Predictive and Prognostic Factors in Melanoma Central Nervous System Metastases—A Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Independent Variables
2.2. Dependent Variables
2.3. Statistical Analysis
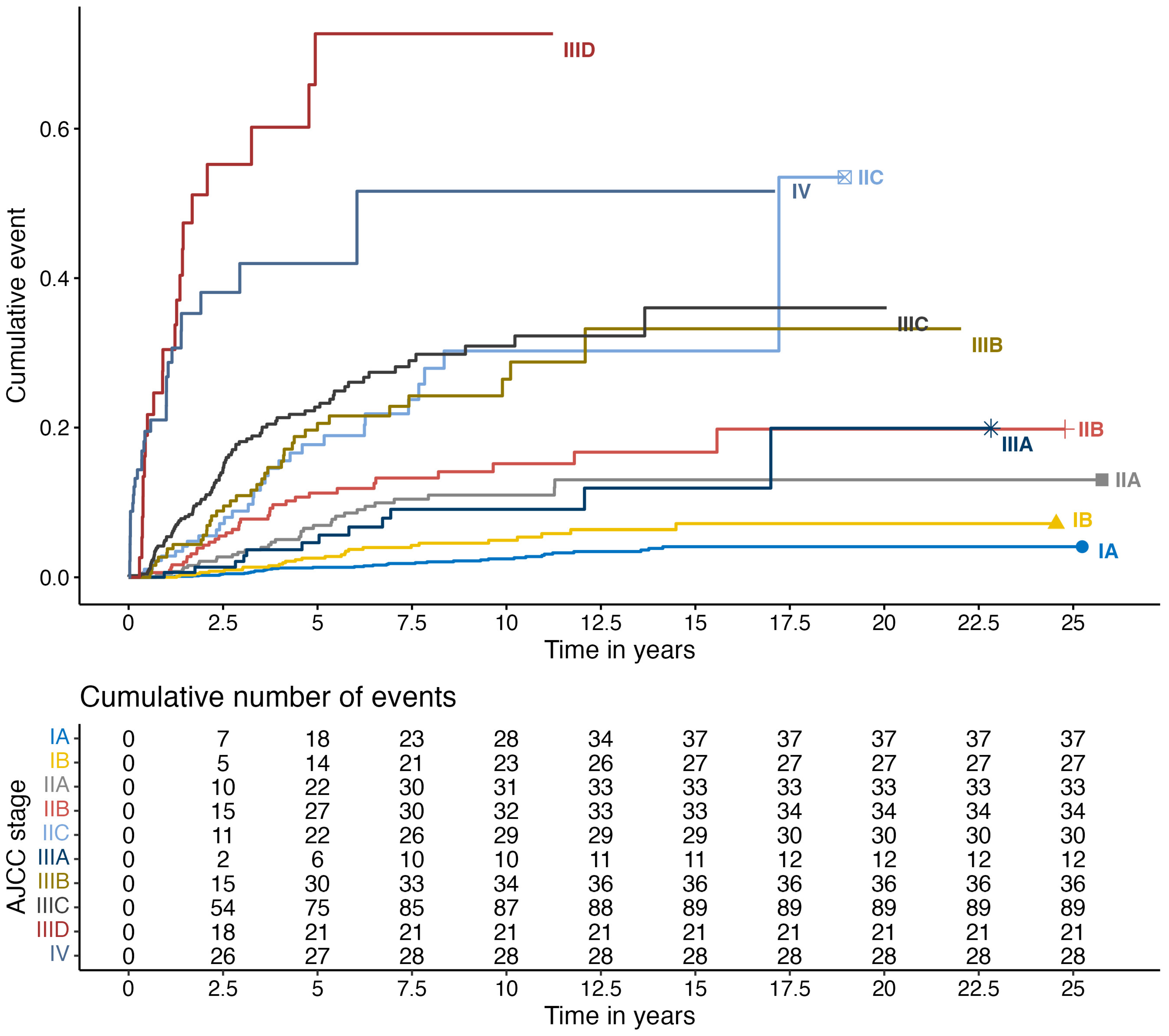
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef] [PubMed]
- Gumusay, O.; Coskun, U.; Akman, T.; Ekinci, A.S.; Kocar, M.; Erceleb, Ö.B.; Yazici, O.; Kaplan, M.A.; Berk, V.; Cetin, B.; et al. Predictive Factors for the Development of Brain Metastases in Patients with Malignant Melanoma: A Study by the Anatolian Society of Medical Oncology. J. Cancer Res. Clin. Oncol. 2014, 140, 151–157. [Google Scholar] [CrossRef]
- Taillibert, S. Le Rhun Epidemiology of Brain Metastases. Cancer/Radiother. 2015, 19, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.V.; Tawbi, H.; Margolin, K.A.; Amravadi, R.; Bosenberg, M.; Brastianos, P.K.; Chiang, V.L.; de Groot, J.; Glitza, I.C.; Herlyn, M.; et al. Melanoma Central Nervous System Metastases: Current Approaches, Challenges, and Opportunities. Pigment Cell Melanoma Res. 2016, 29, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Glitza, I.C.; Heimberger, A.B.; Sulman, E.P.; Davies, M.A. Prognostic Factors for Survival in Melanoma Patients with Brain Metastases. In Brain Metastases from Primary Tumors: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 3, pp. 267–297. ISBN 9780128035597. [Google Scholar]
- Diaz, M.J.; Mark, I.; Rodriguez, D.; Gelman, B.; Tran, J.T.; Kleinberg, G.; Levin, A.; Beneke, A.; Root, K.T.; Tran, A.X.V.; et al. Melanoma Brain Metastases: A Systematic Review of Opportunities for Earlier Detection, Diagnosis, and Treatment. Life 2023, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.J.; Hwu, P.; Bedikian, A. Prognostic Factors for Survival in Melanoma Patients with Brain Metastases. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Shang, D.; Yu, J.; Yuan, S. Incidence and Prognosis of Brain Metastases in Cutaneous Melanoma Patients: A Population-Based Study. Melanoma Res. 2019, 29, 77–84. [Google Scholar] [CrossRef]
