Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Features
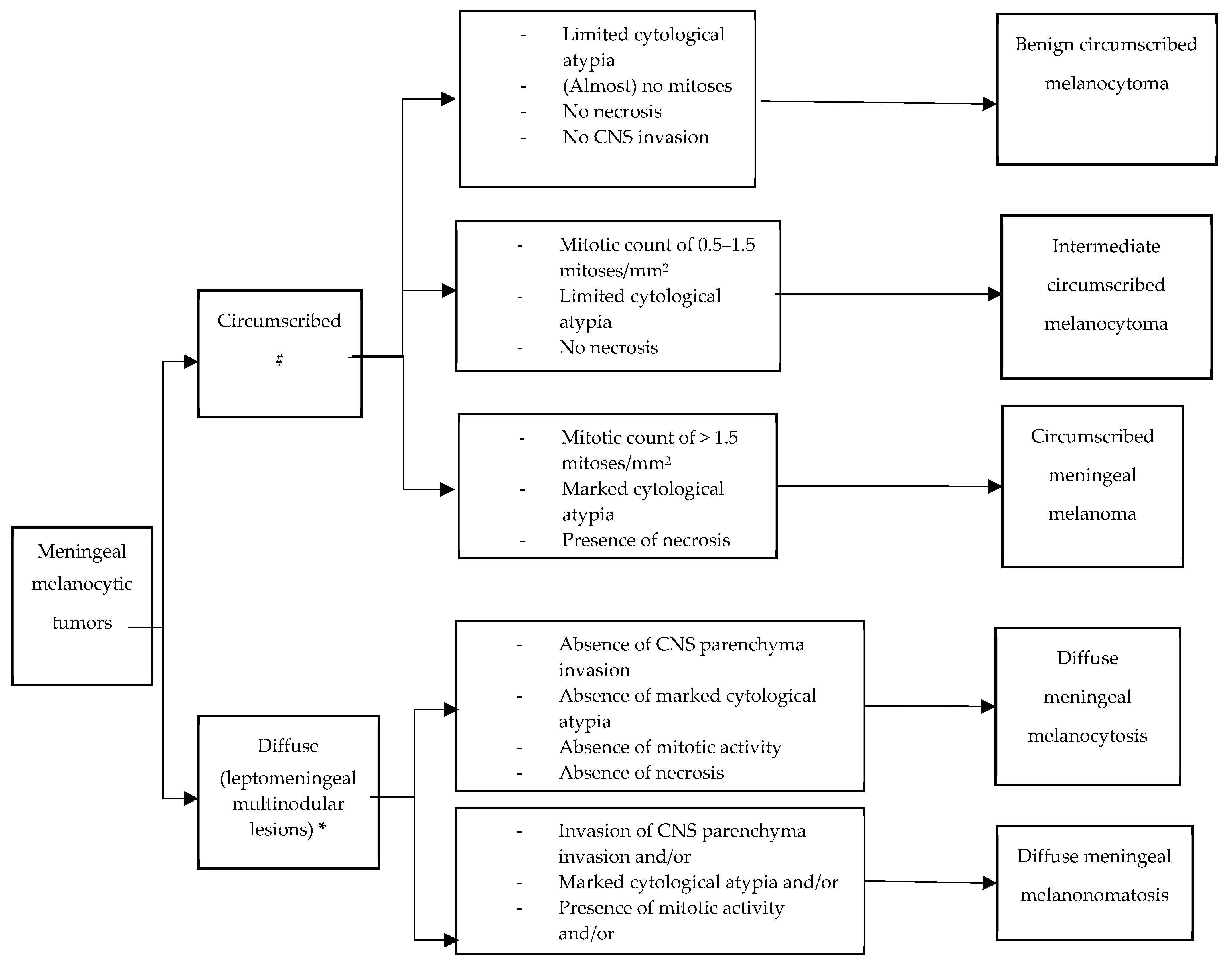
3. Pathology and Molecular Markers
3.1. Circumscribed Meningeal Melanocytic Neoplasms
3.2. Diffuse Meningeal Melanocytic Neoplasms
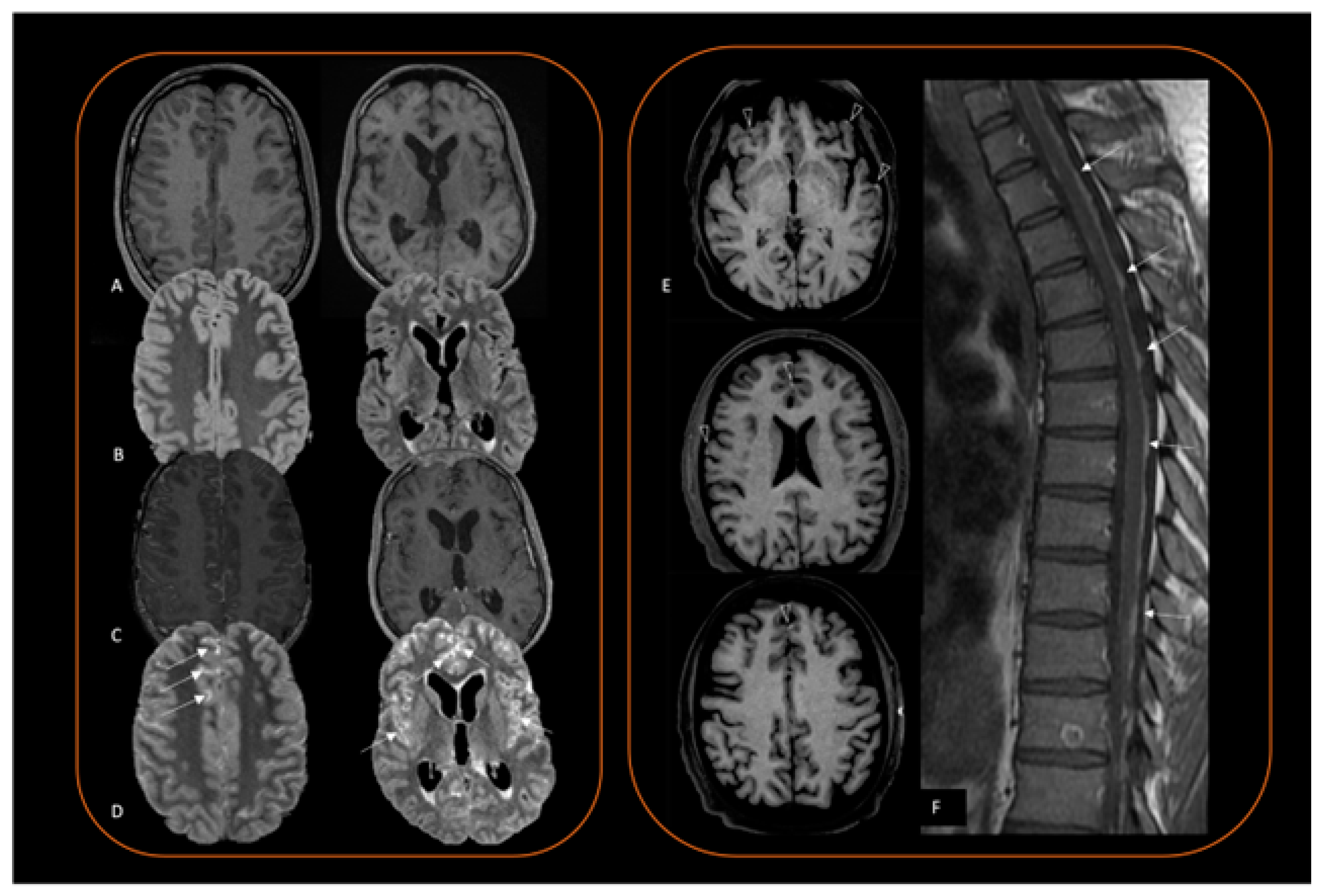
4. Neuroimaging Diagnosis
4.1. Computed Tomography (CT)
4.2. Magnetic Resonance Imaging (MRI)
4.3. Positron Emission Tomography (PET)
5. Treatment Options
5.1. Surgery
5.2. Radiotherapy
5.3. Medical Treatment
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAP1 | BRCA1-associated protein-1 |
| BRAF | V-raf murine sarcoma viral oncogene homolog B |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CTLA4 | Cytotoxic T-lymphocyte associated protein 4 |
| CYSLTR2 | Cysteinyl leukotriene receptor 2 |
| EIF1AX | Eukaryotic translation initiation factor 1A X-linked |
| EURACAN | European Network for Rare Cancers |
| FLAIR | Fluid-attenuated inversion recovery |
| GNAQ | G protein subunit alpha q |
| GNA11 | G protein subunit alpha 11 |
| Gy | Grey |
| HPF | High-power field |
| HRAS | Harvey rat sarcoma virus |
| KIT | KIT proto-oncogene |
| KRAS | Kirsten rat sarcoma virus |
| MEK | Mitogen-activated protein kinase 1 |
| MRI | Magnetic resonance imaging |
| PD-1 | Programmed cell death 1 |
| PLCB4 | Phospholipase C Beta 4 |
| SF3B1 | Splicing factor 3b subunit 1 |
| TERT | Telomerase reverse transcriptase |
| WHO | World Health Organization |
| 18F-FDG PET-CT | 18F-fluorodeoxyglucose positron emission tomography-computed tomography |
| PRKAR1A | Protein kinase cAMP-dependent type I regulatory subunit alpha |
| S100 | Calcium binding protein |
| SOX10 | SRY-box transcription factor 10 |
| CT | Computed tomograph |
References
- Grosshans, H.K.; Huttner, A.J.; Piepmeier, J.M.; Kaulen, L.D.; Fulbright, R.K.; Baehring, J.M.; Karschnia, P. Primary melanotic tumors of the nervous system: A consecutive case series. Eur. J. Neurol. 2020, 27, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Liubinas, S.V.; Maartens, N.; Drummond, K.J. Primary melanocytic neoplasms of the central nervous system. J. Clin. Neurosci. 2010, 17, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Küsters-Vandevelde, H.V.; Willemsen, A.E.; Groenen, P.J.; Küsters, B.; Lammens, M.; Wesseling, P.; Djafarihamedani, M.; Rijntjes, J.; Delye, H.; Willemsen, M.A.; et al. Experimental treatment of NRAS-mutated neurocutaneous melanocytosis with MEK162, a MEK-inhibitor. Acta Neuropathol. Commun. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sarnat, L. Neurocutaneous melanocytosis. Handb. Clin. Neurol. 2013, 111, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Gessi, M.; Wesseling, P.; Bastian, B.C.; Reyes-Múgica, M.; Kölsche, C.; Küsters-Vandevelde, H.V.; Bielle, F. Skin Tumours; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2023. [Google Scholar]
- DeDavid, M.; Orlow, S.J.; Provost, N.; Marghoob, A.A.; Rao, B.K.; Wasti, Q.; Huang, C.L.; Kopf, A.W.; Bart, R.S. Neurocutaneous melanosis: Clinical features of large congenital melanocytic nevi in patients with manifest central nervous system melanosis. J. Am. Acad. Dermatol. 1996, 35, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.D.; Williams, M.L.; Barkovich, A.J.; Hoffman, W.Y.; Mathes, S.J.; Frieden, I.J. Giant congenital melanocytic nevi: The significance of neurocutaneous melanosis in neurologically asymptomatic children. Plast. Reconstr. Surg. 2001, 107, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, F.; Kulkarni, A.; Chau, C.; Nielsen, M.; Sheaff, M.; Steele, J.; van Doorn, R.; Wadt, K.; Hamill, M.; Torr, B.; et al. Clinical practice guidelines for the diagnosis and surveillance of BAP1 tumour predisposition syndrome. Eur. J. Hum. Genet. 2023, 31, 1261–1269. [Google Scholar] [CrossRef]
