Sentinel Lymph Node Detection in Cutaneous Melanoma Using Indocyanine Green-Based Near-Infrared Fluorescence Imaging: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
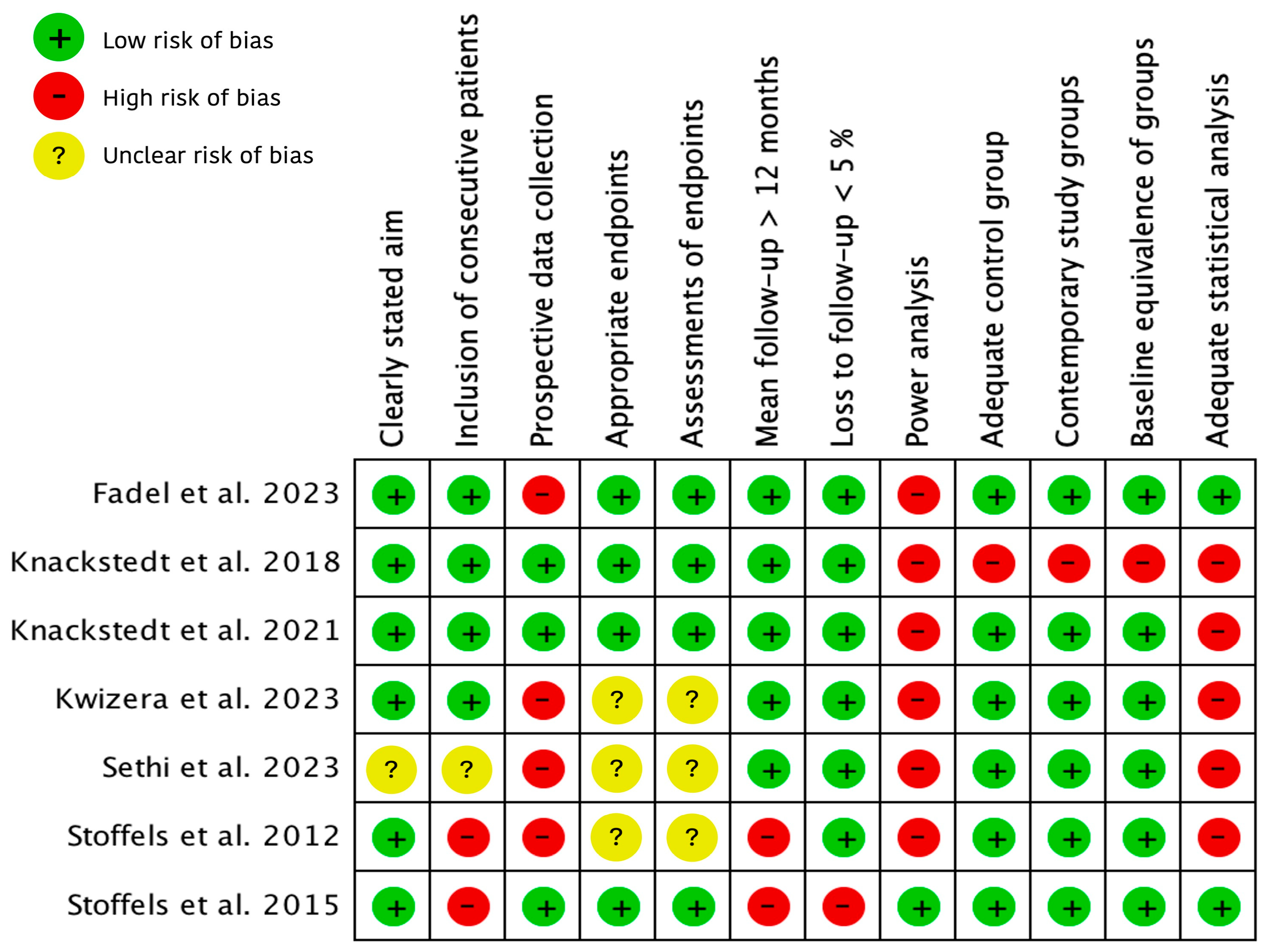
2.3. Quality Assessment
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
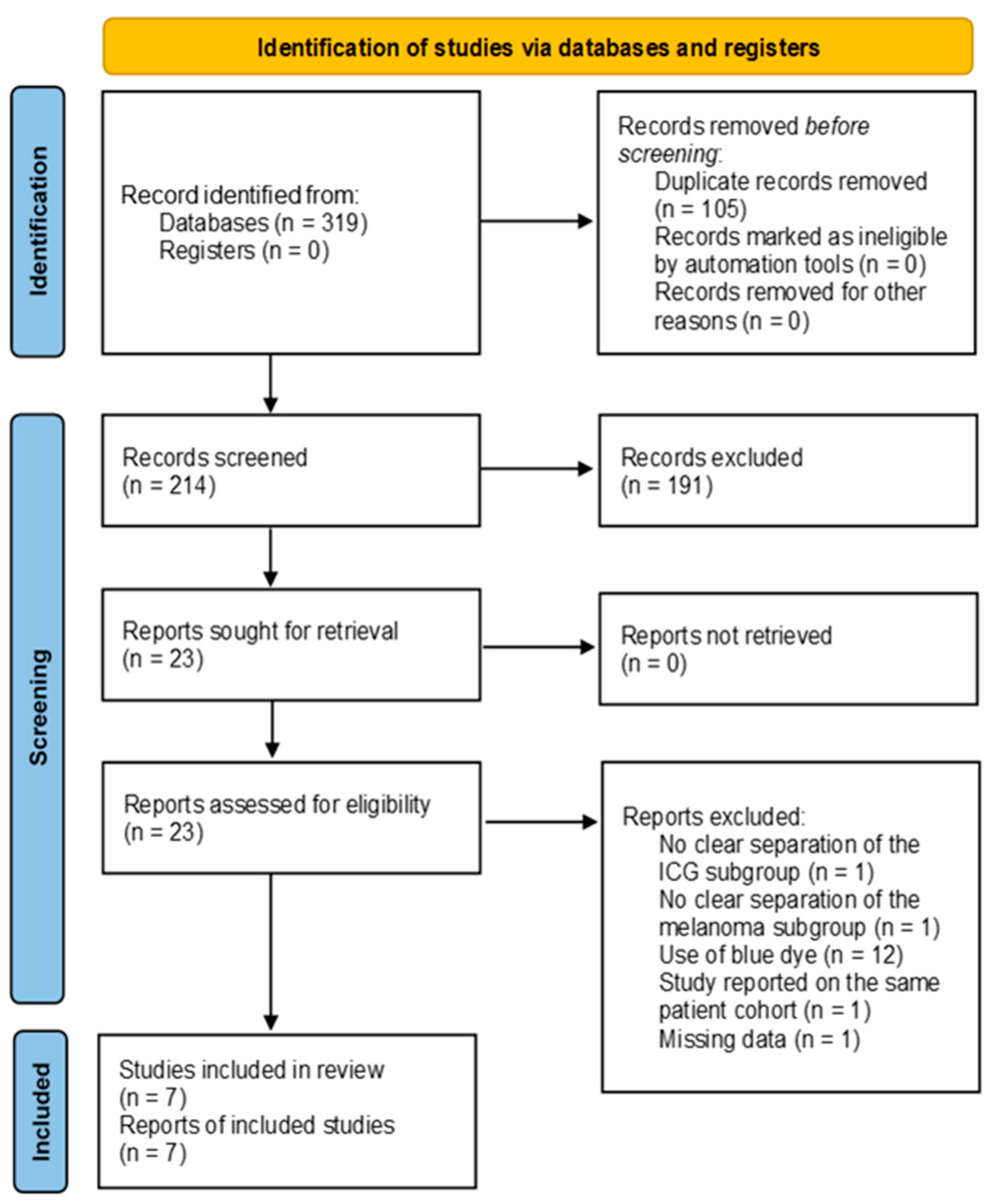
3.1. Literature Search
3.2. Characteristics of Included Studies
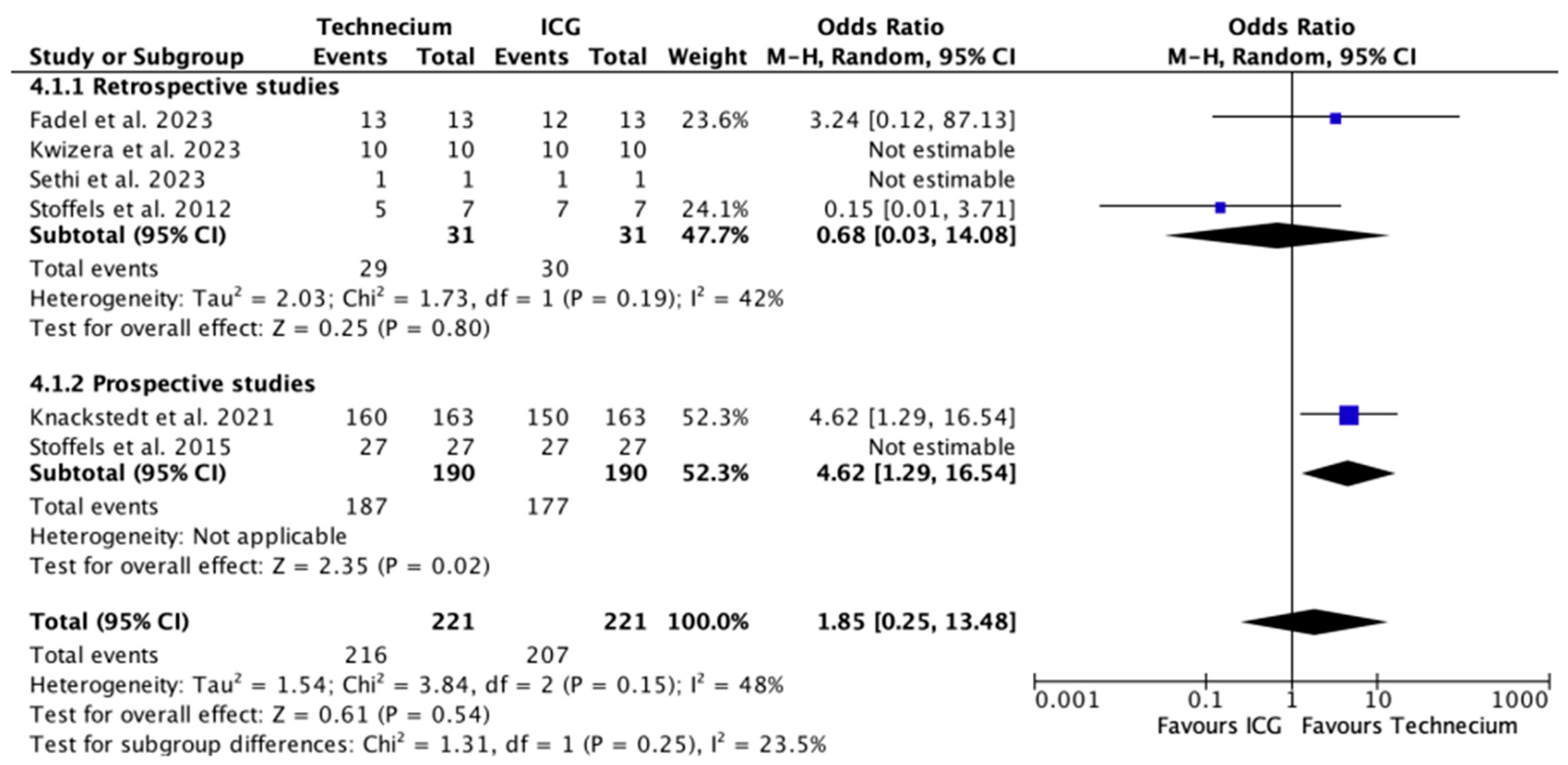
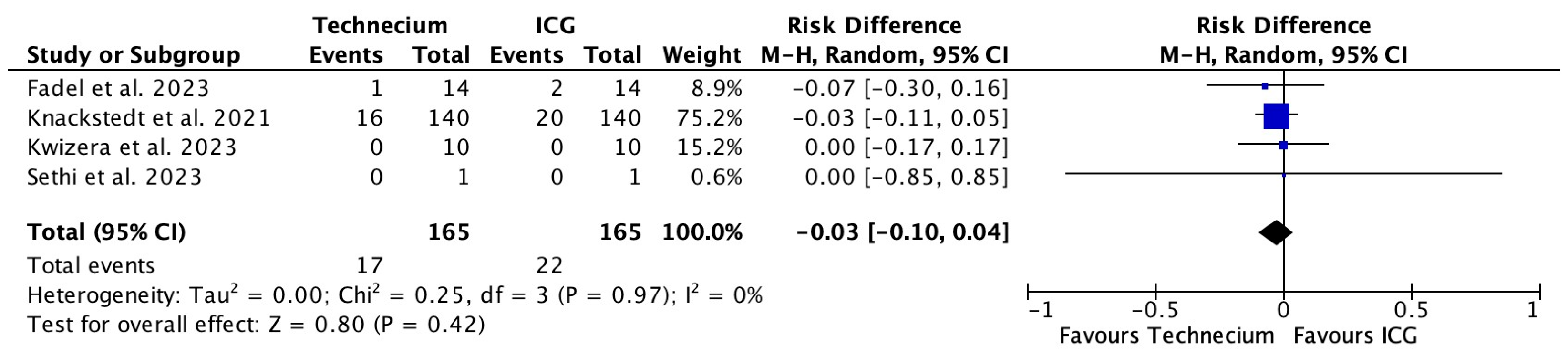
3.3. Characteristics of SLNB Per Tracing Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Morrison, S.; Han, D. Re-evaluation of Sentinel Lymph Node Biopsy for Melanoma. Curr. Treat. Options Oncol. 2021, 22, 22. [Google Scholar] [CrossRef]
- Wong, S.L.; Balch, C.M.; Hurley, P.; Agarwala, S.S.; Akhurst, T.J.; Cochran, A.; Cormier, J.N.; Gorman, M.; Kim, T.Y.; McMasters, K.M.; et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J. Clin. Oncol. 2012, 30, 2912–2918. [Google Scholar] [CrossRef]
- Cattin, F.; Fogacci, T.; Frisoni, G.; Fabiocchi, L.; Dellachiesa, L.; Semprini, G.; Samorani, D. Icg Versus 99tc in Breast Surgery-How to Match Quality Health Care and Costs Reduction: A Cost Effectiveness Study. J. Cancer Sci. Ther. 2017, 9, 340–342. [Google Scholar] [CrossRef]
- Lützen, U.; Naumann, C.M.; Dischinger, J.; Marx, M.; Baumann, R.; Zhao, Y.; Jüptner, M.; Osmonov, D.; Bothe, K.; Jünemann, K.P.; et al. 10-Year experience regarding the reliability and morbidity of radio guided lymph node biopsy in penile cancer patients and the associated radiation exposure of medical staff in this procedure. BMC Urol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Valiveru, R.C.; Agarwal, G.; Agrawal, V.; Gambhir, S.; Mayilvaganan, S.; Chand, G.; Mishra, A.; Agarwal, A.; Mishra, S.K. Low-cost Fluorescein as an Alternative to Radio-colloid for Sentinel Lymph Node Biopsy-a Prospective Validation Study in Early Breast Cancer. World J. Surg. 2020, 44, 3417–3422. [Google Scholar] [CrossRef]
