A Radiomic Approach for Evaluating Intra-Subgroup Heterogeneity in SHH and Group 4 Pediatric Medulloblastoma: A Preliminary Multi-Institutional Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
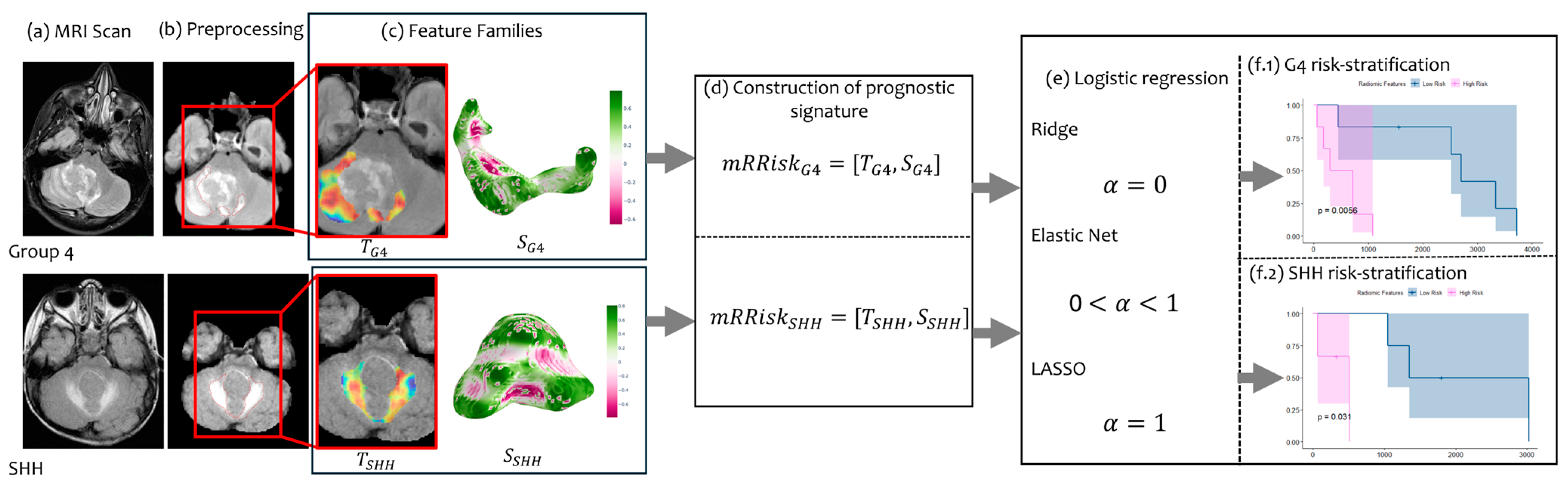
2.1. Overview
2.2. Data Curation
2.3. Pre-Processing and Feature Extraction
2.4. Regression Analysis
3. Results
3.1. Risk-Stratifying MB Patients in Group 4 Subgroup
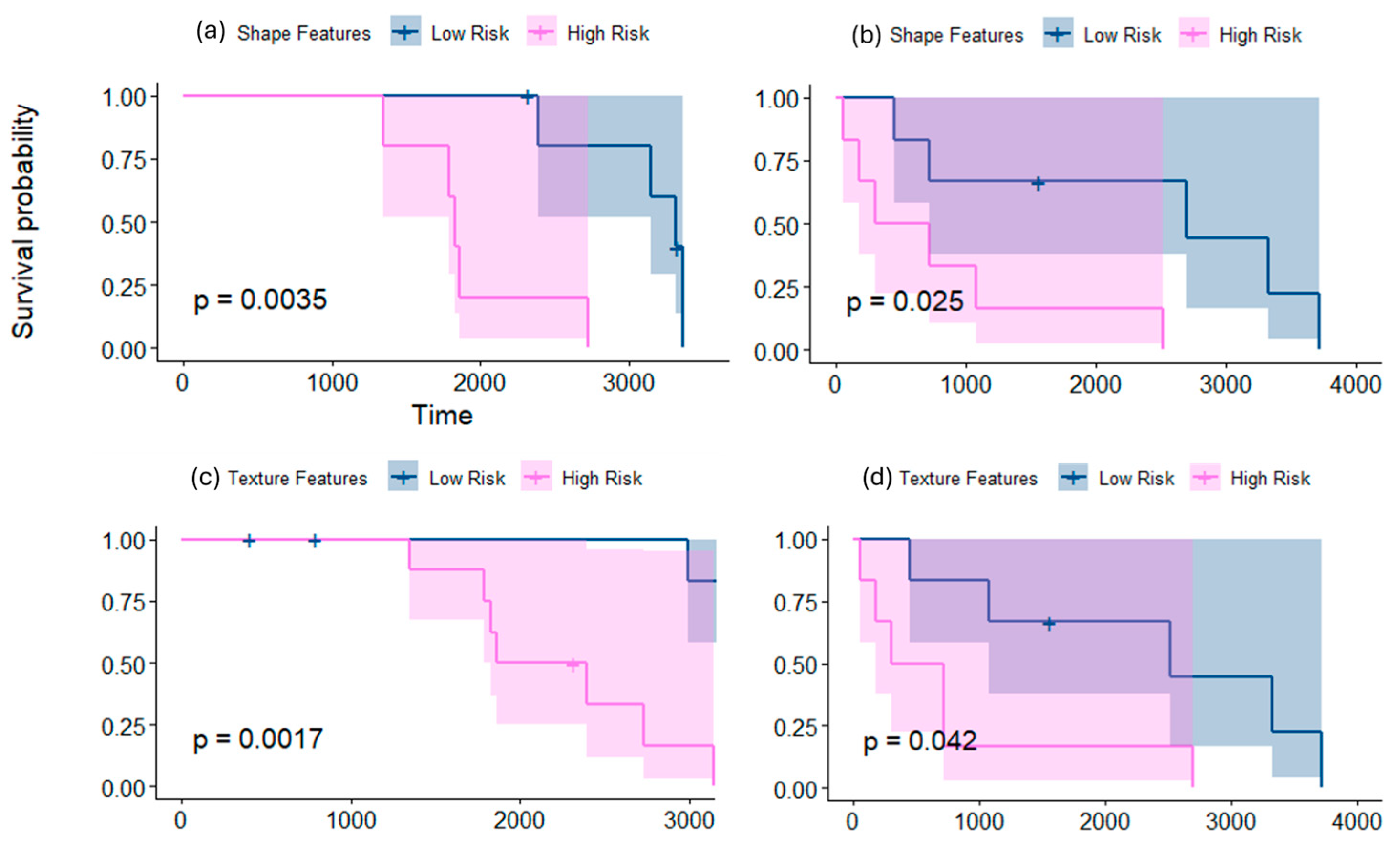
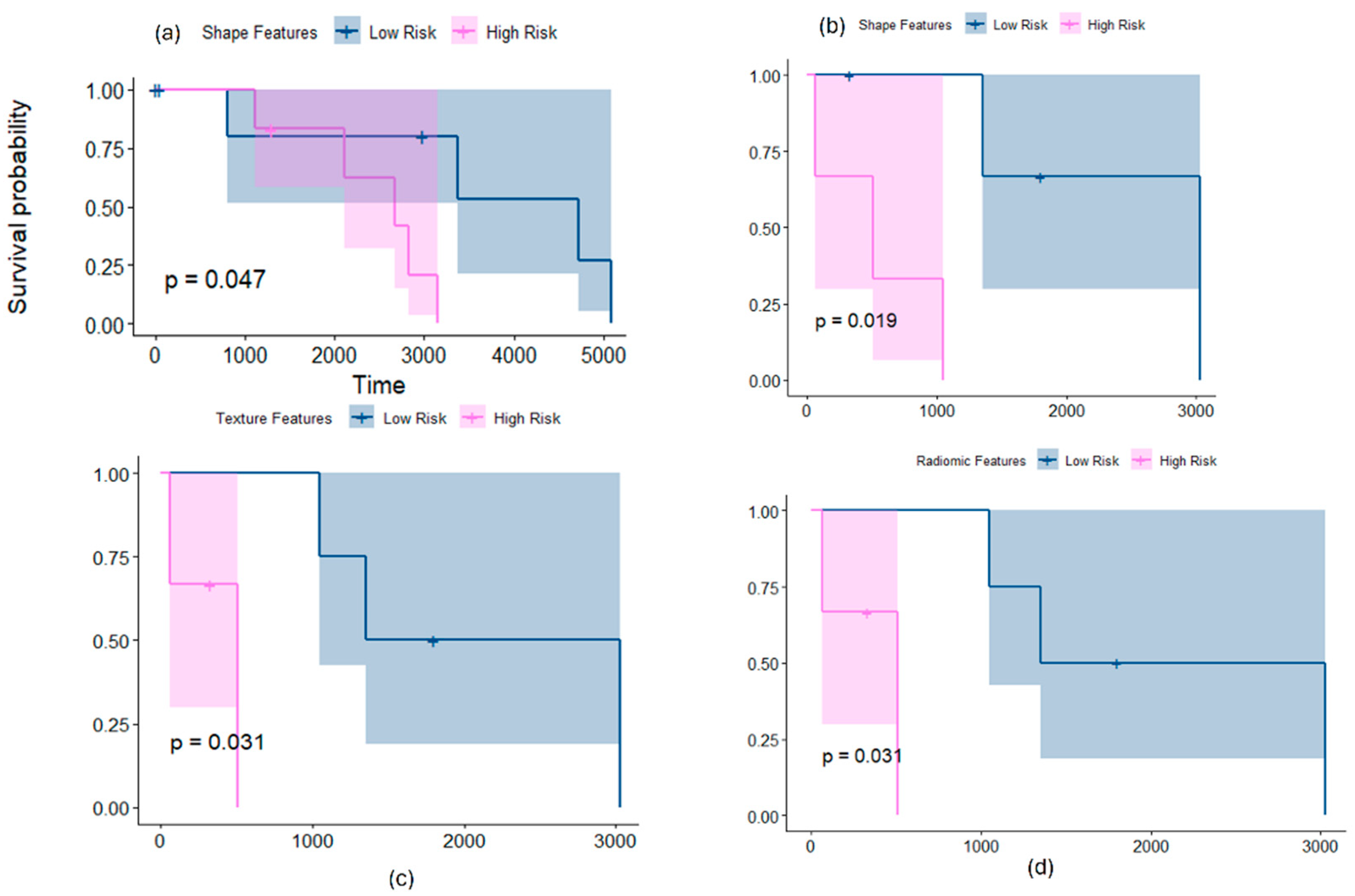
3.1.1. Employing Shape Features Alone for Risk Stratification
3.1.2. Employing Texture Features Alone for Risk Stratification
3.1.3. Employing mRRisk Signature for Risk Stratification
3.2. Risk-Stratifying MB Patients in SHH Subgroup
3.2.1. Employing Shape Features Alone for Risk Stratification
3.2.2. Employing Texture Features Alone for Risk Stratification
3.3. Risk-Stratifying MB Patients in SHH and Group 4 Subgroup Using Chang’s Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, F.C.; Wong, T.T.; Wu, K.S.; Lu, C.F.; Weng, T.W.; Liang, M.L.; Wu, C.C.; Guo, W.Y.; Chen, C.Y.; Hsieh, K.L.C. Magnetic resonance radiomics features and prognosticators in different molecular subtypes of pediatric Medulloblastoma. PLoS ONE 2021, 16, e0255500. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.; Kuijper, S.; Lindsey, J.C.; Petrie, K.; Schwalbe, E.C.; Barker, K.; Boult, J.K.; Williamson, D.; Ahmad, Z.; Hallsworth, A.; et al. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell 2015, 27, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Quaglietta, L.; Vitale, V.; Verrico, A.; Migliorati, R. Treatment of Medulloblastoma: Chemotherapy. In Posterior Fossa Tumors in Children; Springer: Cham, Switzerland, 2015; pp. 349–361. [Google Scholar]
- Dufour, C.; Beaugrand, A.; Pizer, B.; Micheli, J.; Aubelle, M.S.; Fourcade, A.; Couanet, D.; Laplanche, A.; Kalifa, C.; Grill, J. Metastatic medulloblastoma in childhood: Chang’s classification revisited. Int. J. Surg. Oncol. 2012, 2012, 245385. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Ashley, D.; Landi, D. Current medulloblastoma subgroup specific clinical trials. Transl. Pediatr. 2020, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Sursal, T.; Ronecker, J.S.; Dicpinigaitis, A.J.; Mohan, A.L.; Tobias, M.E.; Gandhi, C.D.; Jhanwar-Uniyal, M. Molecular Stratification of Medulloblastoma: Clinical Outcomes and Therapeutic Interventions. Anticancer Res. 2022, 42, 2225–2239. [Google Scholar] [CrossRef] [PubMed]
