Comprehensive Insights into Chondroblastoma Metastasis: Metastatic Patterns and Therapeutic Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Chondroblastoma Metastasis
2.1. Chondroblastoma Metastasis: A Rare Occurrence
2.2. Molecular and Genetic Causes of Metastasis
3. Metastatic Chondroblastoma: Clinical Signs and Diagnostic Criteria
3.1. Patterns and Sites of Metastasis in Chondroblastoma
3.1.1. Bone Metastasis
3.1.2. Pulmonary Metastasis
3.1.3. Soft Tissue Metastasis
3.1.4. Liver and Regional Lymph Node Involvement in Chondroblastoma Metastasis
3.2. Diagnosing Metastatic Chondroblastoma
3.2.1. Histopathological Examination
3.2.2. Immunohistochemical Analysis
3.2.3. Radiographic Imaging
4. Treatment Approaches for Metastatic Chondroblastoma
4.1. Surgical Treatment
4.2. Palliative Care
4.2.1. Radiation Therapy
4.2.2. Systematic Treatment
4.3. Denosumab: A Novel Therapeutic Approach
5. Survival Outcome in Chondroblastoma Metastasis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selvarajah, S.; Zielenska, M.; Squire, J.A.; Park, P.C. Chapter 27—Genetic Aspects of Bone Tumors. In Bone Cancer, 2nd ed.; Heymann, D., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 305–318. [Google Scholar] [CrossRef]
- Suneja, R.; Grimer, R.J.; Belthur, M.; Jeys, L.; Carter, S.R.; Tillman, R.M.; Davies, A.M. Chondroblastoma of bone. J. Bone Jt. Surg. Br. Vol. 2005, 87, 974–978. [Google Scholar] [CrossRef]
- Akhtar, K.; Qadri, S.; Ray, P.S.; Sherwani, R.K. Cytological diagnosis of chondroblastoma: Diagnostic challenge for the cytopathologist. BMJ Case Rep. 2014, 2014, bcr2014204178. [Google Scholar] [CrossRef] [PubMed]
- de Pinieux, G.; Bouvier, C. Chapter 19—Recent Advances in the Biology of Bone Tumors and New Diagnostic Tools. In Bone Cancer; Heymann, D., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 225–234. [Google Scholar]
- Harish, K.; Janaki, M.G.; Alva, N.K. “Primary” aggressive chondroblastoma of the humerus: A case report. BMC Musculoskelet. Disord. 2004, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Fayad, L.M. Revisiting the WHO classification system of bone tumours: Emphasis on advanced magnetic resonance imaging sequences. Part 2. Pol. J. Radiol. 2020, 85, E396–E408. [Google Scholar] [CrossRef] [PubMed]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Araki, Y.; Alkhooly, A.Z.A.; Abd-Elfattah, A.S.; Fouly, E.H.; Elsaid, A.N.S.; Tsuchiya, H. Surgical treatment of chondroblastoma using extended intralesional curettage with phenol as a local adjuvant. J. Orthop. Surg. 2019, 27, 2309499019861031. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Yu, Q.; Liu, Y.; Yang, L. Chondroblastoma of the patella with pathological fracture in an adolescent: A case report. World J. Surg. Oncol. 2019, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Tafti, D.; Rawla, P. Chondroblastoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Brandolini, J.; Bertolaccini, L.; Pardolesi, A.; Salvi, M.; Valli, M.; Solli, P. Chondroblastoma of the rib in a 47-year-old man: A case report with a systematic review of literature. J. Thorac. Dis. 2017, 9, E907–E911. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, W.; Zhu, X.; Wang, J. Chondroblastoma in the long bone diaphysis: A report of two cases with literature review. Chin. J. Cancer 2012, 31, 257–264. [Google Scholar] [CrossRef]
- Chen, W.; DiFrancesco, L.M. Chondroblastoma: An Update. Arch. Pathol. Lab. Med. 2017, 141, 867–871. [Google Scholar] [CrossRef]