- Küsters-Vandevelde, H.V.; Klaasen, A.; Küsters, B.; Groenen, P.J.; van Engen-van Grunsven, I.A.; van Dijk, M.R.; Reifenberger, G.; Wesseling, P.; Blokx, W.A. Activating mutations of the GNAQ gene: A frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010, 119, 317–323. [Google Scholar] [CrossRef]
- van de Nes, J.A.P.; Koelsche, C.; Gessi, M.; Möller, I.; Sucker, A.; Scolyer, R.A.; Buckland, M.E.; Pietsch, T.; Murali, R.; Schadendorf, D.; et al. Activating CYSLTR2 and PLCB4 Mutations in Primary Leptomeningeal Melanocytic Tumors. J. Invest. Dermatol. 2017, 137, 2033–2035. [Google Scholar] [CrossRef]
- Man, W.; Wang, G. Incidence, Outcomes and Predictors of Primary Central Nervous System Melanoma: A SEER-Based Study. World Neurosurg. 2019, 129, e782–e790. [Google Scholar] [CrossRef]
- Neuhold, J.C.; Friesenhahn, J.; Gerdes, N.; Krengel, S. Case reports of fatal or metastasizing melanoma in children and adolescents: A systematic analysis of the literature. Pediatr. Dermatol. 2015, 32, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gebarski, S.S.; Blaivas, M.A. Imaging of normal leptomeningeal melanin. AJNR Am. J. Neuroradiol. 1996, 17, 55–60. [Google Scholar] [PubMed] [PubMed Central]
- Kuo, K.L.; Lin, C.L.; Wu, C.H.; Chang, C.H.; Tsai, H.P.; Loh, J.K.; Lieu, A.S.; Su, Y.F. Meningeal Melanocytoma Associated with Nevus of Ota: Analysis of Twelve Reported Cases. World Neurosurg. 2019, 127, e311–e320. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Giannini, C.; Scheithauer, B.W.; Burger, P.C. Primary melanocytic neoplasms of the central nervous systems. Am. J. Surg. Pathol. 1999, 23, 745–754. [Google Scholar] [CrossRef]
- Knappe, U.J.; Tischoff, I.; Tannapfel, A.; Reinbold, W.D.; Möller, I.; Sucker, A.; Schadendorf, D.; Griewank, K.G.; van de Nes, J.A.P. Intraventricular melanocytoma diagnosis confirmed by gene mutation profile. Neuropathology 2018, 38, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Küsters-Vandevelde, H.V.; Küsters, B.; van Engen-van Grunsven, A.C.; Groenen, P.J.; Wesseling, P.; Blokx, W.A. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol. 2015, 25, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Küsters-Vandevelde, H.V.; Kruse, V.; Van Maerken, T.; Boterberg, T.; Pfundt, R.; Creytens, D.; Van den Broecke, C.; Machielsen, T.C.; Koelsche, C.; von Deimling, A.; et al. Copy number variation analysis and methylome profiling of a GNAQ-mutant primary meningeal melanocytic tumor and its liver metastasis. Exp. Mol. Pathol. 2017, 102, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fang, J.; Li, G.; Jia, W.; Liu, H.; Qi, W.; Xu, Y. Spinal meningeal melanocytomas: Clinical manifestations, radiological and pathological characteristics, and surgical outcomes. J. Neurooncol. 2016, 127, 279–286. [Google Scholar] [CrossRef]
- Kinsler, V.A.; O’Hare, P.; Jacques, T.; Hargrave, D.; Slater, O. MEK inhibition appears to improve symptom control in primary NRAS-driven CNS melanoma in children. Br. J. Cancer. 2017, 116, 990–993. [Google Scholar] [CrossRef]