- Bavle, A.; Parsons, D.W. From one to many: Further refinement of medulloblastoma subtypes offers promise for personalized therapy. Cancer Cell 2017, 31, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013, 31, 2927. [Google Scholar] [CrossRef]
- Cavalli, F.M.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 2017, 31, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Kotrotsou, A.; Zinn, P.O.; Colen, R.R. Radiomics in brain tumors: An emerging technique for characterization of tumor environment. Magn. Reson. Imaging Clin. 2016, 24, 719–729. [Google Scholar] [CrossRef]
- Ammari, S.; Sallé de Chou, R.; Balleyguier, C.; Chouzenoux, E.; Touat, M.; Quillent, A.; Dumont, S.; Bockel, S.; Garcia, G.C.; Elhaik, M.; et al. A predictive clinical-radiomics nomogram for survival prediction of glioblastoma using MRI. Diagnostics 2021, 11, 2043. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Choi, Y.S.; Ahn, S.S.; Chang, J.H.; Kang, S.G.; Kim, E.H.; Kim, S.H.; Lee, S.K. Radiomic MRI phenotyping of glioblastoma: Improving survival prediction. Radiology 2018, 289, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Baid, U.; Rane, S.U.; Talbar, S.; Gupta, S.; Thakur, M.H.; Moiyadi, A.; Mahajan, A. Overall survival prediction in glioblastoma with radiomic features using machine learning. Front. Comput. Neurosci. 2020, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, E.C.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017, 18, 958–971. [Google Scholar] [CrossRef]
- Grist, J.T.; Withey, S.; Bennett, C.; Rose, H.E.; MacPherson, L.; Oates, A.; Powell, S.; Novak, J.; Abernethy, L.; Pizer, B.; et al. Combining multi-site magnetic resonance imaging with machine learning predicts survival in pediatric brain tumors. Sci. Rep. 2021, 11, 18897. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, S.; Li, K.K.; Wang, W.; Li, K.; Duan, W.; Yuan, B.; Wang, L.; Liu, L.; Zhan, Y.; et al. Incremental prognostic value and underlying biological pathways of radiomics patterns in medulloblastoma. eBioMedicine 2020, 61, 103093. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhang, H.; Ge, M.; Hao, X.L.; An, X.; Tian, Y.J. Radiomics signature for the prediction of progression-free survival and radiotherapeutic benefits in pediatric medulloblastoma. Child’s Nerv. Syst. 2022, 38, 1085–1094. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Liu, H.; Ting, G.; Yin, Q.; Li, R.; Liu, M.; Zhang, Y.; Duan, S.; Li, Y.; et al. MRI Radiomics Signature of Pediatric Medulloblastoma Improves Risk Stratification Beyond Clinical and Conventional MR Imaging Features. J. Magn. Reson. Imaging 2022, 58, 236–246. [Google Scholar] [CrossRef]
- Iyer, S.; Ismail, M.; Tamrazi, B.; Salloum, R.; de Blank, P.; Margol, A.; Correa, R.; Chen, J.; Bera, K.; Statsevych, V.; et al. Novel MRI deformation-heterogeneity radiomic features are associated with molecular subgroups and overall survival in pediatric medulloblastoma: Preliminary findings from a multi-institutional study. Front. Oncol. 2022, 12, 915143. [Google Scholar] [CrossRef]
- Iv, M.; Zhou, M.; Shpanskaya, K.; Perreault, S.; Wang, Z.; Tranvinh, E.; Lanzman, B.; Vajapeyam, S.; Vitanza, N.A.; Fisher, P.G.; et al. MR imaging–based radiomic signatures of distinct molecular subgroups of medulloblastoma. Am. J. Neuroradiol. 2019, 40, 154–161. [Google Scholar] [CrossRef]
- Saju, A.C.; Chatterjee, A.; Sahu, A.; Gupta, T.; Krishnatry, R.; Mokal, S.; Sahay, A.; Epari, S.; Prasad, M.; Chinnaswamy, G.; et al. Machine-learning approach to predict molecular subgroups of medulloblastoma using multiparametric MRI-based tumor radiomics. Br. J. Radiol. 2022, 95, 20211359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Qin, B.; Hu, X.; Xiao, W.; Tong, Z.; Li, S.; Jing, Y.; Li, L.; Zhang, Y. Preoperative prediction of sonic hedgehog and group 4 molecular subtypes of pediatric medulloblastoma based on radiomics of multiparametric MRI combined with clinical parameters. Front. Neurosci. 2023, 17, 1157858. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Wang, W.; Zhao, Y.; Li, K.K.; Li, K.; Wang, L.; Yuan, B.; Geng, H.; Zhang, S.; et al. Radiomic features from multi-parameter MRI combined with clinical parameters predict molecular subgroups in patients with medulloblastoma. Front. Oncol. 