- Nishino, M.; Dahlberg, S.E.; Fulton, L.E.; Digumarthy, S.R.; Hatabu, H.; Johnson, B.E.; Sequist, L.V. Volumetric Tumor Response and Progression in EGFR-Mutant NSCLC Patients Treated with Erlotinib or Gefitinib. Acad. Radiol. 2016, 23, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Fife, K.M.; Colman, M.H.; Stevens, G.N.; Firth, I.C.; Moon, D.; Shannon, K.F.; Harman, R.; Petersen-Schaefer, K.; Zacest, A.C.; Besser, M.; et al. Determinants of Outcome in Melanoma Patients with Cerebral Metastases. J. Clin. Oncol. 2004, 22, 1293–1300. [Google Scholar] [CrossRef]
- Staudt, M.; Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Bamberg, M.; Tatagiba, M.; Brossart, P.; Garbe, C. Determinants of Survival in Patients with Brain Metastases from Cutaneous Melanoma. Br. J. Cancer 2010, 102, 1213–1218. [Google Scholar] [CrossRef]
- Bordia, R.; Zhong, H.; Lee, J.; Weiss, S.; Han, S.W.; Osman, I.; Jain, R. Melanoma Brain Metastases: Correlation of Imaging Features with Genomic Markers and Patient Survival. J. Neurooncol. 2017, 131, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Frinton, E.; Tong, D.; Tan, J.; Read, G.; Kumar, V.; Kennedy, S.; Lim, C.; Board, R.E. Metastatic Melanoma: Prognostic Factors and Survival in Patients with Brain Metastases. J. Neurooncol. 2017, 135, 507–512. [Google Scholar] [CrossRef]
- Ostheimer, C.; Bormann, C.; Fiedler, E.; Marsch, W.; Vordermark, D. Malignant Melanoma Brain Metastases: Treatment Results and Prognostic Factors—A Single-Center Retrospective Study. Int. J. Oncol. 2015, 46, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Harary, M.; Zogg, C.K.; Ligon, K.L.; Reardon, D.A.; Hodi, F.S.; Aizer, A.A.; Smith, T.R. Improved Risk-Adjusted Survival for Melanoma Brain Metastases in the Era of Checkpoint Blockade Immunotherapies: Results from a National Cohort. Cancer Immunol. Res. 2018, 6, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients with Melanoma with Active Brain Metastases Treated with Pembrolizumab on a Phase II Trial. J. Clin. Oncol. 2018, 37, 52–60. [Google Scholar] [PubMed]
- Johannet, P.; Simons, M.; Qian, Y.; Azmy, N.; Mehnert, J.M.; Weber, J.S.; Zhong, J.; Osman, I. Risk and Tropism of Central Nervous System (CNS) Metastases in Patients with Stage II and III Cutaneous Melanoma. Cancer 2022, 128, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; Postow, M.A.; et al. Long-Term Outcomes of Patients with Active Melanoma Brain Metastases Treated with Combination Nivolumab