Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.1.1. Entire Cohort
3.1.2. Non-Small Cell Lung Cancer Cohort
3.2. Safety Analysis
3.2.1. Entire Cohort
3.2.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
3.3. Efficacy Analysis
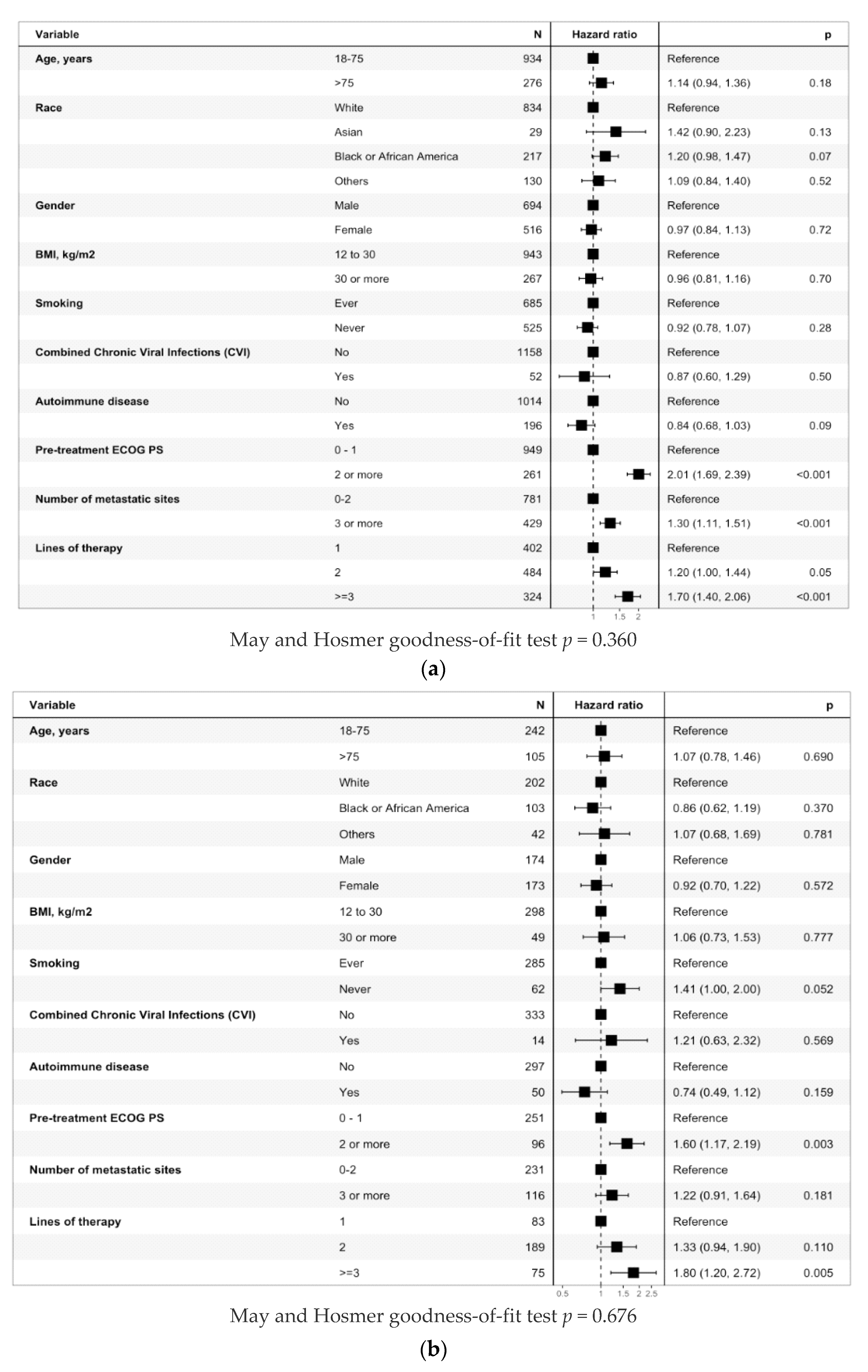
3.3.1. Entire Cohort
3.3.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
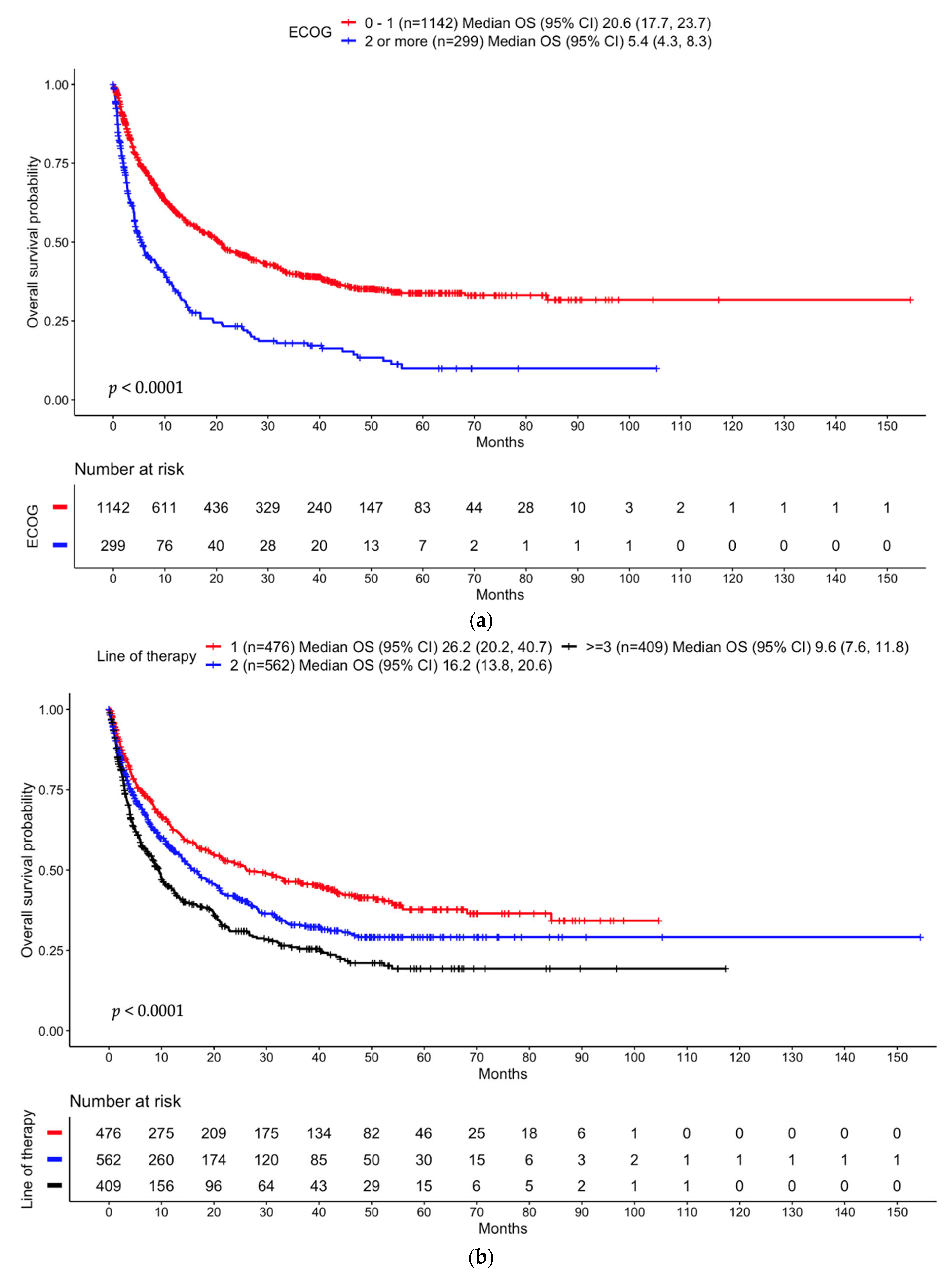
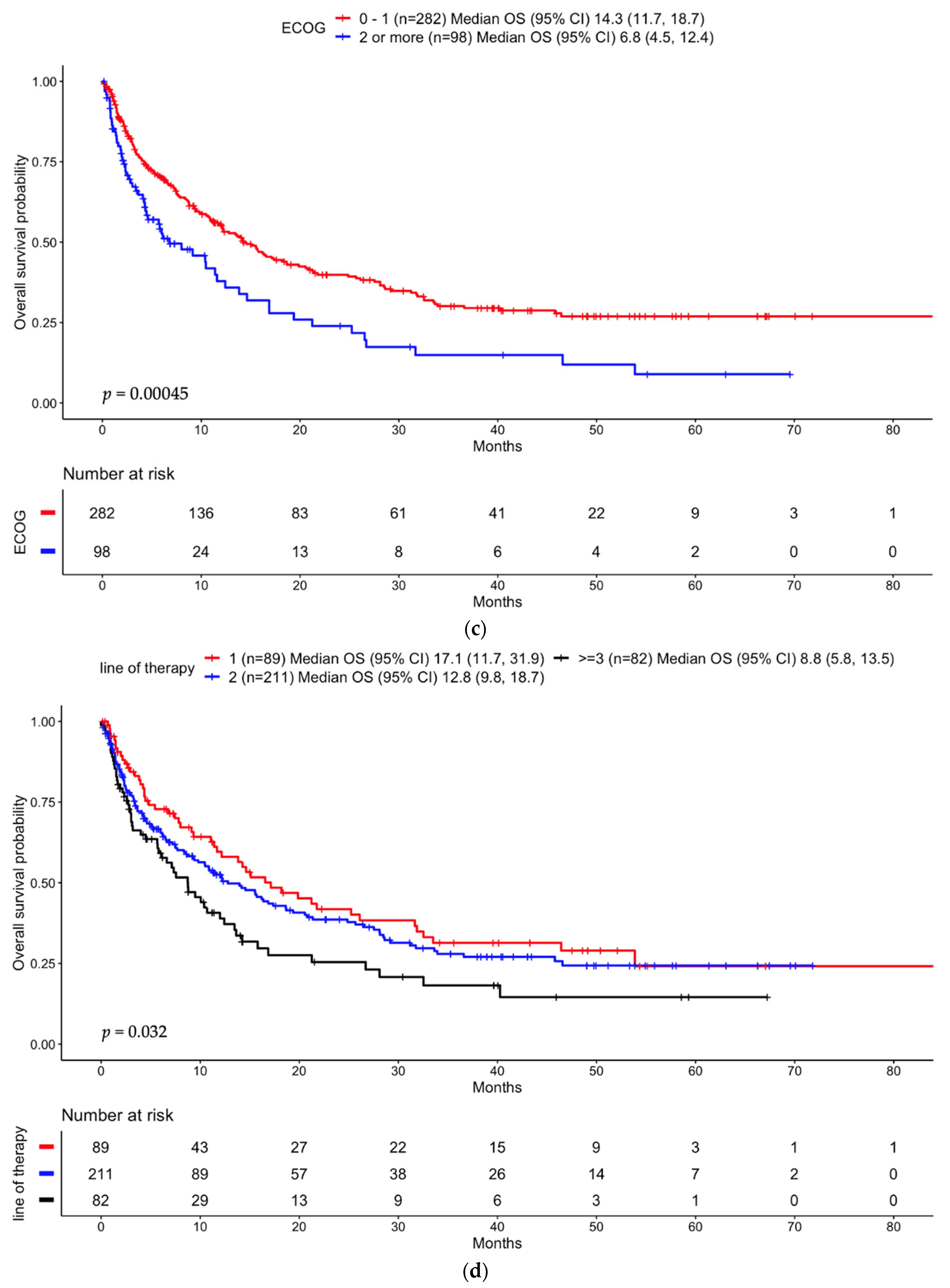
3.4. Time to Treatment Failure (TTF)
3.4.1. Entire Cohort
3.4.2. Anti-PD-(L)1 Monotherapy Non-Small Cell Lung Cancer Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Erratum in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133, Erratum in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Kennedy, E.B.; Alarcon-Rozas, A.E.; Alcindor, T.; Bartley, A.N.; Malowany, A.B.; Bhadkamkar, N.A.; Deighton, D.C.; Janjigian, Y.; Karippot, A.; et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1470–1491, Erratum in J. Clin. Oncol. 2023, JCO2300441. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Sura, S.D.; Shinde, R.; Shi, J.; Singhal, P.K.; Robert, N.J.; Vogelzang, N.J.; Perini, R.F.; Motzer, R.J. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur. Urol. Open Sci. 2023, 49, 110–118. [Google Scholar] [CrossRef]
- Gul, A.; Stewart, T.F.; Mantia, C.M.; Shah, N.J.; Gatof, E.S.; Long, Y.; Allman, K.D.; Ornstein, M.C.; Hammers, H.J.; McDermott, D.F.; et al. Salvage Ipilimumab and Nivolumab in Patients with Metastatic Renal Cell Carcinoma After Prior Immune Checkpoint Inhibitors. J. Clin. Oncol. 2020, 38, 3088–3094. