, G.O.; Mathis, S.L.; Archer, K.R.; Ehrenfeld, J.M.; Miller, M.W.; Homlar, K.C.; Halpern, J.L.; Schwartz, H.S.; Holt, G.E. The financial burden of reexcising incompletely excised soft tissue sarcomas: A cost analysis. Ann. Surg. Oncol. 2013, 20, 2808–2814. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.N.; Jayakumar, P.; Bozic, K.J. Value-based Healthcare: Cost Containment Does Not Equal Value Creation. Clin. Orthop. Relat. Res. 2024, 482, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Bertin, N.; Hebrard, M.; Tirado-Magallanes, R.; Bellis, C.; Lim, W.K.; Chua, C.Y.; Tong, P.M.L.; Chua, R.; Mak, K.; et al. The Singapore National Precision Medicine Strategy. Nat. Genet. 2023, 55, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Studer, G.; Bode, B.; Wellauer, H.; Frei, A.; Theus, C.; Schupfer, G.; Plock, J.; Windegger, H.; Breitenstein, S.; et al. Development of a value-based healthcare delivery model for sarcoma patients. Swiss Med. Wkly. 2021, 151, w30047. [Google Scholar] [CrossRef]
- Porter, M.E. Value-based health care delivery. Ann. Surg. 2008, 248, 503–509. [Google Scholar] [CrossRef]
- Porter, M.E.; Lee, T.H.; Murray, A.C.A. The Value-Based Geography Model of Care. NEJM Catal. 2020, 1. [Google Scholar] [CrossRef]
- Kruiswijk, A.A.; Dorleijn, D.M.J.; Marang-van de Mheen, P.J.; van de Sande, M.A.J.; van Bodegom-Vos, L. Health-Related Quality of Life of Bone and Soft-Tissue Tumor Patients around the Time of Diagnosis. Cancers 2023, 15, 2804. [Google Scholar] [CrossRef] [PubMed]
| Category | Subcategory | Description |
|---|---|---|
| Hospitalization Costs | Individual Costs | |
| Total Individual Costs | Direct individual costs specifically attributed to the patient’s hospital stay. | |
| Medication Costs | Costs specifically incurred for medications uniquely used in this case during hospitalization. | |
| Material and Implant Costs | Costs for materials and implants specifically used for this patient during hospitalization. | |
| Common Costs | ||
| Total Common Costs | General overhead costs that are shared among various patients but are part of the hospitalization expenses. | |
| Operating Room costs | Costs associated with the use of operating rooms include infrastructure, personnel, and minor materials shared among surgical specialists. | |
| Anesthesia and Recovery Room Costs | Costs related to anesthesia services and the recovery room are applicable to multiple patients during hospitalization. | |
| Intensive Care Costs | Costs for intensive care are shared across patients needing such services during hospitalization. | |
| Medical (Fraternity) Staff Costs | Costs for surgical and general medical staff involvement spread over multiple cases during hospitalization. | |
| Nursing Costs | Nursing costs at the ward level, shared among patients during hospitalization. | |
| Surgical Costs | Direct Costs | |
| Operating Room Usage Costs | Costs associated with operating room time (patients arrival until leaving), including all aspects of OR management and nursing costs as well as apportionment of indirect costs. | |