- Unni, K.K.; Inwards, C.Y. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Cohn, A.-M.; Costache, S.; Pop, D.M. Chondroblastoma of bone: Case presentation, immunohistochemistry findings and literature review. Oncolog-Hematolog 2020, 52, 22–29. [Google Scholar] [CrossRef]
- Marques, C. Chapter 19—Tumors of Bone. In Ortner’s Identification of Pathological Conditions in Human Skeletal Remains, 3rd ed.; Buikstra, J.E., Ed.; Academic Press: San Diego, CA, USA, 2019; pp. 639–717. [Google Scholar]
- Bertoni, F.; Unni, K.K.; Beabout, J.W.; Harner, S.G.; Dahlin, D.C. Chondroblastoma of the skull and facial bones. Am. J. Clin. Pathol. 1987, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, R.E.; Sim, F.H.; Krishnan, K. Giant cell tumor of the sacrum. Clin. Orthop. Relat. Res. 1993, 291, 215–221. [Google Scholar] [CrossRef]
- Deventer, N.; Gosheger, G.; de Vaal, M.; Budny, T.; Laufer, A.; Heitkoetter, B.; Luebben, T. Chondroblastoma: Is intralesional curettage with the use of adjuvants a sufficient way of therapy? J. Bone Oncol. 2020, 26, 100342. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Roessl, V.; Leithner, A. Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature. Cancers 2023, 15, 4258. [Google Scholar] [CrossRef] [PubMed]
- Özer, D.; Arıkan, Y.; Gür, V.; Gök, C.; Akman, Y.E. Chondroblastoma: An evaluation of the recurrences and functional outcomes following treatment. Acta Orthop. Traumatol. Turc. 2018, 52, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.K.; Stevenson, J.D.; Evans, S.; Abudu, A.; Sumathi, V.; Jeys, L.M.; Parry, M.C. Chondroblastoma in pelvis and extremities—A signle centre study of 177 cases. J. Bone Oncol. 2019, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.B.; Wood, F.M.; Ackerman, L.V. Malignant chondroblastoma. Report of two cases and review of the literature. Arch. Pathol. 1969, 88, 371–376. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2020; Volume 3. [Google Scholar]
- Green, P.; Whittaker, R. Benign chondroblastoma. Case report with pulmonary metastasis. J. Bone Jt. Surg. Am. 1975, 57, 418–420. [Google Scholar] [CrossRef]
- Huvos, A.G.; Higinbotham, N.L.; Marcove, R.C.; O’Leary, P. Aggressive chondroblastoma. Review of the literature on aggressive behavior and metastases with a report of one new case. Clin. Orthop. Relat. Res. 1977, 126, 266–272. [Google Scholar] [PubMed]
- Baumhoer, D.; Harder, D.; Ameline, B.; Dawson, H.; Kollar, A. Metastasizing chondroblastoma: A rare bone tumor no longer supported by the WHO classification. Skelet. Radiol. 2020, 50, 255–260. [Google Scholar] [CrossRef]
- van Horn, J.R.; Vincent, J.G.; Tilburg, A.M.W.-V.; Pruszczynski, M.; Slooff, T.J.J.H.; Molkenboer, J.F.W.M. Late pulmonary metastases from chondroblastoma of the distal femur. A case report. Acta Orthop. Scand. 1990, 61, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.L.; Johnson, M.E.; Truong, L.D.; Hicks, M.J.; Smith, F.E.; Spjut, H.J. Malignant chondroblastoma presenting as a recurrent pelvic tumor with DNA aneuploidy and p53 mutation as supportive evidence of malignancy. Skelet. Radiol. 1999, 28, 644–650. [Google Scholar]
- Elek, E.M.; Grimer, R.J.; Mangham, D.C.; Davies, A.M.; Carter, S.R.; Tillman, R.M. Malignant Chondroblastoma of the Os Calcis. Sarcoma 1998, 2, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.; Buchanan, R.; Golding, P.; Pringle, J. Chondroblastoma of the rib with widespread bone metastases. Histopathology 1994, 25, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Khalili, K.; White, L.M.; Kandel, R.A.; Wunder, J.S. Chondroblastoma with multiple distant soft tissue metastases. Skelet. Radiol. 1997, 26, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Riddell, R.J.; Louis, C.J.; Bromberger, N.A. Pulmonary metastases from chondroblastoma of the tibia. Report of a case. J. Bone Jt. Surg. Br. 1973, 55, 848–853. [Google Scholar] [CrossRef]