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Spigel, D.R.; McCleod, M.; Jotte, R.M.; Einhorn, L.; Horn, L.; Waterhouse, D.M.; Creelan, B.; Babu, S.; Leighl, N.B.; Chandler, J.C.; et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J. Thorac. Oncol. 2019, 14, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Ardizzoni, A.; Ciuleanu, T.; Cobo, M.; Laktionov, K.; Szilasi, M.; Califano, R.; Carcereny, E.; Griffiths, R.; Paz-Ares, L.; et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur. J. Cancer 2020, 127, 160–172. [Google Scholar] [CrossRef]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; de Castro, G., Jr.; Reck, M.; Wu, Y.L.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, N.; Ochi, N.; Nakagawa, N.; Nagasaki, Y.; Taoka, M.; Ichiyama, N.; Mimura, A.; Nakanishi, H.; Kohara, H.; Yamane, H. Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors? Cancers 2020, 12, 1995. [Google Scholar] [CrossRef]
- Grossi, F.; Crinò, L.; Logroscino, A.; Canova, S.; Delmonte, A.; Melotti, B.; Proto, C.; Gelibter, A.; Cappuzzo, F.; Turci, D.; et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: Results from the Italian cohort of an expanded access programme. Eur. J. Cancer 2018, 100, 126–134. [Google Scholar] [CrossRef]
- Nebhan, C.A.; Cortellini, A.; Ma, W.; Ganta, T.; Song, H.; Ye, F.; Irlmeier, R.; Debnath, N.; Saeed, A.; Radford, M.; et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors among Patients Aged 80 Years or Older with Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021, 7, 1856–1861. [Google Scholar] [CrossRef]