- Bargon, C.A.; Huibers, A.; Young-Afat, D.A.; Jansen, B.A.M.; Borel-Rinkes, I.H.M.; Lavalaye, J.; van Slooten, H.J.; Verkooijen, H.M.; van Swol, C.F.P.; Doeksen, A. Sentinel Lymph Node Mapping in Breast Cancer Patients through Fluorescent Imaging Using Indocyanine Green: The INFLUENCE Trial. Ann. Surg. 2022, 276, 913–920. [Google Scholar] [CrossRef]
- Dumitru, D.; Ghanakumar, S.; Provenzano, E.; Benson, J.R. A Prospective Study Evaluating the Accuracy of Indocyanine Green (ICG) Fluorescence Compared with Radioisotope for Sentinel Lymph Node (SLN) Detection in Early Breast Cancer. Ann. Surg. Oncol. 2022, 29, 3014–3020. [Google Scholar] [CrossRef]
- Yin, R.; Ding, L.Y.; Wei, Q.Z.; Zhou, Y.; Tang, G.Y.; Zhu, X. Comparisons of ICG-fluorescence with conventional tracers in sentinel lymph node biopsy for patients with early-stage breast cancer: A meta-analysis. Oncol. Lett. 2021, 21, 114. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Agoritsas, T.; Merglen, A.; Courvoisier, D.S.; Combescure, C.; Garin, N.; Perrier, A.; Perneger, T.V. Sensitivity and predictive value of 15 PubMed search strategies to answer clinical questions rated against full systematic reviews. J. Med. Internet Res. 2012, 14, e85. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Kretschmer, L.; Bertsch, H.P.; Zapf, A.; Mitteldorf, C.; Satzger, I.; Thoms, K.M.; Völker, B.; Schön, M.P.; Gutzmer, R.; Starz, H. Nodal Basin Recurrence after Sentinel Lymph Node Biopsy for Melanoma: A Retrospective Multicenter Study in 2653 Patients. Medicine 2015, 94, e1433. [Google Scholar] [CrossRef]