plus Ipilimumab (CheckMate 204): Final Results of an Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Hwu, W.J.; Panageas, K.S.; Wilton, A.; Baldwin, D.E.; Bailey, E.; Von Althann, C.; Lamb, L.A.; Alvarado, G.; Bilsky, M.H.; et al. Brain and Leptomeningeal Metastases from Cutaneous Melanoma: Survival Outcomes Based on Clinical Features. Neuro Oncol. 2008, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Ma, M.W.; Fleming, N.H.; Lackaye, D.J.; Hernando, E.; Osman, I.; Shao, Y. Clinicopathological Characteristics at Primary Melanoma Diagnosis as Risk Factors for Brain Metastasis. Melanoma Res. 2013, 23, 461–467. [Google Scholar] [CrossRef][Green Version]
- Zakrzewski, J.; Geraghty, L.N.; Rose, A.E.; Christos, P.J.; Mazumdar, M.; Polsky, D.; Shapiro, R.; Berman, R.; Darvishian, F.; Hernando, E.; et al. Clinical Variables and Primary Tumor Characteristics Predictive of the Development of Melanoma Brain Metastases and Post-Brain Metastases Survival. Cancer 2011, 117, 1711–1720. [Google Scholar] [CrossRef]
- Gorka, E.; Fabó, D.; Gézsi, A.; Czirbesz, K.; Liszkay, G. Distance from Primary Tumor Is the Strongest Predictor for Early Onset of Brain Metastases in Melanoma. Anticancer Res. 2016, 36, 3065–3069. [Google Scholar] [PubMed]
- Frankel, T.L.; Bamboat, Z.M.; Ariyan, C.; Coit, D.; Sabel, M.S.; Brady, M.S. Predicting the Development of Brain Metastases in Patients with Local/Regional Melanoma. J. Surg. Oncol. 2014, 109, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Wei, C.; Detry, M.; Kim, K.B.; Papadopoulos, N.E.; Hwu, W.-J.; Homsi, J.; Davies, M.; McIntyre, S.; Hwu, P. Predictive Factors for the Development of Brain Metastasis in Advanced Unresectable Metastatic Melanoma. Am. J. Clin. Oncol. 2011, 34, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Carter, J.H., Jr.; Friedman, A.H.; Seigler, H.F. Demographics, Prognosis, and Therapy in 702 Patients with Brain Metastases from Malignant Melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-Mc Loughlin, C.; Sandoval-Clavijo, A.; Blanco de Tord, M.; Boada, A.; Alos, L.; García, A.; Carrera, C.; Malvehy, J.; Puig, S.; Toll, A.; et al. Prognostic Role of Microsatellites in Melanoma and Implications in the American Joint Committee on Cancer Classification System: A Cohort Study. J. Am. Acad. Dermatol. 