- Joshi, D.-D.; Anderson, P.M.; Matsumoto, J.; Moir, C.; Shives, T.; Unni, K.; Lennon, V.A. Metastatic chondroblastoma with elevated creatine kinase and paraneoplastic neurologic autoimmunity. J. Pediatr. Hematol. Oncol. 2003, 25, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Kyriakos, M.; Land, V.J.; Penning, H.L.; Parker, S.G. Metastatic chondroblastoma. Report of a fatal case with a review of the literature on atypical, aggressive, and malignant chondroblastoma. Cancer 1985, 55, 1770–1789. [Google Scholar] [CrossRef]
- Jambhekar, N.A.; Desai, P.B.; Chitale, D.A.; Patil, P.; Arya, S. Benign metastasizing chondroblastoma: A case report. Cancer 1998, 82, 675–678. [Google Scholar] [CrossRef]
- Sohn, S.H.; Koh, S.A.; Kim, D.G.; Park, S.W.; Lee, K.H.; Kim, M.K.; Choi, J.H.; Hyun, M.S. A case of spine origin chondroblastoma metastasis to lung. Cancer Res. Treat. 2009, 41, 241–244. [Google Scholar] [CrossRef]
- Focaccia, M.; Gambarotti, M.; Hakim, R.; Paioli, A.; Cesari, M.; Spazzoli, B.; Spinnato, P.; Donati, D.; Rocca, M.; Longhi, A. Chondroblastoma’s Lung Metastases Treated with Denosumab in Pediatric Patient. Cancer Res. Treat. 2021, 53, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Sultanpurkar, G.P.; Duttaluri, R.; Raorane, H.; Vikram, H. Malignant chondroblastoma of extraskeletal origin. Int. J. Appl. Basic Med. Res. 2016, 6, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Ivins, J.C. Benign chondroblastoma: A study of 125 cases. Cancer 1972, 30, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.V.; Kathuria, S. Recurrent and aggressive chondroblastoma of the pelvis with late malignant neoplastic changes. Am. J. Surg. Pathol. 1979, 3, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Oda, M.; Matsumoto, I.; Sawada-Kitamura, S.; Watanabe, G. Chondroblastoma with pulmonary metastasis in a patient presenting with spontaneous bilateral pneumothorax: Report of a case. Surg. Today 2011, 41, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Buhmann, S.; Mussack, T.; Müller-Höcker, J.; Schmitt-Sody, M.; Jansson, V.; Dürr, H.-R. Aggressive scapular chondroblastoma with secondary metastasis—A case report and review of literature. Eur. J. Med. Res. 2006, 11, 128–134. [Google Scholar] [PubMed]
- Binesh, F.; Moghadam, R.N.; Abrisham, J. A fatal case of pure metaphyseal chondroblastoma. BMJ Case Rep. 2013, 2013, bcr2013010315. [Google Scholar] [CrossRef] [PubMed]
- Kunze, E.; Graewe, T.; Peitsch, E. Histology and biology of metastatic chondroblastoma. Report of a case with a review of the literature. Pathol. Res. Pract. 1987, 182, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wirman, J.A.; Crissman, J.D.; Aron, B.F. Metastatic chondroblastoma. Report of an unusual case treated with radiotherapy. Cancer 1979, 44, 87–93. [Google Scholar] [CrossRef]
- Ozkoc, G.; Gonlusen, G.; Ozalay, M.; Kayaselcuk, F.; Pourbagher, A.; Tandogan, R.N. Giant chondroblastoma of the scapula with pulmonary metastases. Skelet. Radiol. 2006, 35, 42–48. [Google Scholar] [CrossRef]
- Wellmann, K.F. Chondroblastoma of the scapula. A case report with ultrastructural observations. Cancer 1969, 24, 408–416. [Google Scholar] [CrossRef]