- Tomasik, B.; Bieńkowski, M.; Braun, M.; Popat, S.; Dziadziuszko, R. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score ≥2—Systematic review and meta-analysis. Lung Cancer 2021, 158, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Goyal, S.; Chen, Z.; Engelhart, A.; Carlisle, J.W.; Beardslee, T.J.; Gill, H.; Odikadze, L.; Liu, Y.; Mishra, M.K.; et al. Efficacy and safety of immune checkpoint blockade in self-identified Black patients with advanced non-small cell lung cancer. Cancer 2020, 126, 5040–5049. [Google Scholar] [CrossRef]
- Florez, M.A.; Kemnade, J.O.; Chen, N.; Du, W.; Sabichi, A.L.; Wang, D.Y.; Huang, Q.; Miller-Chism, C.N.; Jotwani, A.; Chen, A.C.; et al. Persistent ethnicity-associated disparity in anti-tumor effectiveness of immune checkpoint inhibitors despite equal access. Cancer Res. Commun. 2022, 2022, 806–813. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shah, N.J.; Cook, M.; Blackburn, M.; Serzan, M.T.; Advani, S.; Potosky, A.L.; Madhavan, S.; Belouali, A.; Atkins, M.B.; et al. Association between Body Mass Index and Immune-Related Adverse Events (irAEs) among Advanced-Stage Cancer Patients Receiving Immune Checkpoint Inhibitors: A Pan-Cancer Analysis. Cancers 2021, 13, 6109, Erratum in Cancers 2022, 14, 4525. [Google Scholar] [CrossRef] [PubMed]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association Between Body Mass Index and Overall Survival with Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and immune-checkpoint inhibitors in advanced melanoma: A meta-analysis of survival outcomes from clinical studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Fitzgerald, C.; Lee, M.; Valero, C.; Gönen, M.; Shoushtari, A.; Morris, L.G.T. Association Between Toxic Effects and Survival in Patients with Cancer and Autoimmune Disease Treated With Checkpoint Inhibitor Immunotherapy. JAMA Oncol. 