- Altman, D.G. Confidence intervals for the number needed to treat. BMJ 1998, 317, 1309–1312. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Fadel, M.G.; Rauf, S.; Mohamed, H.S.; Yusuf, S.; Hayes, A.J.; Power, K.; Smith, M.J. The Use of Indocyanine Green and Near-Infrared Fluorescence Imaging versus Blue Dye in Sentinel Lymph Node Biopsy in Cutaneous Melanoma: A Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 4333–4340. [Google Scholar] [CrossRef]
- Knackstedt, R.W.; Couto, R.A.; Gastman, B. Indocyanine green fluorescence imaging with lymphoscintigraphy for sentinel node biopsy in head and neck melanoma. J. Surg. Res. 2018, 228, 77–83. [Google Scholar] [CrossRef]
- Knackstedt, R.; Gastman, B.R. Indocyanine Green Fluorescence Imaging with Lymphoscintigraphy Improves the Accuracy of Sentinel Lymph Node Biopsy in Melanoma. Plast. Reconstr. Surg. 2021, 148, 83e–93e. [Google Scholar] [CrossRef]
- Kwizera, A.; Obaid, A.; Tran, D.; Rubarth, C.; Preskitt, J.T. Use of indocyanine green for sentinel lymph node biopsy in melanoma. Proc. (Bayl. Univ. Med. Cent.) 2023, 36, 201–204. [Google Scholar] [CrossRef]
- Sethi, H.K.; Sina, E.M.; Mady, L.J.; Fundakowski, C.E. Sentinel lymph node biopsy for head and neck malignancies utilizing simultaneous radioisotope gamma probe and indocyanine green fluorescence navigation. Head Neck 2024, 46, 212–217. [Google Scholar] [CrossRef]
- Stoffels, I.; von der Stück, H.; Boy, C.; Pöppel, T.; Körber, N.; Weindorf, M.; Dissemond, J.; Schadendorf, D.; Klode, J. Indocyanine green fluorescence-guided sentinel lymph node biopsy in dermato-oncology. J. Dtsch. Dermatol. Ges. 2012, 10, 51–57. [Google Scholar] [CrossRef]