- Sirsat, M.V.; Doctor, V.M. Benign chondroblastoma of bone. Report of a case of malignant transformation. J. Bone Jt. Surg. Br. 1970, 52, 741–745. [Google Scholar] [CrossRef]
- Lin, P.P.; Thenappan, A.; Deavers, M.T.; Lewis, V.O.; Yasko, A.W. Treatment and Prognosis of Chondroblastoma. Clin. Orthop. Relat. Res. 2005, 438, 103–109. [Google Scholar] [CrossRef]
- De Mattos, C.B.R.; Angsanuntsukh, C.; Arkader, A.; Dormans, J.P. Chondroblastoma and chondromyxoid fibroma. J. Am. Acad. Orthop. Surg. 2013, 21, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bloem, J.L.; Mulder, J.D. Chondroblastoma: A clinical and radiological study of 104 cases. Skelet. Radiol. 1985, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ningegowda, R.V.; Subramanian, K.; Suresh, I. Chondroblastoma of the talus. J. Foot Ankle Surg. 2013, 52, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Accadbled, F.; Brouchet, A.; Salmeron, F.; Darodes, P.; Cahuzac, J.P.; De Gauzy, J.S. Recurrent aggressive chondroblastoma: Two cases and a review of the literature. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2001, 87, 718–723. [Google Scholar] [PubMed]
- Narhari, P.; Haseeb, A.; Lee, S.; Singh, V.A. Spontaneous Conventional Osteosarcoma Transformation of a Chondroblastoma: A Case Report and Literature Review. Indian J. Orthop. 2018, 52, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Pansuriya, T.C.; van Eijk, R.; D’Adamo, P.; van Ruler, M.A.J.H.; Kuijjer, M.L.; Oosting, J.; Cleton-Jansen, A.-M.; van Oosterwijk, J.G.; Verbeke, S.L.J.; Meijer, D.; et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat. Genet. 2011, 43, 1256–1261. [Google Scholar] [CrossRef]
- Yin, L.; Cai, W.-J.; Liu, C.-X.; Chen, Y.-Z.; Hu, J.-M.; Jiang, J.-F.; Li, H.-A.; Cui, X.-B.; Chang, X.-Y.; Zhang, W.J.; et al. Analysis of PTEN methylation patterns in soft tissue sarcomas by MassARRAY spectrometry. PLoS ONE 2013, 8, e62971. [Google Scholar] [CrossRef]
- Venneker, S.; Szuhai, K.; Hogendoorn, P.C.W.; Bovée, J.V.M.G. Mutation-driven epigenetic alterations as a defining hallmark of central cartilaginous tumours, giant cell tumour of bone and chondroblastoma. Virchows Arch. 2019, 476, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Gan, H.; Lee, J.-H.; Han, J.; Wang, Z.; Riester, S.M.; Jin, L.; Chen, J.; Zhou, H.; Wang, J.; et al. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science 2016, 352, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Mermel, C.H.; Robinson, J.T.; Garraway, L.A.; Golub, T.R.; Meyerson, M.; Gabriel, S.B.; Lander, E.S.; Getz, G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014, 505, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jain, S.U.; Hoelper, D.; Bechet, D.; Molden, R.C.; Ran, L.; Murphy, D.; Venneti, S.; Hameed, M.; Pawel, B.R.; et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science 2016, 352, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Berisha, F.; Mozela, R.; Gibbons, R.; Guttridge, A.; O’Donnell, P.; Baumhoer, D.; Tirabosco, R.; Flanagan, A.M. The H3F3 K36M mutant antibody is a sensitive and specific marker for the diagnosis of chondroblastoma. Histopathology 2016, 69, 121–127. [Google Scholar] [CrossRef]
- Cleven, A.H.; Höcker, S.; Bruijn, I.B.-D.; Szuhai, K.; Cleton-Jansen, A.-M.; Bovée, J.V. Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. Am. J. Surg. Pathol. 2015, 39, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Patel, A.; Hughes, S.; Rehousek, P.; Drake, J.; Sumathi, V.; Botchu, R.; Davies, A.M. Bone metastases from chondroblastoma: A rare pattern of metastatic disease in an adult. Skelet. Radiol. 2023, 53, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.-M.; Unni, K.K.; Sim, F.H.; McLeod, R.A. Chondroblastoma of bone. Hum. Pathol. 1989, 20, 965–976. [Google Scholar] [CrossRef]