2022, 8, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Pruitt, S.L.; Xuan, L.; Gerber, D.E. Prevalence of Autoimmune Disease Among Patients with Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol. 2016, 2, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019, 71, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bhatia, S.; Thompson, J.A.; Grivas, P. Preexisting Autoimmune Disease: Implications for Immune Checkpoint Inhibitor Therapy in Solid Tumors. J. Natl. Compr. Cancer Netw. 2019, 17, 750–757. [Google Scholar] [CrossRef]
- Zarif, T.E.; Nassar, A.; Adib, E.; Fitzgerald, B.; Huang, J.; Mouhieddine, T.; Nonato, T.; McKay, R.; Li, M.; Mittra, A.; et al. 437 Safety and efficacy of immune checkpoint inhibitors (ICI) in patients living with HIV (PLWH) and metastatic non-small cell lung cancer (NSCLC): A matched cohort study from the international CATCH-IT consortium. J. Immuno Therapy Cancer 2022, 10 (Suppl. S2), A457–A458. [Google Scholar]
- Shah, N.J.; Al-Shbool, G.; Blackburn, M.; Cook, M.; Belouali, A.; Liu, S.V.; Madhavan, S.; He, A.R.; Atkins, M.B.; Gibney, G.T.; et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J. Immunother. Cancer 2019, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.R.; Kim, C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients with HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019, 5, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- CTCAE V4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 1 March 2024).