2023, 88, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.J.; Ward, M.; Andtbacka, R.H.I.; Boucher, K.M.; Bowen, G.M.; Bowles, T.L.; Cohen, A.L.; Grossmann, K.; Hitchcock, Y.J.; Holmen, S.L.; et al. Risk Factors for Development of Melanoma Brain Metastasis and Disease Progression: A Single-Center Retrospective Analysis. Melanoma Res. 2017, 27, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gugger, A.; Barnhill, R.L.; Seifert, B.; Dehler, S.; Moch, H.; Lugassy, C.; Marques-Maggio, E.; Rushing, E.J.; Mihic-Probst, D. Cutaneous Melanoma with Brain Metastasis: Report of 193 Patients with New Observations. PLoS ONE 2016, 11, e0156115. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.E.; Burmeister, B.H.; Burmeister, E.A.; Foote, M.C.; Thomas, J.M.; Meakin, J.A.; Smithers, B.M. Melanoma Brain Metastases: The Impact of Nodal Disease. Clin. Exp. Metastasis 2014, 31, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, S.; Spagnolo, F.; Merlo, D.F.; Signori, A.; Acquati, M.; Pronzato, P.; Queirolo, P. The Treatment of Melanoma Brain Metastases before the Advent of Targeted Therapies: Associations between Therapeutic Choice, Clinical Symptoms and Outcome with Survival. Melanoma Res. 2014, 24, 61–67. [Google Scholar] [CrossRef]
- Podlipnik, S.; Potrony, M.; Puig, S. Genetic Markers for Characterization and Prediction of Prognosis of Melanoma Subtypes: A 2021 Update. Ital. J. Dermatol. Venereol. 2021, 156, 322–330. [Google Scholar] [CrossRef]
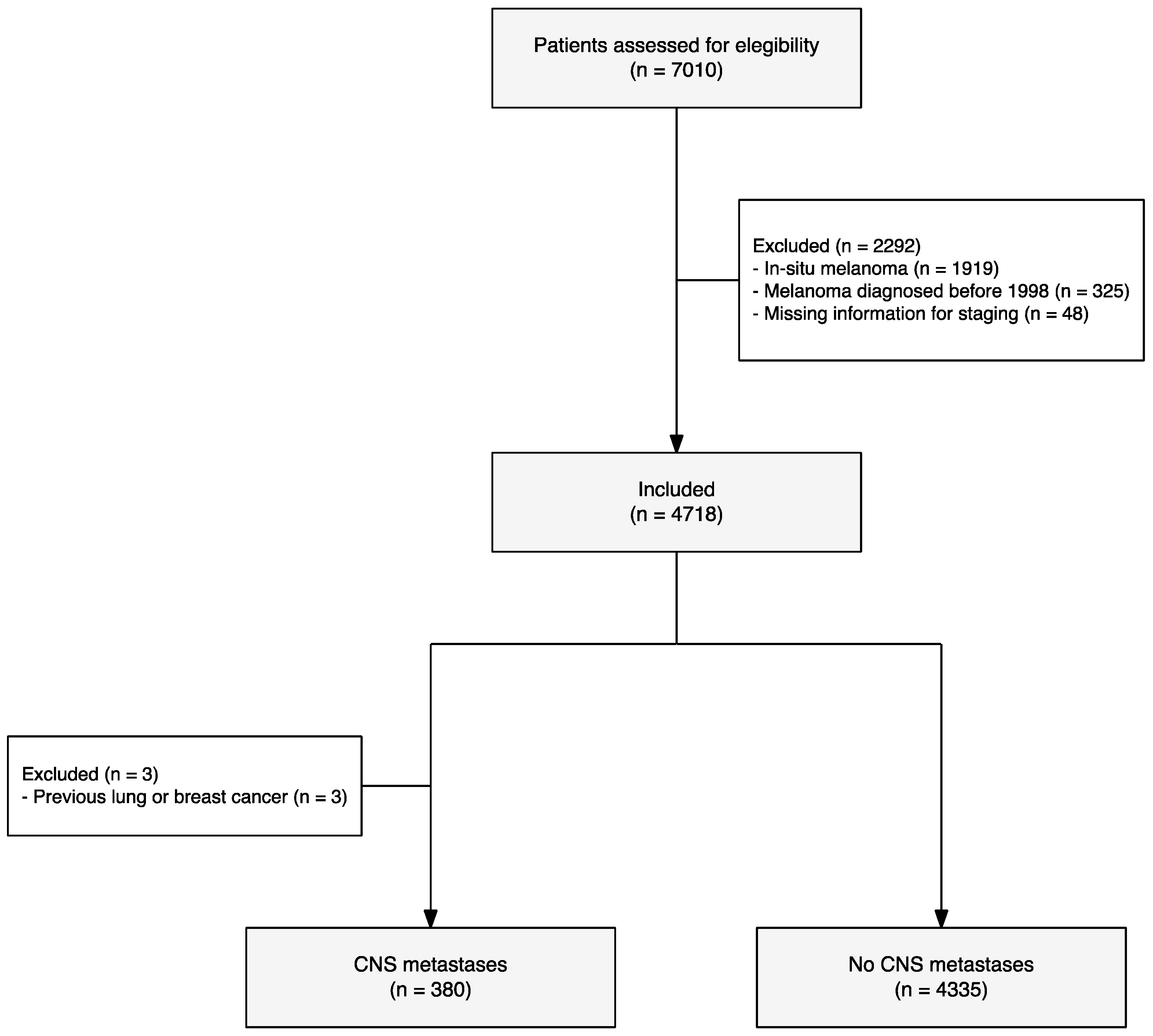
| Characteristic | Overall, N = 4715 | Stratified Groups | p-Value | q-Value | |
|---|---|---|---|---|---|
| No Brain Metastasis, N = 4335 | Brain Metastasis, N = 380 | ||||
| Gender, n (%) | <0.001 | <0.001 | |||
| Female | 2410 (51.1%) | 2249 (51.9%) | 161 (42.4%) | ||
| Male | 2305 (48.9%) | 2086 (48.1%) | 219 (57.6%) | ||
| Age, Median (IQR) | 56.8 (42.8–70.3) | 56.8 (42.9–70.6) | 56.6 (41.3–67.8) | 0.114 | 0.110 |
| Missing values | 9 | 9 | 0 | ||
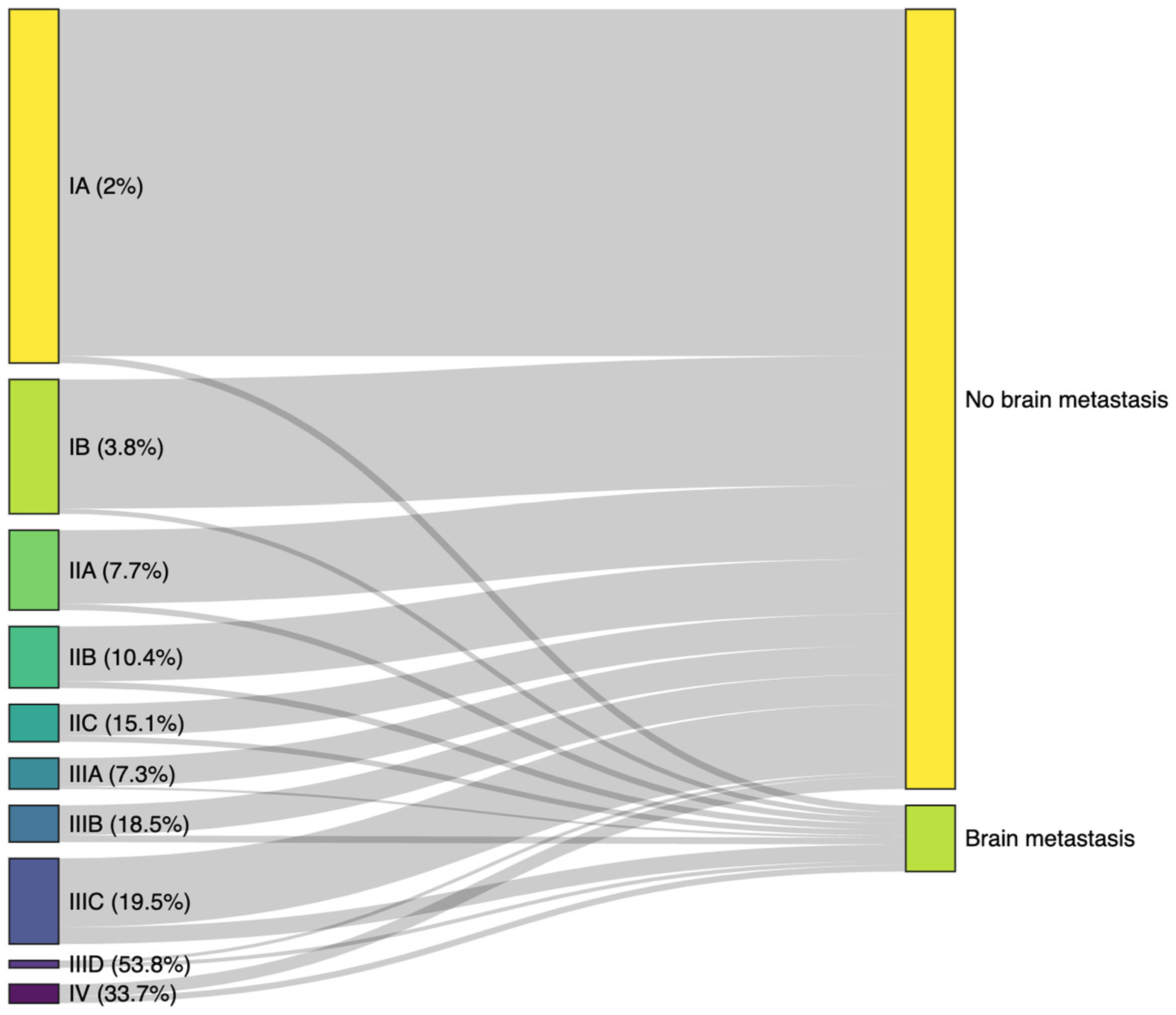
| 8th version AJCC, n (%) | - | - | |||
| IA | 1889 (41.8%) | 1852 (44.5%) | 37 (10.5%) | ||
| IB | 717 (15.9%) | 690 (16.6%) | 27 (7.6%) | ||
| IIA | 426 (9.4%) | 393 (9.4%) | 33 (9.3%) | ||
| IIB | 328 (7.3%) | 294 (7.1%) | 34 (9.6%) | ||