- Ramappa, A.J.; Lee, F.Y.; Tang, P.; Carlson, J.R.; Gebhardt, M.C.; Mankin, H.J. Chondroblastoma of bone. J. Bone Jt. Surg. Am. 2000, 82, 1140–1145. [Google Scholar] [CrossRef]
- Coleman, S.S. Benign Chondroblastoma with Recurrent Soft-Tissue and Intra-Articular Lesions: Report of a case. JBJS 1966, 48, 1554–1560. [Google Scholar] [CrossRef]
- Schajowicz, F.; Gallardo, H. Epiphysial chondroblastoma of bone. A clinico-pathological study of sixty-nine cases. J. Bone Jt. Surg. Br. 1970, 52, 205–226. [Google Scholar] [CrossRef]
- Seline, M.P.C.; Jaskierny, M.D.J. Cutaneous metastases from a chondroblastoma initially presenting as unilateral palmar hyperhidrosis. J. Am. Acad. Dermatol. 1999, 40, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Gulia, A.; Puri, A.; Jain, S.; Dhanda, S.; Gujral, S. Chondrosarcoma of the bone with nodal metastasis: The first case report with review of literature. Indian J. Med. Sci. 2011, 65, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kaim, A.H.; Hügli, R.; Bonél, H.M.; Jundt, G. Chondroblastoma and clear cell chondrosarcoma: Radiological and MRI characteristics with histopathological correlation. Skelet. Radiol. 2001, 31, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Din, N.-U.; Ahmed, A.; Pervez, S.; Ahmed, R.; Kayani, N. Chondroblastoma: A clinico-pathological analysis. J. Coll. Physicians Surg. Pak. 2014, 24, 898–901. [Google Scholar]
- de Silva, M.; Reid, R. Chondroblastoma: Varied histologic appearance, potential diagnostic pitfalls, and clinicopathologic features associated with local recurrence. Ann. Diagn. Pathol. 2003, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rekhi, B.; Ghate, S.; Shah, A.; Ramadwar, M.; Gulia, A. Immunohistochemical analysis of 36 cases of chondroblastomas: A single institutional experience. Ann. Diagn. Pathol. 2020, 44, 151440. [Google Scholar] [CrossRef] [PubMed]
- Amary, F.; Berisha, F.; Ye, H.; Gupta, M.; Gutteridge, A.; Baumhoer, D.; Gibbons, R.; Tirabosco, R.; O’donnell, P.; Flanagan, A.M. H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone. Am. J. Surg. Pathol. 2017, 41, 1059–1068. [Google Scholar] [CrossRef]
- Konishi, E.; Nakashima, Y.; Iwasa, Y.; Nakao, R.; Yanagisawa, A. Immunohistochemical analysis for Sox9 reveals the cartilaginous character of chondroblastoma and chondromyxoid fibroma of the bone. Hum. Pathol. 2010, 41, 208–213. [Google Scholar] [CrossRef]
- Rehkämper, J.; Steinestel, K.; Jeiler, B.; Elges, S.; Hekeler, E.; Huss, S.; Sperveslage, J.; Hardes, J.; Streitbürger, A.; Gosheger, G.; et al. Diagnostic tools in the differential diagnosis of giant cell-rich lesions of bone at biopsy. Oncotarget 2018, 9, 30106–30114. [Google Scholar] [CrossRef]
- Reid, L.B.; Wong, D.S.; Lyons, B. Chondroblastoma of the temporal bone: A case series, review, and suggested management strategy. Skull Base Rep. 2011, 1, 71–82. [Google Scholar] [CrossRef]
- Blancas, C.; Llauger, J.; Palmer, J.; Valverde, S.; Bagué, S. Imaging findings in chondroblastoma. Radiologia 2008, 50, 416–423. [Google Scholar] [CrossRef]