| Surgical Staff Costs | Direct costs for medical fraternity present in the operating room, calculated based on surgical service time. | |
| Anesthesia Costs | Anesthesia costs during surgery and for the (anesthesia-led) recovery room. During surgery, the cost is calculated in one of four categories, depending on the type of anesthesia. In the recovery room, a minute-based cost is calculated. | |
| Indirect Costs | ||
| Anesthesia Costs | Apportionment of indirect costs. |
| Overall n = 356 | Institution A n = 132 | Institution B n = 121 | Institution C n = 103 | p-Value | |
|---|---|---|---|---|---|
| Age | 51.0 (34.0, 65.0) | 52.5 (37.0, 67.0) | 51.0 (34.0, 62.0) | 48.0 (33.0, 63.0) | 0.33 |
| Female | 157 (44.1%) | 56 (42.4%) | 45 (46.3%) | 45 (43.7%) | 0.82 |
| Dignity Benign Intermediate Malignant | 115 (32.3%) 80 (22.5%) 161 (45.2%) | 42 (31.8%) 31 (23.5%) 59 (44.7%) | 50 (41.3%) 26 (21.5%) 45 (37.2%) | 23 (22.3%) 23 (22.3%) 57 (55.4%) | 0.03 |
| Complexity score 1 2 3 4 | 25 (7.0%) 77 (21.6%) 110 (30.9%) 118 (33.1%) | 8 (6.0%) 34 (25.8%) 33 (25.0%) 46 (34.8%) | 14 (11.6%) 31 (25.6%) 40 (33.1%) 29 (24.0%) | 3 (2.9%) 12 (11.7%) 37 (35.9%) 43 (41.7%) | 0.002 |
| Case mix index (CMI) | 0.86 (0.71, 1.37) | 0.85 (0.70, 1.38) | 0.85 (0.70, 1.30) | 1.09 (0.77, 1.63) | 0.001 |
| Characteristic | Overall | Institution A | Institution B | Institution C | p-Value |
|---|---|---|---|---|---|
| Duration of Stay | 4.0 (2.0–8.0) | 4.0 (2.0–8.0) | 3.0 (2.0–7.0) | 5.0 (3.0–11.5) | <0.001 |
| By Tumor Dignity -benign -intermediate -malignant | 2.0 (2.0–3.5) 4.0 (2.0–7.0) 8.0 (5.0–13.0) | 2.0 (2.0, 3.0) 4.0 (3.0, 7.0) 8.0 (4.5, 13.0) | 2.0 (1.0, 3.0) 3.0 (2.0, 6.0) 7.0 (5.0, 9.0) | 3.0 (2.0, 4.0) 4.0 (2.5, 7.5) 9.0 (5.0, 15.0) | <0.001 |
| By Complexity Score -Score 1 -Score 2 -Score 3 -Score 4 | 2.0 (1.0–3.0) 2.0 (2.0–3.0) 4.0 (2.25–7.0) 9.0 (5.0–13.0) | 1.5 (1.0, 2.25) 2.0 (2.0, 3.0) 4.0 (3.0, 7.0) 9.0 (5.25, 13.0) | 2.0 (2.0, 3.0) 2.0 (1.0, 2.5) 4.0 (2.0, 7.0) 7.0 (5.0, 9.0) | 2.0 (2.0, 2.0) 3.0 (2.0, 4.0) 3.0 (2.0, 6.0) 10.0 (5.5, 14.5) | <0.001 |
| Characteristics | Overall | Institution A | Institution B | Institution C | p-Value |
|---|---|---|---|---|---|
| Duration of surgery | 89.5 (56.0–165.0) | 83.5 (61.0, 176.0) | 89.0 (56.0, 147.0) | 90.0 (53.0, 199.5) | 0.76 |
| Tumor Dignity -benign -intermediate -malignant | 60.0 (43.0–82.3) 86.0 (58.0–123.5) 150.5 (80.3–277.5) | 62.5 (40.0–75.8) 82.0 (61.5–120.0) 185.0 (87.0–374.0) | 57.5 (47.3, 94.8) 87.5 (58.8, 108.8) 144.0 (93.0, 237.0) | 60.0 (41.5, 89.3) 90.0 (40.0, 167.0) 147.0 (60.5, 255.5) | <0.001 |
| Complexity Score -Score 1 -Score 2 -Score 3 -Score 4 | 44.0 (33.0–59.0) 57.5 (43.3–75.8) 90.0 (58.0–136.0) 163.0 (103.8–336.0) | 46.5 (38.0–78.8) 62.0 (41.0–75.8) 77.0 (62.0–106.0) 198.5 (112.0–383.5) | 48.5 (38.0, 58.8) 55.0 (46.5, 73.0) 106.0 (74.0, 152.5) 144.0 (93.0, 246.0) | 32.0 (31.0, 36.0) 52.0 (42.0, 81.0) 77.5 (41.3, 161.8) 158.0 (113.0, 281.0) | <0.001 |
| Factor | Overall [CHF] | Institution A [CHF] | Institution B [CHF] | Institution C [CHF] | p-Value |
|---|---|---|---|---|---|
| Costs Surgery | 3519 (2502–5945) | 3118 (2228–6142) | 3408 (2464–5050) | 4611 (2957–7885) | 0.001 |