| IIC | 199 (4.4%) | 169 (4.1%) | 30 (8.5%) | ||
| IIIA | 165 (3.7%) | 153 (3.7%) | 12 (3.4%) | ||
| IIIB | 195 (4.3%) | 159 (3.8%) | 36 (10.2%) | ||
| IIIC | 457 (10.1%) | 368 (8.8%) | 89 (25.2%) | ||
| IIID | 39 (0.9%) | 18 (0.4%) | 21 (5.9%) | ||
| IV | 101 (2.2%) | 67 (1.6%) | 34 (9.6%) | ||
| Missing values | 199 | 172 | 27 | ||
| Melanoma of unknown primary, n (%) | 130 (2.8%) | 91 (2.1%) | 39 (10.3%) | <0.001 | <0.001 |
| Missing values | 9 | 9 | 0 | ||
| Breslow index (mm), Median (IQR) | 1.2 (0.7–2.7) | 1.1 (0.6–2.5) | 3.2 (1.5–6.0) | <0.001 | <0.001 |
| Missing values | 123 | 84 | 39 | ||
| Ulceration, n (%) | <0.001 | <0.001 | |||
| Absent | 3360 (75.7%) | 3220 (78.3%) | 140 (42.6%) | ||
| Present | 1081 (24.3%) | 892 (21.7%) | 189 (57.4%) | ||
| Missing values | 274 | 223 | 51 | ||
| Mitotic index mm2, n (%) | <0.001 | <0.001 | |||
| Zero Mitosis | 1738 (44.7%) | 1681 (46.7%) | 57 (20.1%) | ||
| One or more mitosis | 2146 (55.3%) | 1919 (53.3%) | 227 (79.9%) | ||
| Missing values | 831 | 735 | 96 | ||
| Histological subtype, n (%) | <0.001 | <0.001 | |||
| Superficial spreading | 2916 (65.5%) | 2773 (67.1%) | 143 (44.0%) | ||
| Lentiginous malignant | 289 (6.5%) | 273 (6.6%) | 16 (4.9%) | ||
| Nodular | 695 (15.6%) | 582 (14.1%) | 113 (34.8%) | ||
| Other | 299 (6.7%) | 279 (6.8%) | 20 (6.2%) | ||
| Acral lentiginous | 256 (5.7%) | 223 (5.4%) | 33 (10.2%) | ||
| Missing values | 260 | 205 | 55 | ||
| Satellitosis, n (%) | <0.001 | <0.001 | |||
| Absent | 4581 (97.2%) | 4234 (97.7%) | 347 (91.3%) | ||
| Present | 134 (2.8%) | 101 (2.3%) | 33 (8.7%) | ||
| Regression, n (%) | <0.001 | <0.001 | |||
| <50% | 966 (31.5%) | 915 (32.0%) | 51 (24.6%) | ||
| >50% | 369 (12.0%) | 361 (12.6%) | 8 (3.9%) | ||
| None | 1734 (56.5%) | 1586 (55.4%) | 148 (71.5%) | ||
| Missing values | 1646 | 1473 | 173 | ||
| Location, n (%) | - | - | |||
| Trunk | 2044 (44.9%) | 1867 (44.4%) | 177 (51.0%) | ||
| Head and neck | 644 (14.2%) | 579 (13.8%) | 65 (18.7%) | ||
| Lower limbs | 916 (20.1%) | 879 (20.9%) | 37 (10.7%) | ||
| Upper limbs | 546 (12.0%) | 522 (12.4%) | 24 (6.9%) | ||
| Acral | 333 (7.3%) | 297 (7.1%) | 36 (10.4%) | ||
| Mucosa | 64 (1.4%) | 56 (1.3%) | 8 (2.3%) | ||
| Other | 3 (0.1%) | 3 (0.1%) | 0 (0.0%) | ||