- Cajaiba, M.M.; Benjamin, D.; Halaban, R.; Reyes-Múgica, M. Metastatic peritoneal neurocutaneous melanocytosis. Am. J. Surg. Pathol. 2008, 32, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Gessi, M.; Bastian, B.C.; Kölsche, C.; Küsters-Vandevelde, H.V. Chapter 9—Circumscribed meningeal melanocytic neoplasms: Melanocytoma and melanoma. In WHO Classification of Tumours, 5th ed.; Central Nervous system Tumours; International Agency for Research on Cancer: Lyon, France, 2021; pp. 344–348. [Google Scholar]
- Chaharyn, B.; Yip, S.; Maguire, J.A. Amelanotic melanocytoma of the cervicomedullary junction. Clin. Neuropathol. 2022, 41, 3–5. [Google Scholar] [CrossRef]
- Nicolaides, P.; Newton, R.W.; Kelsey, A. Primary malignant melanoma of meninges: Atypical presentation of subacute meningitis. Pediatr. Neurol. 1995, 12, 172–174. [Google Scholar] [CrossRef]
- Makin, G.W.; Eden, O.B.; Lashford, L.S.; Moppett, J.; Gerrard, M.P.; Davies, H.A.; Powell, C.V.; Campbell, A.N.; Frances, H. Leptomeningeal melanoma in childhood. Cancer 1999, 86, 878–886. [Google Scholar] [CrossRef]
- El-Khashab, M.; Koral, K.; Bowers, D.C.; Johnson-Welch, S.; Swift, D.; Nejat, F. Intermediate grade meningeal melanocytoma of cervical spine. Childs Nerv. Syst. 2009, 25, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Bocquillon, P.; Berteloot, A.S.; Maurage, C.A.; Mackowiak-Cordoliani, M.A.; Pasquier, F.; Bombois, S. Mélanome malin leptoméningé primitif: Une étiologie rare de pachy- et leptoméningite [Primitive leptomeningeal malignant melanoma: A rare etiology of pachymeningitis and leptomeningitis]. Rev. Neurol. 2010, 166, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.D.; Syndikus, I.; Clark, A.; Baborie, A. Diffuse primary leptomeningeal melanocytosis in a patient receiving a novel cancer cell vaccine for prostate cancer. BMJ Case Rep. 2010, 2010, bcr11.2009.2495. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, B.C.; Hwang, S.W.; Cho, S.K.; Kim, H.W.; Lee, S.W.; Hwang, J.H.; Lee, J. F-18 fluorodeoxyglucose PET/CT and post hoc PET/MRI in a case of primary meningeal melanomatosis. Korean J. Radiol. 2013, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.; Medina-Flores, R.; Taylor, C.L.; Yonas, H.; Chohan, M.O. Primary melanocytic tumors of the central nervous system: Report of two cases and review of literature. Surg. Neurol. Int. 2014, 5, 147. [Google Scholar]
- Angelino, G.; De Pasquale, M.D.; De Sio, L.; Serra, A.; Massimi, L.; De Vito, R.; Marrazzo, A.; Lancella, L.; Carai, A.; Antonelli, M.; et al. NRAS(Q61K) mutated primary leptomeningeal melanoma in a child: Case presentation and discussion on clinical and diagnostic implications. BMC Cancer 2016, 16, 512. [Google Scholar] [CrossRef]
- Carey, A.R.; Bermudez-Magner, J.A.; Dubovy, S.R.; Schatz, N.J.; Sternau, L.L.; Sklar, E.M.; Lam, B.L. “TB or Not TB?” That is the Question. J. Neuroophthalmol. 2016, 36, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Szathmari, A.; Perbet, R.; Hermier, M.; Di Rocco, F.; Frappaz, D.; Mottolese, C. Primary Amelanotic Leptomeningeal Melanomatosis in a Child: A Rare but Severe Disease. World Neurosurg. 2016, 92, e15–e581. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Delfort, F.; Heliette, C.; Renard, D. Brain 18F-choline PET/CT in primary diffuse leptomeningeal melanomatosis. Acta Neurol. Belg. 2016, 116, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Gocmen, R.; Acar, N.P.; Khasiyev, F.; Gumeler, E.; Soylemezoglu, F.; Tuncer, A.; Arsava, E.M.; Topçuoglu, M.A.; Unal Cevik, I. Two cases of primary leptomeningeal melanomatosis mimicking subacute meningitis. Neuroradiol. J. 2018, 31, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Sakai, K.; Higashiyama, F.; Oya, F.; Maejima, T.; Miyake, T. Primary central nervous system malignant melanoma with leptomeningeal melanomatosis: A case report and review of the literature. Neurosurg. Rev. 2018, 41, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nicolotto, E.; Presas-Rodriguez, S.; Morra, I.; Franchino, F.; Magistrello, M.; Pellerino, A.; Massaro, F.; Pinessi, L.; Rudà, R.; Soffietti, R. Spinal melanocytoma with leptomeningeal spread and long survival following surgery and chemotherapy. J. Neurosurg. Sci. 2018, 62, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Achua, J.K.; Locatelli, E.; Shah, A.H.; Komotar, R.J.; Ghods, A.J. Primary Diffuse Leptomeningeal Melanomatosis: Case Report and Review of the Literature. World Neurosurg. 2019, 122, 648–655. [Google Scholar] [CrossRef]
- Hou, W.; Yu, J.; Gao, S.; Chu, Y. Primary cervical meningeal melanocytoma with a dumbbell shape: Case report and review of the literature. Medicine 2023, 102, e33435. [Google Scholar] [CrossRef]
- Baumgartner, A.; Stepien, N.; Mayr, L.; Madlener, S.; Dorfer, C.; Schmook, M.T.; Traub-Weidinger, T.; Lötsch-Gojo, D.; Kirchhofer, D.; Reisinger, D.; et al. Novel Insights into Diagnosis, Biology and Treatment of Primary Diffuse Leptomeningeal Melanomatosis. J. Pers. Med. 2021, 11, 292. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Bastian, B.C.; Folberg, R.; Verdijk, R.M.; Weis, E.; De Klein, A.; Beenakker, J.W.; Kiilgaard, J.; Cassoux, N. Chapter 4—Choroidal and ciliary body melanomas. In WHO Classification of Tumours, 5th ed.; Eye and Orbit Tumours; International Agency for Research on Cancer: Lyon, France, 2023. [Google Scholar]
- Vader, M.J.C.; Madigan, M.C.; Versluis, M.; Suleiman, H.M.; Gezgin, G.; Gruis, N.A.; Out-Luiting, J.J.; Bergman, W.; Verdijk, R.M.; Jager, M.J.; et al. GNAQ and GNA11 mutations and downstream YAP activation in choroidal nevi. Br. J. Cancer. 2017, 117, 884–887. [Google Scholar] [CrossRef]
- Koelsche, C.; Hovestadt, V.; Jones, D.T.; Capper, D.; Sturm, D.; Sahm, F.; Schrimpf, D.; Adeberg, S.; Böhmer, K.; Hagenlocher, C.; et al. Melanotic tumors of the nervous system are characterized by distinct mutational, chromosomal and epigenomic profiles. Brain Pathol. 2015, 25, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Wu, C.; Zhang, Z.; Qin, T. Melanocytomas of the central nervous system: A clinicopathological and molecular study. Eur. J. Clin. Investig. 2013, 43, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Gessi, M.; Hammes, J.; Lauriola, L.; Dörner, E.; Kirfel, J.; Kristiansen, G.; zur Muehlen, A.; Denkhaus, D.; Waha, A.; Pietsch, T. GNA11 and N-RAS mutations: Alternatives for MAPK pathway activating GNAQ mutations in primary melanocytic tumours of the central nervous system. Neuropathol. Appl. Neurobiol. 2013, 39, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gessi, M.; van de Nes, J.; Griewank, K.; Barresi, V.; Buckland, M.E.; Kirfel, J.; Caltabiano, R.; Hammes, J.; Lauriola, L.; Pietsch, T.; et al. Absence of TERT promoter mutations in primary melanocytic tumours of the central nervous system. Neuropathol. Appl. Neurobiol. 2014, 40, 794–797. [Google Scholar] [CrossRef]