- May, S.; Hosmer, D.W. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998, 4, 109–120. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. 2022. Available online: https://www.r-project.org/ (accessed on 1 March 2024).
- Kim, C.M.; Lee, J.B.; Shin, S.J.; Ahn, J.B.; Lee, M.; Kim, H.S. The efficacy of immune checkpoint inhibitors in elderly patients: A meta-analysis and meta-regression. ESMO Open 2022, 7, 100577. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tapia Rico, G.; Chan, M.M.; Loo, K.F. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat. Rev. 2020, 86, 102011. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.-G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
| Characteristics | Entire Cohort N = 1453 n (%) | PD-(L)1 Monotherapy NSCLC Cohort N = 384 n (%) |
|---|---|---|
| Age, median (IQR) years | 65.8 (56.6, 74.3) | 70.1 (61.8, 76.3) |
| 18–75 | 1118 (77.2) | 264 (68.9) |
| >75 | 330 (22.8) | 119 (31.1) |
| Race | ||
| Asian | 39 (2.7) | 13 (3.4) |
| Black | 237 (16.3) | 111 (28.9) |
| White a | 1012 (69.6) | 227 (59.1) |
| Others | 165 (11.4) | 33 (8.6) |
| Gender | ||
| Male | 838 (57.8) | 195 (50.8) |
| Female | 612 (42.2) | 189 (49.2) |
| BMI, kg/m2 | ||
| 12 ≤ BMI < 30 | 1110 (77.9) | 322 (85) |
| BMI ≥ 30 | 315 (22.1) | 57 (15) |
| Smoking Status | ||
| Ever Smoker b | 817 (56.5) | 319 (83.1) |
| Never Smoker | 628 (43.5) | 65 (16.9) |
| Chronic Viral Infections (CVI) | ||
| Combined CVI c | 68 (4.7) | 16 (4.2) |
| Hepatitis B (HBV) | 25 (1.7) | 3 (0.8) |
| Hepatitis C (HCV) | 32 (2.2) | 6 (1.6) |
| HIV | 18 (1.2) | 8 (2.1) |
| History of AID d | 228 (15.7) | 54 (14.1) |
| Pre-treatment ECOG PS | ||
| 0 | 383 (26.5) | 78 (20.4) |
| 1 | 761 (52.7) | 205 (53.7) |
| ≥2 | 300 (20.8) | 99 (25.9) |
| ICIs | ||
| Atezolizumab | 47 (3.2) | 26 (6.8) |
| Avelumab | 3 (0.2) | 1 (0.3) |
| Durvalumab | 17 (1.2) | 9 (2.3) |
| Ipilimumab | 163 (11.2) | - |
| Nivolumab | 539 (37.1) | 245 (63.8) |
| Nivolumab + ipilimumab | 192 (13.2) | - |
| Pembrolizumab | 323 (22.2) | 103 (26.8) |
| Pembrolizumab + ipilimumab | 14 (1) | - |
| IO plus chemo | 38 (2.6) | - |
| Others e | 117 (8.1) | - |
| Cancer types | ||
| Lung cancer | 499 (34.4) f | 384 (100) |
| Adenocarcinoma | 312 (62.5) | 256 (66.7) |
| Squamous | 116 (23.2) | 105 (27.3) |
| Others | 69 (13.8) | - |
| Melanoma | 403 (27.8) g | - |
| Cutaneous | 293 (72.7) | - |
| Others | 75 (18.6) | - |
| GI cancers | 104 (7.2) | - |
| Kidney cancers | 100 (6.9) | - |
| Others | 346 (23.8) | - |
| Characteristic | Entire Cohort | |||
|---|---|---|---|---|
| Any Grade irAEs OR (95% CI) | p-Value | Grade ≥ 3 irAEs OR (95% CI) | p-Value | |
| Age, years | 0.446 | 0.425 | ||
| 18–75 | ref | ref | ||
| >75 | 1.11 (0.85, 1.43) | 0.85 (0.57, 1.25) | ||
| Race | ||||