2020, 10, 558162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wong, S.W.; Wright, J.N.; Wagner, M.W.; Toescu, S.; Han, M.; Tam, L.T.; Zhou, Q.; Ahmadian, S.S.; Shpanskaya, K.; et al. MRI radiogenomics of pediatric medulloblastoma: A multicenter study. Radiology 2022, 304, 406–416. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gupta, T.; Pungavkar, S.; Shirsat, N.; Epari, S.; Chinnaswamy, G.; Mahajan, A.; Janu, A.; Moiyadi, A.; Kannan, S.; et al. Nomograms based on preoperative multiparametric magnetic resonance imaging for prediction of molecular subgrouping in medulloblastoma: Results from a radiogenomics study of 111 patients. Neuro-Oncology 2019, 21, 115–124. [Google Scholar] [CrossRef]
- Chen, X.; Fan, Z.; Li, K.K.; Wu, G.; Yang, Z.; Gao, X.; Liu, Y.; Wu, H.; Chen, H.; Tang, Q.; et al. Molecular subgrouping of medulloblastoma based on few-shot learning of multitasking using conventional MR images: A retrospective multicenter study. Neuro-Oncol. Adv. 2020, 2, vdaa079. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhuang, Y.; Zhang, S.; Wang, J.; Teng, S.; Zeng, H. Multiparametric MRI-based radiomics signature with machine learning for preoperative prediction of prognosis stratification in pediatric medulloblastoma. Acad. Radiol. 2024, 31, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, Q.; Peng, H.; Chen, F.; Zheng, Y.; Jiao, Z.; Gong, J.; Li, W. Automatic image segmentation and online survival prediction model of medulloblastoma based on machine learning. Eur. Radiol. 2023, 34, 3644–3655. [Google Scholar] [CrossRef]
- Juntu, J.; Sijbers, J.; Van Dyck, D.; Gielen, J. Bias field correction for MRI images. In Computer Recognition Systems: Proceedings of the 4th International Conference on Computer Recognition Systems CORES’05; Springer: Berlin/Heidelberg, Germany, 2005; pp. 543–551. [Google Scholar]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Richards, J.E.; Xie, W. Brains for all the ages: Structural neurodevelopment in infants and children from a life-span perspective. Adv. Child Dev. Behav. 2015, 48, 1–52. [Google Scholar]