| Missing values | 165 | 132 | 33 | ||
| Characteristic | N | OR (95% CI) 1 | p-Value | q-Value 2 |
|---|---|---|---|---|
| Age | 4401 | <0.001 | <0.001 | |
| <47.5 | — | |||
| 47.5–66 | 0.78 (0.58 to 1.04) | |||
| >66 | 0.47 (0.35 to 0.64) | |||
| Gender | 4401 | 0.28 | 0.28 | |
| female | — | |||
| male | 1.14 (0.89 to 1.47) | |||
| T score | 4401 | <0.001 | <0.001 | |
| pT1 | — | |||
| pT2 | 2.77 (1.87 to 4.13) | |||
| pT3 | 3.67 (2.42 to 5.60) | |||
| pT4 | 6.19 (4.07 to 9.53) | |||
| Ulceration | 4401 | <0.001 | <0.001 | |
| absent | — | |||
| present | 2.41 (1.83 to 3.19) | |||
| Location | 4401 | <0.001 | <0.001 | |
| trunk | — | |||
| head and neck | 1.00 (0.72 to 1.39) | |||
| lower limbs | 0.45 (0.30 to 0.65) | |||
| upper limbs | 0.52 (0.32 to 0.82) | |||
| acral | 0.84 (0.55 to 1.26) | |||
| mucosa | 0.62 (0.24 to 1.37) | |||
| Satellitosis | 4401 | 0.007 | 0.009 | |
| absent | — | |||
| present | 1.90 (1.20 to 2.95) |
| Characteristic | N = 380 |
|---|---|
| Gender, n (%) | |
| Female | 161 (42.4%) |
| Male | 219 (57.6%) |
| Age at metastasis, Median (IQR) | 59.5 (46.5–70.6) |
| Brain metastasis location, n (%) | |
| Supratentorial | 249 (71.3%) |
| Multiple locations | 81 (23.2%) |
| Infratentorial | 14 (4.0%) |
| Leptomeninges Exclusively | 5 (1.4%) |
| Missing values | 31 |
| Leptomeningeal involvement, n (%) | |
| No leptomeningeal involvement | 326 (93.4%) |
| Leptomeningeal metastasis | 23 (6.6%) |
| Missing values | 31 |
| Brain hemorrage, n (%) | |
| Absent | 256 (72.3%) |
| Present | 98 (27.7%) |
| Missing values | 26 |
| Previous metastasis, n (%) | |
| Debut | 82 (22.5%) |
| Previous metastasis | 282 (77.5%) |
| Missing values | 16 |
| Number of other organs affected, n (%) | |
| None | 77 (21.4%) |
| 1 | 103 (28.7%) |
| 2–3 | 114 (31.8%) |
| >3 | 65 (18.1%) |
| Missing values | 21 |
| Symptoms, n (%) | |
| Asymptomatic | 169 (47.2%) |
| Symptomatic | 189 (52.8%) |
| Missing values | 22 |
| Diametre of largest brain metastasis, n (%) | |
| <10 mm | 103 (33.9%) |
| 10–25 mm | 113 (37.2%) |
| >25 mm | 88 (28.9%) |
| Missing values | 76 |
| Lactate dehydrogenase level (LDH), n (%) | |
| ≤ULN | 186 (61.6%) |
| >ULN | 116 (38.4%) |
| Missing values | 78 |