- Drabarek, W.; Yavuzyigitoglu, S.; Obulkasim, A.; van Riet, J.; Smit, K.N.; van Poppelen, N.M.; Vaarwater, J.; Brands, T.; Eussen, B.; Verdijk, R.M.; et al. Rotterdam Ocular Melanoma Study Group. Multi-Modality Analysis Improves Survival Prediction in Enucleated Uveal Melanoma Patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- van de Nes, J.; Gessi, M.; Sucker, A.; Möller, I.; Stiller, M.; Horn, S.; Scholz, S.L.; Pischler, C.; Stadtler, N.; Schilling, B.; et al. Targeted next generation sequencing reveals unique mutation profile of primary melanocytic tumors of the central nervous system. J. Neurooncol. 2016, 127, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Küsters-Vandevelde, H.V.; van Engen-van Grunsven, I.A.; Coupland, S.E.; Lake, S.L.; Rijntjes, J.; Pfundt, R.; Küsters, B.; Wesseling, P.; Blokx, W.A.; Groenen, P.J. Mutations in g protein encoding genes and chromosomal alterations in primary leptomeningeal melanocytic neoplasms. Pathol. Oncol. Res. 2015, 21, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Rushing, E.J.; Smirniotopoulos, J.G. Pigmented lesions of the central nervous system: Radiologic-pathologic correlation. Radiographics 2009, 29, 1503–1524. [Google Scholar] [CrossRef]
- Saadeh, Y.S.; Hollon, T.C.; Fisher-Hubbard, A.; Savastano, L.E.; McKeever, P.E.; Orringer, D.A. Primary diffuse leptomeningeal melanomatosis: Description and recommendations. J. Clin. Neurosci. 2018, 50, 139–143. [Google Scholar] [CrossRef]
- Kinsler, V.A.; Thomas, A.C.; Ishida, M.; Bulstrode, N.W.; Loughlin, S.; Hing, S.; Chalker, J.; McKenzie, K.; Abu-Amero, S.; Slater, O.; et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J. Investig. Dermatol. 2013, 133, 2229–2236, Erratum in J. Investig. Dermatol. 2016, 136, 2326. [Google Scholar] [CrossRef]
- Pedersen, M.; Küsters-Vandevelde, H.V.N.; Viros, A.; Groenen, P.J.T.A.; Sanchez-Laorden, B.; Gilhuis, J.H.; van Engen-van Grunsven, I.A.; Renier, W.; Schieving, J.; Niculescu-Duvaz, I.; et al. Primary melanoma of the CNS in children is driven by congenital expression of oncogenic NRAS in melanocytes. Cancer Discov. 2013, 3, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Basu, D.; Nikiforova, M.; Bauer, B.S.; Johnson, D.; Rundell, V.; Grunwaldt, L.J.; Reyes-Múgica, M. BRAF mutations are also associated with neurocutaneous melanocytosis and large/giant congenital melanocytic nevi. Pediatr. Dev. Pathol. 2015, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.A.; Marquez, H.J.; Veltkamp, D.L.; Xie, S.Q.; Klesse, L.J.; Timmons, C.F.; Pfeifer, C.M. CT and MRI findings in leptomeningeal melanocytosis. Radiol. Case Rep. 2019, 15, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kinsler, V.A.; Polubothu, S.; Calonje, J.E.; Chong, W.K.; Thompson, D.; Jacques, T.S.; Morrogh, D. Copy number abnormalities in new or progressive ‘neurocutaneous melanosis’ confirm it to be primary CNS melanoma. Acta Neuropathol. 2017, 133, 329–331. [Google Scholar] [CrossRef] [PubMed]
- van Engen-van Grunsven, A.C.; Rabold, K.; Küsters-Vandevelde, H.V.; Rijntjes, J.; Djafarihamedani, M.; Hehir-Kwa, J.Y.; Küsters, B.; Willemsen, M.A.; van der Burgt, I.; Wesseling, P.; et al. Copy number variations as potential diagnostic and prognostic markers for CNS melanocytic neoplasms in neurocutaneous melanosis. Acta Neuropathol. 2017, 133, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Basu, D.; Nikiforova, M.; Hamilton, R.L.; Gehris, R.; Jakacki, R.; Panigrahy, A.; Yatsenko, S.; Reyes-Múgica, M. Amplification of mutated NRAS leading to congenital melanoma in neurocutaneous melanocytosis. Melanoma Res. 2015, 25, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.D. Malignant melanoma and the central nervous system. A guide for classification based on the clinical findings. J. Neurol. Neurosurg. Psychiatry. 1976, 39, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Warakaulle, D.R.; Anslow, P. Differential Diagnosis of Intracranial Lesions with High Signal on T1 or Low Signal on T2-weighted MRI. Clin. Radiol. 2003, 58, 922–933. [Google Scholar] [CrossRef]