- Vinciguerra, A.; Verillaud, B.; Eliezer, M.; Kaci, R.; Kania, R.; Herman, P. Functional treatment of temporal bone chondroblastoma: Retrospective analysis of 3 cases. Eur. Arch. Otorhinolaryngol. 2021, 278, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guan, J.; Zhang, K.; Chen, H.; Wu, W.; Zhang, J. Rare chondroblastoma of the 6th left rib, video-assisted thoracoscopy resected: One case report and literature review. J. Cardiothorac. Surg. 2021, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Gulia, A.; Byregowda, S.; Panda, P.K. Palliative Care in Musculoskeletal Oncology. Indian J. Palliat. Care 2016, 22, 244–251. [Google Scholar] [CrossRef]
- Liu, J.; Ahmadpour, A.; Bewley, A.F.; Lechpammer, M.; Bobinski, M.; Shahlaie, K. Chondroblastoma of the Clivus: Case Report and Review. J. Neurol. Surg. Rep. 2015, 76, e258–e264. [Google Scholar] [CrossRef] [PubMed]
- Öçal, F.C.A.; Satar, B.; Çelik, E.; Bozlar, U.; Beyzadeoğlu, M. Promising Outcome of Radiation Therapy for Chondroblastoma of Temporal Bone in Childhood: A Case Report. Turk. Arch. Otorhinolaryngol. 2022, 60, 109–113. [Google Scholar] [CrossRef]
- Atalar, H.; Basarir, K.; Yildiz, Y.; Erekul, S.; Saglik, Y. Management of chondroblastoma: Retrospective review of 28 patients. J. Orthop. Sci. 2007, 12, 334–340. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, Y.; Dai, H.; Zhang, S.; Li, G.; Yu, B. Chondroblastoma of the Talus: A Case Report and Literature Review. J. Foot Ankle Surg. 2012, 51, 262–265. [Google Scholar] [CrossRef]
- Borkowska, A.M.; Szumera-Ciećkiewicz, A.; Szostakowski, B.; Pieńkowski, A.; Rutkowski, P.L. Denosumab in Giant Cell Tumor of Bone: Multidisciplinary Medical Management Based on Pathophysiological Mechanisms and Real-World Evidence. Cancers 2022, 14, 2290. [Google Scholar] [CrossRef]
- Xu, S.; Adams, B.; Yu, X.; Xu, M. Denosumab and Giant Cell Tumour of Bone—A Review and Future Management Considerations. Curr. Oncol. 2013, 20, 442–447. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Wessel, L.E.; Strike, S.A.; Singh, A.; Bernthal, N.M.; Athanasian, E.A. The Role of Denosumab in the Treatment of Primary Tumors of Bone. J. Hand Surg. 2023, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Samargandi, R.; Bernard, M.; Miquelestorena-Standley, E.; Le Nail, L.R. Efficacy of denosumab treatment for lung metastasis secondary to proximal humerus chondroblastoma. Saudi Med. J. 2024, 45, 633. [Google Scholar] [CrossRef]
- Cao, X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018, 6, 35. [Google Scholar] [CrossRef]
- Cheng, M.L.; Fong, L. Effects of RANKL-Targeted Therapy in Immunity and Cancer. Front. Oncol. 2014, 3, 329. [Google Scholar] [CrossRef]
- Won, K.Y.; Kalil, R.K.; Kim, Y.W.; Park, Y.-K. RANK signalling in bone lesions with osteoclast-like giant cells. Pathology 2011, 43, 318–321. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.Y.; Chow, L.T.; Zheng, M.H.; Kumta, S.M. Receptor activator of NF-kappaB ligand (RANKL) is expressed in chondroblastoma: Possible involvement in osteoclastic giant cell recruitment. Mol. Pathol. 2003, 56, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Suster, D.I.; Kurzawa, P.; Neyaz, A.; Jarzembowski, J.A.; Lozano-Calderon, S.; Raskin, K.; Schwab, J.; Choy, E.; Chebib, I.; Deshpande, V. Chondroblastoma Expresses RANKL by RNA In Situ Hybridization and May Respond to Denosumab Therapy. Am. J. Surg. Pathol. 2020, 44, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Calvert, N.; Wood, D. Use of denosumab in recurrent chondroblastoma of the squamous temporal bone: A case report. Clin. Case Rep. 2017, 5, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Visgauss, J.D.; Lazarides, A.; Dickson, B.; Cardona, D.; Sheth, M.; DeWitt, S.B.; Somarelli, J.A.; Eward, W.C. Treatment of Chondroblastoma with Denosumab: A Case Report with a Correlative Analysis of Effect on the RANK Signaling Pathway. JBJS Case Connect 2021, 11, e20.00178. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, Z.; Witwicki, T. Chondroblastoma malignum primarium. Patol. Pol. 1965, 16, 181–189. [Google Scholar]