- Stoffels, I.; Dissemond, J.; Pöppel, T.; Schadendorf, D.; Klode, J. Intraoperative Fluorescence Imaging for Sentinel Lymph Node Detection: Prospective Clinical Trial to Compare the Usefulness of Indocyanine Green vs Technetium Tc 99m for Identification of Sentinel Lymph Nodes. JAMA Surg. 2015, 150, 617–623. [Google Scholar] [CrossRef]
- Manca, G.; Romanini, A.; Rubello, D.; Mazzarri, S.; Boni, G.; Chiacchio, S.; Tredici, M.; Duce, V.; Tardelli, E.; Volterrani, D.; et al. A critical reappraisal of false negative sentinel lymph node biopsy in melanoma. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 105–113. [Google Scholar]
- Wang, B.; Ma, X.; Zhang, X.; Zhang, X.; Guan, S.; Xiao, T.; Li, X. Application value of a hybrid tracer during sentinel lymph node biopsy for head and neck malignancies: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 108340. [Google Scholar] [CrossRef]
- Whitman, G.J.; AlHalawani, R.H.; Karbasian, N.; Krishnamurthy, R. Sentinel Lymph Node Evaluation: What the Radiologist Needs to Know. Diagnostics 2019, 9, 12. [Google Scholar] [CrossRef]
- Wilke, L.G.; McCall, L.M.; Posther, K.E.; Whitworth, P.W.; Reintgen, D.S.; Leitch, A.M.; Gabram, S.G.; Lucci, A.; Cox, C.E.; Hunt, K.K.; et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann. Surg. Oncol. 2006, 13, 491–500. [Google Scholar] [CrossRef]
- Roaten, J.B.; Pearlman, N.; Gonzalez, R.; Gonzalez, R.; McCarter, M.D. Identifying risk factors for complications following sentinel lymph node biopsy for melanoma. Arch. Surg. 2005, 140, 85–89. [Google Scholar] [CrossRef]
- Puza, C.J.; Josyula, S.; Terando, A.M.; Howard, J.H.; Agnese, D.M.; Mosca, P.J.; Lee, W.T.; Beasley, G.M. Does the number of sentinel lymph nodes removed affect the false negative rate for head and neck melanoma? J. Surg. Oncol. 2018, 117, 1584–1588. [Google Scholar] [CrossRef]