- Haller, S.; Haacke, E.M.; Thurnher, M.M.; Barkhof, F. Susceptibility-weighted Imaging: Technical Essentials and Clinical Neurologic Applications. Radiology 2021, 299, 3–26. [Google Scholar] [CrossRef]
- Straub, S.; Laun, F.B.; Freitag, M.T.; Kölsche, C.; von Deimling, A.; Denoix, M.; Bendszus, M.; Schlemmer, H.P.; Ladd, M.E.; Schneider, T.M. Assessment of Melanin Content and its Influence on Susceptibility Contrast in Melanoma Metastases. Clin. Neuroradiol. 2020, 30, 607–614. [Google Scholar] [CrossRef]
- Schwarz, D.; Niederle, T.; Münch, P.; Hielscher, T.; Hassel, J.C.; Schlemmer, H.P.; Platten, M.; Winkler, F.; Wick, W.; Heiland, S.; et al. Susceptibility-weighted imaging in malignant melanoma brain metastasis. J. Magn. Reson. Imaging. 2019, 50, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Isiklar, I.; Leeds, N.E.; Fuller, G.N.; Kumar, A.J. Intracranial metastatic melanoma: Correlation between MR imaging characteristics and melanin content. Am. J. Roentgenol. 1995, 165, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Pappenheim, E. Primary Melanoma of the Central Nervous System: Clinical-Pathological Report of a Case, with Survey and Discussion of the Literature. Arch. Neurol. 1962, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Varela-Poblete, J.; Vidal-Tellez, A.; Cruz-Quiroga, J.P.; Montoya-Salvadores, F.; Medina-Escobar, J. Melanocytic lesions of the central nervous system: A case series. Arq. Neuropsiquiatr. 2022, 80, 153–160. [Google Scholar] [CrossRef]
- Mohapatra, A.; Choudhury, P. An Uncommon Case of Primary Leptomeningeal Melanoma in a 66-Year-Old White Caucasian Male. Cureus 2020, 12, e10793. [Google Scholar] [CrossRef] [PubMed]
- Naul, L.G.; Hise, J.H.; Bauserman, S.C.; Todd, F.D. CT and MR of meningeal melanocytoma. AJNR Am. J. Neuroradiol. 1991, 12, 315–316. [Google Scholar]
- Gamoh, S.; Tsuno, T.; Akiyama, H.; Kotaki, S.; Nakanishi, T.; Tsuji, K.; Yoshida, H.; Shimizutani, K. Intracranial meningeal melanocytoma diagnosed using an interdisciplinary approach: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 177. [Google Scholar] [CrossRef]
- Wang, F.; Ling, S. Primary Meningeal Melanocytoma in Sellar Region, Simulating a Nonfunctioning Pituitary Adenoma: Case Report and Literature Review. World Neurosurg. 2018, 112, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kremer, S.; Abu Eid, M.; Bierry, G.; Bogorin, A.; Koob, M.; Dietemann, J.L.; Fruehlich, S. Accuracy of delayed post-contrast FLAIR MR imaging for the diagnosis of leptomeningeal infectious or tumoral diseases. J. Neuroradiol. 2006, 33, 285–291. [Google Scholar] [CrossRef]
- Cakirer, S.; Karaarslan, E.; Arslan, A. Spontaneously T1-hyperintense lesions of the brain on MRI: A pictorial review. Curr. Probl. Diagn. Radiol. 2003, 32, 194–217. [Google Scholar] [CrossRef]
- Sąsiadek, M.J. Intracranial Lesions with Low Signal Intensity on T2-weighted MR Images—Review of Pathologies. Pol. J. Radiol. 2015, 80, 40–50. [Google Scholar] [CrossRef]