- Sweetnam, R.; Ross, K. Surgical treatment of pulmonary metastases from primary tumours of bone. J. Bone Jt. Surg. Br. 1967, 49, 74–79. [Google Scholar] [CrossRef]

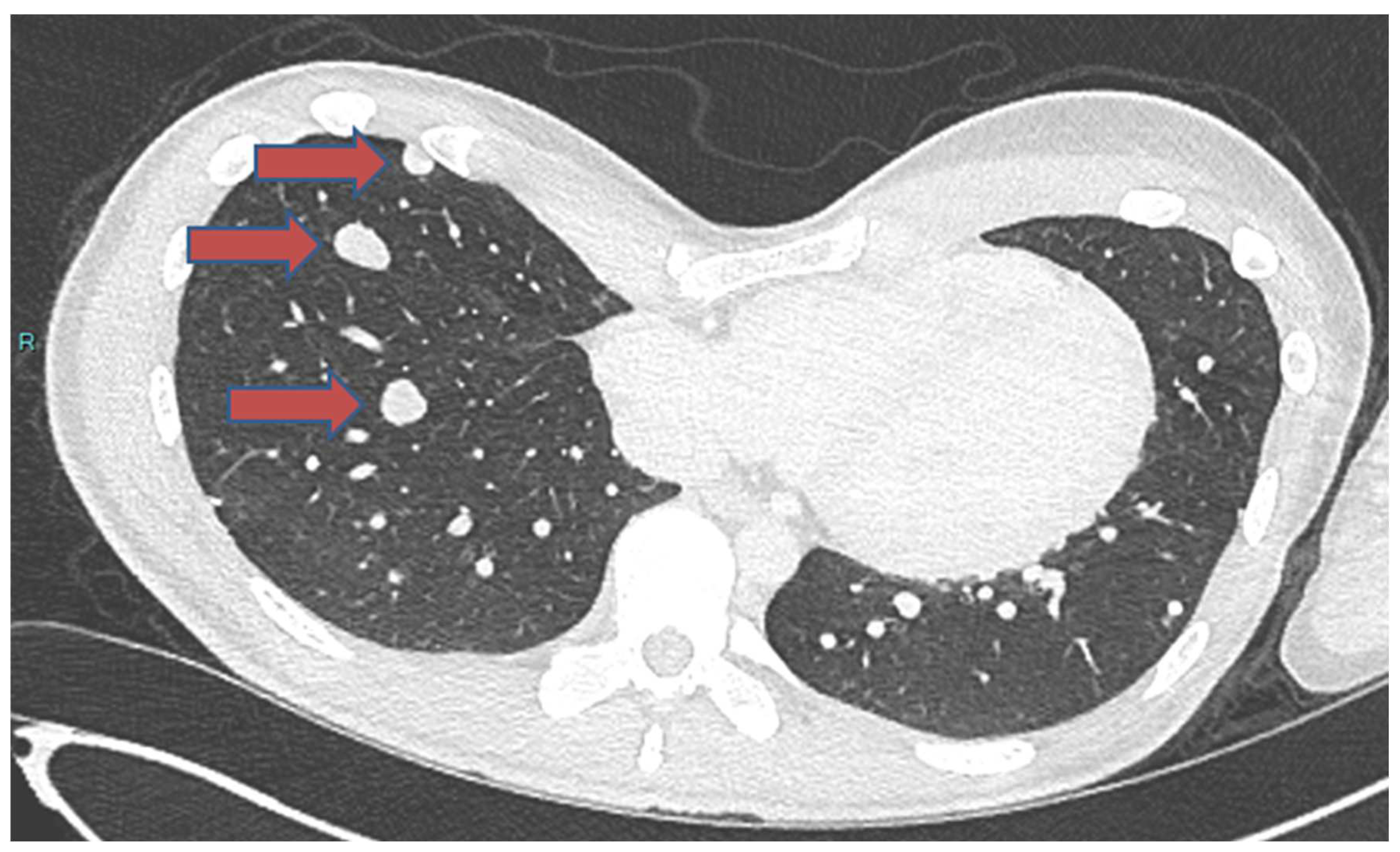
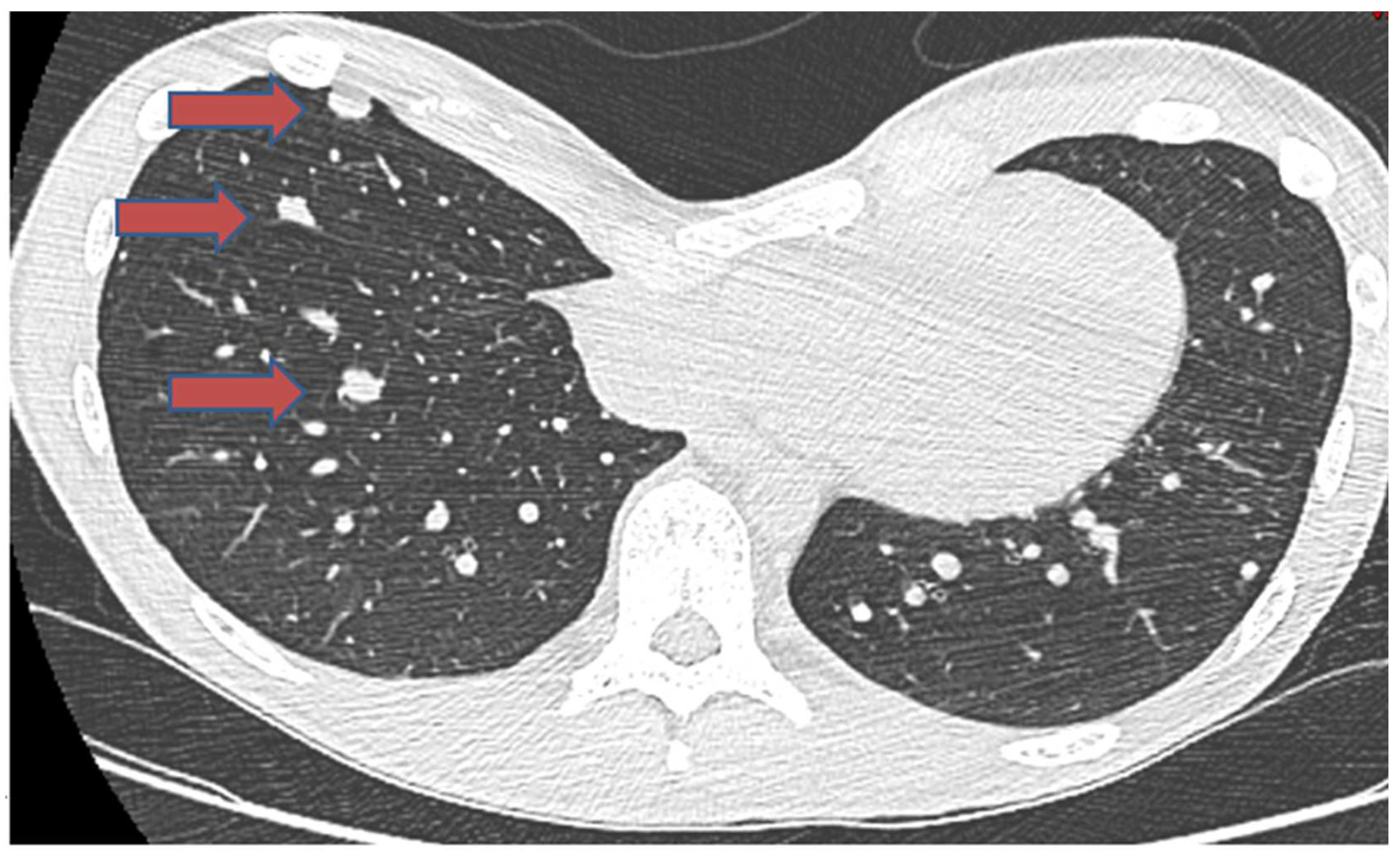
| Author | Age/Sex | Primary Location | Site of Metastasis | Metastasis Occurrence | Histology of Bone | Interval to Metastasis | Follow-up after Metastasis | Survival Status | Treatment of Metastasis |
|---|---|---|---|---|---|---|---|---|---|
| Dahlin D.C. [39] | - | Pelvis | Lung | Late metastasis without local recurrence | Benign | 58 months | - | Deceased | - |
| Gawlik Z. [101] | 6/F | Prox. Humerus | Lung | Late metastasis without local recurrence | Benign | 3 months | Deceased | Metastasectomy | |
| Green P. [24] | 13/F | Prox. femur | Lung | Occurred at recurrence | NR | 20 months | 10 years | Alive at final follow-up | Thoracotomy |
| Huvos A.G. [25] | 16/F | Dist. femur | Lung | Occurred at recurrence | Benign | 14 months | 5 years | Alive at final follow-up | Metastasectomy |
| Jambhekar N.A. [35] | 27/F | Talus | Lung | Occurred at initial diagnosis | Benign | 4 years | Alive at final follow-up | Metastasectomy (3 surgical resection) | |
| Joshi D.D [33] | 17/F | Fibula | Lung | Occurred at initial diagnosis | Benign | 3 years | Alive at final follow-up | Thoracotomy | |
| Kahn L.B. [22] | 13/M | Pelvis | Lung, liver, diaphragm, subcutaneous | Occurred at recurrence | Malignant | 9 years | 6 years | Deceased 6 years later | No treatment |
| Khalili K. [31] | 43/M | Rib | Scalp, soft tissue of neck area, forearm soft tissue, buttock | Late metastasis without local recurrence | Benign | 5 years | 8 years | Alive at final follow-up | Resection |
| Kunze E. [44] | 33/M | Pelvis | Lung | Late metastasis without local recurrence | Benign | 12 years | 6 years | Alive at final follow-up | Exploratory thoracotomy but not all nodules were removed |
| Kyriakos M. [34] | 9/M | Prox. tibia | Lung | Occurred at the initial diagnosis | Benign | 4 years and 9 months | Deceased 5 years after diagnosis | Exploratory thoracotomy, vincristine, cyclophosphamide, and doxorubicin for progression | |
| Riddell R.J. [32] | 14/F | Prox. tibia | Lung | Occurred at recurrence | Benign | 34 months | 9 years | Alive at final follow-up | Excision of 1 nodule |
| Schajowicz F. [69] | 32/F | Dist. metatarsus | Subcutaneous metastasis of the gluteal region, thigh, neck | Occurred at recurrence | Malignant | 5 months | Deceased 6 years after 1st diagnosis | No treatment | |
| Sirsat M.V. [48] | 15/M | Rt. Prox. tibia | Liver | Occurred at recurrence | Benign | 2 years | Deceased 9 years after 1st diagnosis and shorty after metastasis | - | |
| Sweetnam R. [102] | 19/M | Prox. fibula | Lung | Late metastasis without local recurrence | Malignant | 36 months | 5.8 years | Alive at final follow-up | Lobectomy |
| Van Horn J.R. [27] | 38/M | Dist. femur | Lung | Late metastasis without local recurrence | Benign | 2 years | 1.6 years | Alive at final follow-up | Metastasectomy |
| Wellmann K. [47] | 29/M | Scapula | Lung | Late metastasis without local recurrence | Benign | 14 years | 1 year | Alive at final follow-up | Lobectomy |