- Miller, M.W.; Vetto, J.T.; Monroe, M.M.; Weerasinghe, R.; Andersen, P.E.; Gross, N.D. False-negative sentinel lymph node biopsy in head and neck melanoma. Otolaryngol. Head Neck Surg. 2011, 145, 606–611. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Wu, C.E.; Hsieh, C.H.; Chang, C.J.; Yeh, J.T.; Kuo, T.T.; Yang, C.H.; Lo, Y.F.; Lin, K.J.; Lin, Y.C.; Chang, J.W. Prognostic factors for Taiwanese patients with cutaneous melanoma undergoing sentinel lymph node biopsy. J. Formos. Med. Assoc. 2015, 114, 415–421. [Google Scholar] [CrossRef]
- Sinnamon, A.J.; Neuwirth, M.G.; Bartlett, E.K.; Zaheer, S.; Etherington, M.S.; Xu, X.; Elder, D.E.; Czerniecki, B.J.; Fraker, D.L.; Karakousis, G.C. Predictors of false negative sentinel lymph node biopsy in trunk and extremity melanoma. J. Surg. Oncol. 2017, 116, 848–855. [Google Scholar] [CrossRef]
- Leitlinien. S3—Leitlinie zur Diagnostik, Therapie und Nachsorge des Melanoms. J. Dtsch. Dermatol. Ges. 2020, 18, 1079–1208. [Google Scholar] [CrossRef]
- Panasiti, V.; Devirgiliis, V.; Curzio, M.; Roberti, V.; Gobbi, S.; Rossi, M.; Bottoni, U.; Clerico, R.; Scuderi, N.; Calvieri, S. Predictive factors for false negative sentinel lymph node in melanoma patients. Dermatol. Surg. 2010, 36, 1521–1528. [Google Scholar] [CrossRef]
- Couto, R.A.; Lamaris, G.A.; Knackstedt, R.; Alleyne, B.; Durand, P.; Rueda, S.; Gastman, B. Determining the False-Negative Rate Using Fluorescence Image-Assisted Sentinel Lymph Node Biopsy in Cutaneous Melanoma. Ann. Plast. Surg. 2018, 80, 54–58. [Google Scholar] [CrossRef]
- Lese, I.; Constantinescu, M.A.; Leckenby, J.I.; Zubler, C.; Alberts, I.; Hunger, R.E.; Wartenberg, J.; Olariu, R. Transcutaneous sentinel lymph node detection in cutaneous melanoma with indocyanine green and near-infrared fluorescence: A diagnostic sensitivity study. Medicine 2022, 101, e30424. [Google Scholar] [CrossRef]