| Melanoma-Specific Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Values | N (%) | HR (Univariable) | HR (Multivariable) | HR (Univariable) | HR (Multivariable) |
| Sex | female | |||||
| male | 161 (42.8) | 1.00 (0.80–1.24, p = 0.978) | 0.96 (0.71–1.30, p = 0.782) | 1.00 (0.81–1.25, p = 0.967) | 0.96 (0.71–1.29, p = 0.773) | |
| Age | <47.5 | 215 (57.2) | ||||
| 47.5–66 | 130 (34.6) | 1.27 (0.98–1.64, p = 0.069) | 1.08 (0.76–1.55, p = 0.658) | 1.28 (0.99–1.65, p = 0.061) | 1.08 (0.75–1.54, p = 0.684) | |
| >66 | 135 (35.9) | 1.31 (0.99–1.72, p = 0.055) | 1.18 (0.81–1.72, p = 0.391) | 1.30 (0.99–1.71, p = 0.058) | 1.14 (0.78–1.66, p = 0.487) | |
| T score | pT1 | 111 (29.5) | ||||
| pT2 | 44 (13.1) | 0.80 (0.54–1.17, p = 0.249) | 1.01 (0.61–1.68, p = 0.967) | 0.79 (0.54–1.17, p = 0.246) | 1.00 (0.60–1.66, p = 0.993) | |
| pT3 | 75 (22.3) | 0.81 (0.55–1.17, p = 0.262) | 0.91 (0.55–1.51, p = 0.717) | 0.84 (0.58–1.21, p = 0.346) | 0.94 (0.57–1.56, p = 0.818) | |
| pT4 | 87 (25.8) | 0.82 (0.58–1.17, p = 0.276) | 0.93 (0.58–1.51, p = 0.778) | 0.84 (0.59–1.19, p = 0.317) | 0.93 (0.57–1.50, p = 0.751) | |
| Symptoms | Asymptomatic | 131 (38.9) | ||||
| Symptomatic | 169 (47.5) | 2.33 (1.86–2.92, p < 0.001) | 2.34 (1.67–3.29, p < 0.001) | 2.31 (1.85–2.90, p < 0.001) | 2.31 (1.65–3.25, p < 0.001) | |
| LDH levels | ≤ULN | 187 (52.5) | ||||
| >ULN | 186 (61.6) | 1.94 (1.51–2.48, p < 0.001) | 1.85 (1.32–2.58, p < 0.001) | 1.92 (1.50–2.46, p < 0.001) | 1.82 (1.30–2.54, p < 0.001) | |
| Brain hemorrhage | absent | 116 (38.4) | ||||
| present | 256 (72.7) | 1.44 (1.13–1.83, p = 0.003) | 1.25 (0.86–1.82, p = 0.242) | 1.42 (1.11–1.80, p = 0.005) | 1.25 (0.86–1.81, p = 0.249) | |
| Number of brain metastasis | 1 lesion | 96 (27.3) | ||||
| 2–5 lesions | 121 (35.3) | 2.07 (1.57–2.72, p < 0.001) | 1.53 (1.05–2.22, p = 0.025) | 2.09 (1.59–2.75, p < 0.001) | 1.55 (1.07–2.25, p = 0.020) | |
| >5 lesions | 125 (36.4) | 2.38 (1.77–3.20, p < 0.001) | 2.45 (1.65–3.65, p < 0.001) | 2.43 (1.81–3.26, p < 0.001) | 2.44 (1.64–3.63, p < 0.001) | |
| Diameter of largest brain metastasis | <10 mm | 97 (28.3) | ||||
| 10–25 mm | 103 (34.0) | 1.52 (1.14–2.04, p = 0.005) | 0.99 (0.69–1.43, p = 0.953) | 1.50 (1.13–2.01, p = 0.005) | 0.97 (0.67–1.39, p = 0.861) | |