- Madabhushi, A.; Udupa, J.K. Interplay between intensity standardization and inhomogeneity correction in MR image processing. IEEE Trans. Med. Imaging 2005, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Ghalati, M.K.; Nunes, A.; Ferreira, H.; Serranho, P.; Bernardes, R. Texture analysis and its applications in biomedical imaging: A survey. IEEE Rev. Biomed. Eng. 2021, 15, 222–246. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Tiwari, P.; Madabhushi, A. Co-occurrence of local anisotropic gradient orientations (CoLlAGe): A new radiomics descriptor. Sci. Rep. 2016, 6, 37241. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, R.; Fischl, B.; Caviness, V.; Makris, N.; Grant, P.E. A methodology for analyzing curvature in the developing brain from preterm to adult. Int. J. Imaging Syst. Technol. 2008, 18, 42–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Jacobucci, R. Regularized structural equation modeling to detect measurement bias: Evaluation of lasso, adaptive lasso, and elastic net. Struct. Equ. Model. A Multidiscip. J. 2020, 27, 722–734. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Wagner, P.; Merlo, J. The median hazard ratio: A useful measure of variance and general contextual effects in multilevel survival analysis. Stat. Med. 2017, 36, 928–938. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’agostino, R.B. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat. Med. 2004, 23, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Steck, H.; Krishnapuram, B.; Dehing-Oberije, C.; Lambin, P.; Raykar, V.C. On ranking in survival analysis: Bounds on the concordance index. In Proceedings of the Advances in Neural Information Processing Systems, Vancouver, BC, Canada, 3–6 December 2007; Volume 20. [Google Scholar]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Pfister, S.M. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, I. Choosing the level of significance: A decision-theoretic approach. Abacus 2021, 57, 27–71. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Craig, S.; Ahmed, R.; de Blank, P.; Tiwari, P. Opportunities and Advances in Radiomics and Radiogenomics for Pediatric Medulloblastoma Tumors. Diagnostics 2023, 13, 2727. [Google Scholar] [CrossRef] [PubMed]