- Namikawa, K.; Tsutsumida, A.; Tanaka, R.; Kato, J.; Yamazaki, N. Limitation of indocyanine green fluorescence in identifying sentinel lymph node prior to skin incision in cutaneous melanoma. Int. J. Clin. Oncol. 2014, 19, 198–203. [Google Scholar] [CrossRef]
- Gilmore, D.M.; Khullar, O.V.; Gioux, S.; Stockdale, A.; Frangioni, J.V.; Colson, Y.L.; Russell, S.E. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann. Surg. Oncol. 2013, 20, 2357–2363. [Google Scholar] [CrossRef]
- Murawa, D.; Hirche, C.; Dresel, S.; Hünerbein, M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br. J. Surg. 2009, 96, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Korn, J.M.; Tellez-Diaz, A.; Bartz-Kurycki, M.; Gastman, B. Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast. Reconstr. Surg. 2014, 133, 914–922. [Google Scholar] [CrossRef] [PubMed]



| Kwizera 2023 [21] | Fadel 2023 [18] | Sethi 2023 [22] | Stoffels 2015 [24] | Stoffels 2012 [23] | Knackstedt 2021 [20] | Knackstedt 2018 [19] | Total (%) or Weighted Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| Type | Cohort Study | Cohort Study | Cohort Study | Cohort Study | Cohort Study | Cohort Study | Cohort Study | |
| Design | Retrospective | Retrospective | Retrospective | Prospective | Retrospective | Prospective | Prospective | |
| Location | Monocentric | Monocentric | Monocentric | Monocentric | Monocentric | Monocentric | Monocentric | |
| Study period (months) | NR | 51 | 46 | 18 | NR | 72 | 36 | |
| No. patients | 52 | 122 | 10 | 80 | 22 | 594 | 61 | 941 (100) |
| Men (%) | 26 (50) | 47 (38.5) | NR | 52 (65) | 10 (45.5) | 337 (56.7) | 45 (73.8) | 517 (55.6) |
| Women (%) | 26 (50) | 75 (61.5) | NR | 28 (35) | 12 (54.5) | 257(43.3) | 15 (24.6) | 413 (44.4) |