| >25 mm | 112 (37.0) | 1.80 (1.32–2.45, p < 0.001) | 0.91 (0.59–1.42, p = 0.686) | 1.76 (1.30–2.39, p < 0.001) | 0.89 (0.57–1.39, p = 0.611) | |
| Location | Supratentorial | 88 (29.0) | ||||
| Other location | 248 (71.5) | 1.21 (0.94–1.55, p = 0.140) | 0.66 (0.44–0.99, p = 0.045) | 1.20 (0.94–1.54, p = 0.139) | 0.66 (0.44–0.98, p = 0.041) | |
| Leptomeningeal involvement | No leptomeningeal involvement | 99 (28.5) | ||||
| Leptomeningeal metastasis | 324 (93.4) | 1.94 (1.27–2.97, p = 0.002) | 1.97 (0.99–3.93, p = 0.054) | 1.92 (1.25–2.93, p = 0.003) | 1.90 (0.96–3.79, p = 0.067) | |
| Previous metastasis | Debut | 23 (6.6) | ||||
| Previous metastasis | 79 (21.9) | 1.60 (1.22–2.10, p = 0.001) | 1.87 (1.26–2.78, p = 0.002) | 1.63 (1.24–2.14, p < 0.001) | 1.92 (1.30–2.86, p = 0.001) | |
| Year of diagnosis | <2011 | 281 (78.1) | ||||
| ≥2011 | 114 (30.3) | 0.74 (0.59–0.93, p = 0.010) | 0.71 (0.52–0.98, p = 0.040) | 0.73 (0.58–0.92, p = 0.008) | 0.70 (0.51–0.97, p = 0.031) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, E.; Abarzua-Araya, Á.; Arance, A.; Martin-Huertas, R.; Aya, F.; Olondo, M.L.; Rizo-Potau, D.; Malvehy, J.; Puig, S.; Carrera, C.; et al. Predictive and Prognostic Factors in Melanoma Central Nervous System Metastases—A Cohort Study. Cancers 2024, 16, 2272. https://doi.org/10.3390/cancers16122272
Serra E, Abarzua-Araya Á, Arance A, Martin-Huertas R, Aya F, Olondo ML, Rizo-Potau D, Malvehy J, Puig S, Carrera C, et al. Predictive and Prognostic Factors in Melanoma Central Nervous System Metastases—A Cohort Study. Cancers. 2024; 16(12):2272. https://doi.org/10.3390/cancers16122272
Chicago/Turabian StyleSerra, Estefania, Álvaro Abarzua-Araya, Ana Arance, Roberto Martin-Huertas, Francisco Aya, María Lourdes Olondo, Daniel Rizo-Potau, Josep Malvehy, Susana Puig, Cristina Carrera, and et al. 2024. "Predictive and Prognostic Factors in Melanoma Central Nervous System Metastases—A Cohort Study" Cancers 16, no. 12: 2272. https://doi.org/10.3390/cancers16122272
APA StyleSerra, E., Abarzua-Araya, Á., Arance, A., Martin-Huertas, R., Aya, F., Olondo, M. L., Rizo-Potau, D., Malvehy, J., Puig, S., Carrera, C., & Podlipnik, S. (2024). Predictive and Prognostic Factors in Melanoma Central Nervous System Metastases—A Cohort Study. Cancers, 16(12), 2272. https://doi.org/10.3390/cancers16122272