| Total Cost by Dignity -benign -intermediate -malignant | 2570 (1998–3321) 3545 (2571–5062) 5502.7 (3270–10,281) | 2259 (1868–2743) 3317 (2385–4325) 6137 (3076–10694) | 2612 (2016–3578) 3262 (2332–4001) 4616 (3709–7871) | 3087 (2532–3841) 4693 (3388–7044) 5349 (3074–12,762) | <0.001 |
| Total Cost by Complexity Score -Score 1 -Score 2 -Score 3 -Score 4 | 2207 (1584–2760) 2431 (1969–3035) 3531 (2627–5250) 5964 (3948–11,747) | 2247 (1455–2709) 2259 (1889–2915) 3010 (2366–3796) 6300 (4108–11,491) | 2041 (1689–2511) 2473 (2064–2987) 3846 (2918–5375) 4616 (3709–8039) | 3087 (2074–3278) 2990 (2342–3631) 3767 (2924–5559) 6291 (4811–13,715) | <0.001 |
| Factor | Overall Median Cost [CHF] | Institution A [CHF] | Institution B [CHF] | Institution C [CHF] | p-Value |
|---|---|---|---|---|---|
| Total Costs | 10,378 (6865–18,806) | 8827 (6116–16,719) | 10,564 (6852–17,310) | 11,990 (8121–26,390) | 0.005 |
| Cost by Tumor Dignity –Benign –Intermediate –malignant | 6828 (5419–8739) 10,062 (7119–13,004) 18,374 (10,160–37,228) | 5825 (4939–7291) 8745 (6567–12,226) 20,123 (8878–38,691) | 7189 (5569–10,875) 9616 (6954–12,449) 18,079 (12,877–31,054) | 7363 (6161–8816) 12,221 (9467–16,404) 18,627 (10,292–40,374) | <0.0001 |
| Cost by Complexity Score –Score 1 –Score 2 –Score 3 –Score 4 | 5650 (5217–8007) 6568 (5431–7814) 10,367 (7118–15,369) 22,779 (11,752–39,198) | 5535 (4988–5708) 5942 (4939–7761) 8705 (6619–12,186) 23,526 (10,692–41,764) | 5882 (5235–9202) 6852 (5604–7775) 13,817 (8459–17,281) 18,079 (12,368–26,846) | 6525 (5609–7568) 7312 (6120–8001) 10,876 (7019–14,072) 25,677 (12,579–43,020) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schelling, G.; Heesen, P.; Tautermann, B.; Wepf, M.; Di Federico, B.; Frei, A.; van Oudenaarde, K.; Giovanoli, P.; Bode-Lesniewska, B.; Studer, G.; et al. Impact of Institutional Practices and Surgical Complexity on Sarcoma Surgery Costs: Driving Efficiency in Value-Based Healthcare. Cancers 2024, 16, 2209. https://doi.org/10.3390/cancers16122209
Schelling G, Heesen P, Tautermann B, Wepf M, Di Federico B, Frei A, van Oudenaarde K, Giovanoli P, Bode-Lesniewska B, Studer G, et al. Impact of Institutional Practices and Surgical Complexity on Sarcoma Surgery Costs: Driving Efficiency in Value-Based Healthcare. Cancers. 2024; 16(12):2209. https://doi.org/10.3390/cancers16122209
Chicago/Turabian StyleSchelling, Georg, Philip Heesen, Boris Tautermann, Markus Wepf, Barbara Di Federico, Annika Frei, Kim van Oudenaarde, Pietro Giovanoli, Beata Bode-Lesniewska, Gabriela Studer, and et al. 2024. "Impact of Institutional Practices and Surgical Complexity on Sarcoma Surgery Costs: Driving Efficiency in Value-Based Healthcare" Cancers 16, no. 12: 2209. https://doi.org/10.3390/cancers16122209
APA StyleSchelling, G., Heesen, P., Tautermann, B., Wepf, M., Di Federico, B., Frei, A., van Oudenaarde, K., Giovanoli, P., Bode-Lesniewska, B., Studer, G., Fuchs, B., & on behalf of the Swiss Sarcoma Network. (2024). Impact of Institutional Practices and Surgical Complexity on Sarcoma Surgery Costs: Driving Efficiency in Value-Based Healthcare. Cancers, 16(12), 2209. https://doi.org/10.3390/cancers16122209