| Mean age (years) | 63 | 60.5 | 65 | 55.5 | 51.6 | 61.2 | 64.3 | 61 (2) |
| Primary tumor location | ||||||||
| Head and Neck (%) | 11 (21.2) | 13 (10.7) | 10 (100) | 0 (0) | 2 (9.1) | 136 (22.9) | 61 (100) | 233 (24.8) |
| Trunk (%) | 20 (38.5) | 38 (31.1) | 0 (0) | 40 (50) | 9 (40.9) | 163 (27.4) | 0 (0) | 270 (28.7) |
| Extremities (%) | 21 (40.4) | 71 (58.2) | 0 (0) | 40 (50) | 11 (50) | 295 (49.7) | 0 (0) | 438 (46.5) |
| Camera system | Stryker Elite, SPY-PHI | SPY-PHI | SPY Elite | Fluobeam | PDE | SPY, PDE, Quest | SPY, PDE | |
| ICG dose (mg/mL) | 2.5 | 2.5 | 2.5 | NR | NR | 2.5 | 2.5 |
| Kwizera 2023 [21] | Fadel 2023 [18] | Sethi 2023 [22] | Stoffels 2015 [24] | Stoffels 2012 [23] | Knackstedt 2021 [20] | Knackstedt 2018 [19] | Total (%) or Weighted Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| No. SLNs sampled (%) | 139 | 204 | 10 | 149 | 61 | 1827 | 198 | 2588 (100) |
| identified by both methods | 137 (98.6) | 159 (77.9) | 10 (100) | 139 (93.3) | 50 (82) | 1556 (85.2) | 172 (86.9) | 2223 (85.9) |
| identified only by Tc | 0 (0) | 11 (5.4) | 0 (0) | 8 (5.4) | 0 (0) | 255 (14) | 18 (9.1) | 292 (11.3) |
| identified only by ICG | 2 (1.4) | 34 (16.7) | 0 (0) | 2 (1.3) | 11 (18) | 16 (0.9) | 8 (4) | 73 (2.8) |
| Follow-up (months) | ||||||||
| Mean | NR | NR | 16.2 | NR | 1.2 | 34.4 | 30.6 | 31 (7) |
| Median | 24 | 24 | NR | NR | NR | NR | NR | |
| No. metastatic patients (%) | 10 | 13 | 1 | 24 | 6 | 128 | 10 | 192 (100) |
| identified by both methods | 10 (100) | 12 (92.3) | 1 (100) | 24 (100) | 4 (66.7) | 116 (90.6) | NR | 167 (91.8) |
| identified only by Tc | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 8 (6.3) | NR | 9 (4.9) |
| identified only by ICG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 4 (3.1) | NR | 6 (3.3) |