| Site | CCHMC | CHLA | CHOP | ||||
|---|---|---|---|---|---|---|---|
| N | G4 N = 14 | SHH N = 8 | G4 N = 18 | SHH N = 13 | G4 N = 16 | SHH N = 1 | |
| Age, mean (SD) | 7.4 (3.8) | 9.6 (6.6) | 8.1 (4) | 3.4 (3.3) | 10.7 (3.45) | 8.9 | |
| Sex | Male | 12 (85.7) | 5 (62.5%) | 13 (72.2%) | 5 (38.4%) | 14 (87.5%) | |
| Female | 2 (14.3) | 3 (37.5%) | 5 (27.8%) | 8 (61.6%) | 2 (12.5%) | 1 (100%) | |
| Scan type | T1-FFE axial post-contrast | T1-FFE axial post-contrast | T1-FFE axial post-contrast | ||||
| MR acquisition type | 2D | 2D | 2D | ||||
| Scanning sequence | Gradient-recalled | Gradient-recalled | Spin-echo | ||||
| Sequence variant | Steady-state | Steady-state | Segmented k-space/Spoiled/Oversampling phase | ||||
| Pixel spacing (mm) | 0.46–1 | 0.46–1 | 2 | ||||
| Slice thickness (mm) | Mean = 5.4 mm | Mean = 5.4 mm | Mean = 5.4 mm | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.; Um, H.; Salloum, R.; Hollnagel, F.; Ahmed, R.; de Blank, P.; Tiwari, P. A Radiomic Approach for Evaluating Intra-Subgroup Heterogeneity in SHH and Group 4 Pediatric Medulloblastoma: A Preliminary Multi-Institutional Study. Cancers 2024, 16, 2248. https://doi.org/10.3390/cancers16122248
Ismail M, Um H, Salloum R, Hollnagel F, Ahmed R, de Blank P, Tiwari P. A Radiomic Approach for Evaluating Intra-Subgroup Heterogeneity in SHH and Group 4 Pediatric Medulloblastoma: A Preliminary Multi-Institutional Study. Cancers. 2024; 16(12):2248. https://doi.org/10.3390/cancers16122248
Chicago/Turabian StyleIsmail, Marwa, Hyemin Um, Ralph Salloum, Fauzia Hollnagel, Raheel Ahmed, Peter de Blank, and Pallavi Tiwari. 2024. "A Radiomic Approach for Evaluating Intra-Subgroup Heterogeneity in SHH and Group 4 Pediatric Medulloblastoma: A Preliminary Multi-Institutional Study" Cancers 16, no. 12: 2248. https://doi.org/10.3390/cancers16122248
APA StyleIsmail, M., Um, H., Salloum, R., Hollnagel, F., Ahmed, R., de Blank, P., & Tiwari, P. (2024). A Radiomic Approach for Evaluating Intra-Subgroup Heterogeneity in SHH and Group 4 Pediatric Medulloblastoma: A Preliminary Multi-Institutional Study. Cancers, 16(12), 2248. https://doi.org/10.3390/cancers16122248