| Asian | 0.43 (0.19, 0.90) | 0.033 | 0.56 (0.13, 1.60) | 0.344 |
| Black | 0.54 (0.39, 0.73) | <0.001 | 0.49 (0.28, 0.81) | 0.008 |
| White | ref | - | ref | - |
| Other | 0.81 (0.57, 1.14) | 0.233 | 0.79 (0.46, 1.3) | 0.383 |
| Gender | 0.356 | 0.439 | ||
| Male | ref | ref | ||
| Female | 1.11 (0.89, 1.38) | 0.88 (0.63, 1.21) | ||
| BMI, kg/m2 | <0.001 | 0.001 | ||
| 12 ≤ BMI < 30 | ref | ref | ||
| BMI ≥ 30 | 1.44 (1.11, 1.86) | 1.61 (1.13, 2.28) | ||
| ECOG PS | <0.001 | 0.001 | ||
| 0–1 | ref | ref | ||
| ≥2 | 0.46 (0.34, 0.62) | 0.45 (0.27, 0.71) | ||
| Characteristic | Anti-PD-(L)1 NSCLC Cohort | |||
|---|---|---|---|---|
| Any Grade irAEs OR (95% CI) | p-Value | Grade ≥ 3 irAEs OR (95% CI) | p-Value | |
| Age, years | 0.097 | 0.093 | ||
| 18–75 | ref | ref | ||
| >75 | 1.52 (0.93, 2.47) | 1.98 (0.89, 4.42) | ||
| Race | ||||
| Black | 0.53 (0.30, 0.90) | 0.023 | 0.44 (0.14, 1.16) | 0.123 |
| White | ref | - | ref | - |
| Other | 0.56 (0.25, 1.15) | 0.128 | 0.70 (0.16, 2.21) | 0.583 |
| Gender | 0.136 | 0.292 | ||
| Male | ref | ref | ||
| Female | 1.43 (0.89, 2.28) | 1.53 (0.70, 3.50) | ||
| BMI, kg/m2 | - | - | 0.252 | |
| 12 ≤ BMI < 30 | ref | |||
| BMI ≥ 30 | 1.72 (0.64, 4.17) | |||
| Combined CVI a | 0.006 | 0.041 | ||
| Yes | ref | ref | ||
| No | 0.22 (0.07, 0.64) | 0.23 (0.06, 1.12) | ||
| History of AID b | 0.037 | 0.293 | ||
| Yes | 1.93 (1.03, 3.59) | 1.65 (0.61, 4.00) | ||
| No | ref | ref | ||
| ECOG PS | 0.036 | - | - | |
| 0–1 | ref | |||
| ≥2 | 0.55 (0.31, 0.95) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.J.; Della Pia, A.; Wu, T.; Williams, A.; Weber, M.; Sinclaire, B.; Gourna Paleoudis, E.; Alaoui, A.; Lev-Ari, S.; Adams, S.; et al. Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers 2024, 16, 2223. https://doi.org/10.3390/cancers16122223
Shah NJ, Della Pia A, Wu T, Williams A, Weber M, Sinclaire B, Gourna Paleoudis E, Alaoui A, Lev-Ari S, Adams S, et al. Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers. 2024; 16(12):2223. https://doi.org/10.3390/cancers16122223
Chicago/Turabian StyleShah, Neil J., Alexandra Della Pia, Tianmin Wu, Aquino Williams, Melinda Weber, Brittany Sinclaire, Elli Gourna Paleoudis, Adil Alaoui, Shaked Lev-Ari, Shari Adams, and et al. 2024. "Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials" Cancers 16, no. 12: 2223. https://doi.org/10.3390/cancers16122223
APA StyleShah, N. J., Della Pia, A., Wu, T., Williams, A., Weber, M., Sinclaire, B., Gourna Paleoudis, E., Alaoui, A., Lev-Ari, S., Adams, S., Kaufman, J., Parikh, S. B., Tonti, E., Muller, E., Serzan, M., Cheruku, D., Lee, A., Sridhar, A., Hee, B. P., ... Atkins, M. B. (2024). Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers, 16(12), 2223. https://doi.org/10.3390/cancers16122223