| No. metastatic SLNs (%) | 10 | 13 | 1 | 27 | 7 | 163 | NR | 221 (100) |
| identified by both methods | 10 (100) | 12 (92.3) | 1 (100) | 27 (100) | 5 (71.4) | 147 (90.2) | NR | 202 (91.4) |
| identified only by Tc | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 13 (8) | NR | 14 (6.3) |
| identified only by ICG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (28.6) | 3 (1.8) | NR | 5 (2.3) |
| No. recurrences (1) | 0 | 1 | 0 | NR | 0 | 12 | 1 | 14 (100) |
| FNR ICG (%) | 0 | 14 | 0 | NR | NR | 14 | NR | 13 (4) |
| FNR Tc (%) | 0 | 7 | 0 | NR | NR | 11 | NR | 10 (3) |
| FNR | ICG | Tc | RR (95% CI) | NNH (95% CI) | p Value |
|---|---|---|---|---|---|
| Pooled FNR for all patients (n = 778) | 2.8% | 2.2% | 1.3 (0.7, 2.4) | 155.6 (45.5, ∞) | 0.419 |
| Pooled FNR for metastatic patients (n = 165) | 13.3% | 10.3% | 1.3 (0.7, 2.3) | 33 (10.0, ∞) | 0.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wölffer, M.; Liechti, R.; Constantinescu, M.; Lese, I.; Zubler, C. Sentinel Lymph Node Detection in Cutaneous Melanoma Using Indocyanine Green-Based Near-Infrared Fluorescence Imaging: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2523. https://doi.org/10.3390/cancers16142523
Wölffer M, Liechti R, Constantinescu M, Lese I, Zubler C. Sentinel Lymph Node Detection in Cutaneous Melanoma Using Indocyanine Green-Based Near-Infrared Fluorescence Imaging: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(14):2523. https://doi.org/10.3390/cancers16142523
Chicago/Turabian StyleWölffer, Marcus, Rémy Liechti, Mihai Constantinescu, Ioana Lese, and Cédric Zubler. 2024. "Sentinel Lymph Node Detection in Cutaneous Melanoma Using Indocyanine Green-Based Near-Infrared Fluorescence Imaging: A Systematic Review and Meta-Analysis" Cancers 16, no. 14: 2523. https://doi.org/10.3390/cancers16142523