- Freudenstein, D.; Wagner, A.; Bornemann, A.; Ernemann, U.; Bauer, T.; Duffner, F. Primary melanocytic lesions of the CNS: Report of five cases. Zentralbl Neurochir. 2004, 65, 146–153. [Google Scholar] [CrossRef]
- Jaiswal, S.; Vij, M.; Tungria, A.; Jaiswal, A.K.; Srivastava, A.K.; Behari, S. Primary melanocytic tumors of the central nervous system: A neuroradiological and clinicopathological study of five cases and brief review of literature. Neurol. India. 2011, 59, 413–419. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.Z.; Wang, Y.J.; Zhang, S.G.; Xing, D.G. Microsurgery for the treatment of primary malignant intracranial melanoma: A surgical series and literature review. Eur. J. Surg. Oncol. 2014, 40, 1062–1071. [Google Scholar] [CrossRef]
- Ma, Y.; Gui, Q.; Lang, S. Intracranial malignant melanoma: A report of 7 cases. Oncol. Lett. 2015, 10, 2171–2175. [Google Scholar] [CrossRef]
- Puyana, C.; Denyer, S.; Burch, T.; Bhimani, A.D.; McGuire, L.S.; Patel, A.S.; Mehta, A.I. Primary Malignant Melanoma of the Brain: A Population-Based Study. World Neurosurg. 2019, 130, e1091–e1097. [Google Scholar] [CrossRef]
- Liu, X.L.; Run-Hua, Z.; Pan, J.X.; Li, Z.J.; Yu, L.; Li, Y.L. Emerging therapeutic strategies for metastatic uveal melanoma: Targeting driver mutations. Pigment. Cell Melanoma Res. 2024; ahead of print. [Google Scholar] [CrossRef]
- Zadro, I.; Brinar, V.V.; Barun, B.; Ozretić, D.; Pazanin, L.; Grahovac, G.; Habek, M. Primary diffuse meningeal melanomatosis. Neurologist 2010, 16, 117–119. [Google Scholar] [CrossRef]
- Koziorowska-Gawron, E.; Kaczorowski, M.; Bladowska, J.; Budrewicz, S.; Koszewicz, M.; Hałoń, A. Diagnostic Difficulties in Primary Pauci-Melanotic Leptomeningeal Melanomatosis. Eur. Neurol. 2018, 80, 68–70. [Google Scholar] [CrossRef]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Diagnosis and Treatment of Peripheral and Cranial Nerve Tumors with Expert Recommendations: An EUropean Network for RAre CANcers (EURACAN) Initiative. Cancers 2023, 15, 1930. [Google Scholar] [CrossRef]
- Kurita, H.; Segawa, H.; Shin, M.; Ueki, K.; Ichi, S.; Sasaki, T.; Tago, M.; Kirino, T. Radiosurgery of meningeal melanocytoma. J. Neurooncol. 2000, 46, 57–61. [Google Scholar] [CrossRef]
- Ferreira de Araújo, M.G.; Almondes Santana Lemos, L.E.; Negromonte Guerra, P.L.; Dos Santos Lima Didjurgeit, F.M.; Batista Cezar, A., Jr.; Faquini, I.V.; Cirne de Azevedo Filho, H.R. Supratentorial meningeal melanocytoma mimicking meningioma: Case report and literature review. Pathol. Oncol. Res. 2024, 29, 1611482. [Google Scholar] [CrossRef] [PubMed]

| Authors | Sex/Age (Years) | Symptoms | Distinct Mass on Imaging Workup | Diagnosis | Medical Treatment | OS |
|---|---|---|---|---|---|---|
| Nicolaides et al., 1995 [25] | M/5 | Increased ICP; headache, nausea, vomiting, intermittent fever, anorexia and weight loss | Yes, left parietal lobe | Parietal biopsy | Local PNET protocol | Not reported |
| Makin et al., 1999 [26] | M/5 | Increased ICP | No, only leptomeningeal spread | Meningeal biopsy | Carboplatin + etoposide + vincristine | 6 months |
| Tosaka et al., 2001 [27] | M/20 | Increased ICP, hydrocephalus | No, only leptomeningeal spread | Second CSF tap test (cytology) | Dacarbazine + numistine + vincristine + intrathecal methotrexate | 4.5 months |
| Bocquillon et al., 2010 [28] | F/32 | Increased ICP, transient hemiparesis | No, only leptomeningeal spread | Meningeal biopsy | Fotemustine | 4 months |