| Wirmann J.A. [45] | 38/M | Rt. acromion | Lung | Late metastasis without local recurrence | Benign | 34 years. | 1 year | Deceased 9 months after the diagnosis of metastasis | Refusal of treatment |
| Ozkoc G. [46] | 53/M | Scapula | Lung | Late metastasis without local recurrence | Benign | 12 years | 3 years | Deceased | Palliative treatment |
| Baumhoer D. [26] | 54/M | Acromion | Lung, T5, soft tissue (muscles), 3rd rib, craniovertevral junction | Late metastasis without local recurrence | Benign | 9 years | 3 years | Alive at final follow-up | Debulking surgery and radiation for bone, doxorubicin, pembrolizumab |
| Binesh F. [43] | 9/M | Distal tibia | Lung | Late metastasis without local recurrence | Benign | 2 months | Few weeks | Deceased 2 months later after surgery | Chemotherapy with doxorubicin, vincristine and cyclophosphamide |
| Sohn SH. [36] | 21/M | L4 spine | Lung | Occurred at initial diagnosis | Benign | 3 years | Alive at final follow-up | Pulmonary wedge resection | |
| Tamura M. [41] | 21/M | Ischium | Lung | Late metastasis without local recurrence | Benign | 20 months | - | Alive at final follow-up | Partial metastasectomy (debulking) |
| Ostrowski M.L. [28] | 28/M | Pelvis | Lung | Late metastasis without local recurrence | Malignant | 18 years | 9 months | Deceased 8 months later | Chemotherapy |
| Reyes [40] | 32/M | Pelvis | Lung, scalp, nose | Late metastasis without local recurrence | Malignant | 32 years | 6 months | Alive at final follow-up | Excision of metastasis of nose, scalp |
| Emil M. Elek. [29] | 12/M | Calcaneum | Lung, tibia soft tissue of calf | Late metastasis without local recurrence | Benign | 3 months | 3 years | Alive at final follow-up | Chemotherapy, metastasectomy, radiotherapy of lung |
| Focaccia. M. [37] | 16/M | Proximal humerus | Lung | Occurred at initial diagnosis | Benign | 2 years | Alive at final follow-up | Denosumab treatment | |
| Duttaluri, R. [38] | 61/F | Extraskeletal soft tissue posterolateral aspect of the left knee | Lung | Occurred at initial diagnosis | Malignant | - | Alive at time of publication | Palliative radiotherapy | |
| Birch PJ. [30] | 37/F | rib | Skull bone, acetabulum, scapula | Occurred at recurrence | Benign | 23 years | 3 years | Alive at final follow-up | Radiotherapy |
| Samargandi. [93] | 19/M | Proximal humerus | Lung, shoulder metastasis to lung | Late metastasis without local recurrence | Benign | 1 year 3 months | 2 years | Alive at last follow-up | Partial metastasectomy and denosumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samargandi, R.; Bafail, A.; Le Nail, L.-R.; Berhouet, J. Comprehensive Insights into Chondroblastoma Metastasis: Metastatic Patterns and Therapeutic Approaches. Cancers 2024, 16, 2283. https://doi.org/10.3390/cancers16122283
Samargandi R, Bafail A, Le Nail L-R, Berhouet J. Comprehensive Insights into Chondroblastoma Metastasis: Metastatic Patterns and Therapeutic Approaches. Cancers. 2024; 16(12):2283. https://doi.org/10.3390/cancers16122283
Chicago/Turabian StyleSamargandi, Ramy, Abrar Bafail, Louis-Romée Le Nail, and Julien Berhouet. 2024. "Comprehensive Insights into Chondroblastoma Metastasis: Metastatic Patterns and Therapeutic Approaches" Cancers 16, no. 12: 2283. https://doi.org/10.3390/cancers16122283
APA StyleSamargandi, R., Bafail, A., Le Nail, L.-R., & Berhouet, J. (2024). Comprehensive Insights into Chondroblastoma Metastasis: Metastatic Patterns and Therapeutic Approaches. Cancers, 16(12), 2283. https://doi.org/10.3390/cancers16122283