| Michael et al., 2010 [29] | M/75 | Vomiting, poor balance, gait disturbance, cognitive decline | Yes, left parietal lesion | Gross-total section | Goserelin+ bicalutamide + docetaxel | 16 days |
| Lee et al., 2013 [30] | M/17 | Headache, nausea, vomiting | Yes, left heel | Left heel biopsy | WBRT + dacarbazine + carmustine + cisplatin + tamoxifen | Not reported |
| Trinh et al., 2014 [31] | M/51 | Headache, nausea, vomiting, hip pain, fatigue | Yes, multinodular diffuse parenchymal enhancement | Meningeal biopsy | CSI + temozolomide | 3 months |
| Angelino et al., 2016 [32] | F/2 | Mild, intermittent diplopia | No, only leptomeningeal enhancement | Meningeal biopsy | Temozolomide + cisplatin + vindesine + PET-interferon alfa-2B | 11 months |
| Carey et al., 2016 [33] | M/36 | Headache, bilateral leg numbness, bilateral decreased visual acuity | No, only leptomeningeal enhancement | Meningeal biopsy | Ipilimumab | 1 month |
| Szathmani et al., 2016 [34] | F/5 | Headache, vomiting, seizures | Yes, bulky left parietal lesion | Left parietal biopsy | Etoposide + temozolomide + carboplatin | 24 months |
| Jacob et al., 2016 [35] | M/55 | Headache, nausea, fatigue | No, only leptomeningeal spread | Meningeal biopsy | Temozolomide | Not reported |
| Aslan et al., 2018 [36] | M/21 | Headache, vomiting, fever | No, only leptomeningeal enhancement | Meningeal biopsy | Cisplatin + dacarbazine + WBRT | 12 months |
| Aslan et al., 2018 [36] | F/44 | Nausea, vomiting, weight loss, mental slowness, poor concentration, impairment in short-term memory | No, only leptomeningeal diffusion | Meningeal biopsy | Temozolomide | 4 months |
| Fujimory et al., 2018 [37] | M/37 | Headache | Yes, left temporal lesion | Partial resection | 1st: WBRT + vemurafenib 2nd: nivolumab | 5 months |
| Nicolotto et al., 2018 [38] | M/36 | Back pain, headache, mental confusion, cervicalgia, tinnitus, facial paresis | Yes, lumbar (L2) bulky lesion with spinal compression | Gross-total resection | 1st: temozolomide 2nd: fotemustine | 24 months |
| Eichberg et al., 2019 [39] | F/44 | Mental confusion, left facial palsy, slurred speech, left hemiplegia, hydrocephalus | No, only leptomeningeal diffusion | Meningeal biopsy | Nivolumab + ipilimumab | 4 months |
| Tamura et al., 2020 [40] | F/49 | Hydrocephalus | No, only leptomeningeal diffusion | 3rd CSF cytology | Nivolumab + ipilimumab | 4 months |
| Baumgartner et al., 2020 [41] | M/15 | Increased ICP, bilateral papilledema | Yes, parieto-occipital lesion | Parenchymal biopsy | 1st: trametinib 2nd: tametinib + everolimus 3rd: nivolumab + ipilimumab 4th: WBRT | 6.5 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN). Cancers 2024, 16, 2508. https://doi.org/10.3390/cancers16142508
Pellerino A, Verdijk RM, Nichelli L, Andratschke NH, Idbaih A, Goldbrunner R. Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN). Cancers. 2024; 16(14):2508. https://doi.org/10.3390/cancers16142508
Chicago/Turabian StylePellerino, Alessia, Robert M. Verdijk, Lucia Nichelli, Nicolaus H. Andratschke, Ahmed Idbaih, and Roland Goldbrunner. 2024. "Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN)" Cancers 16, no. 14: 2508. https://doi.org/10.3390/cancers16142508
APA StylePellerino, A., Verdijk, R. M., Nichelli, L., Andratschke, N. H., Idbaih, A., & Goldbrunner, R. (2024). Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN). Cancers, 16(14), 2508. https://doi.org/10.3390/cancers16142508








