Multiparametric Whole-Body MRI: A Game Changer in Metastatic Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
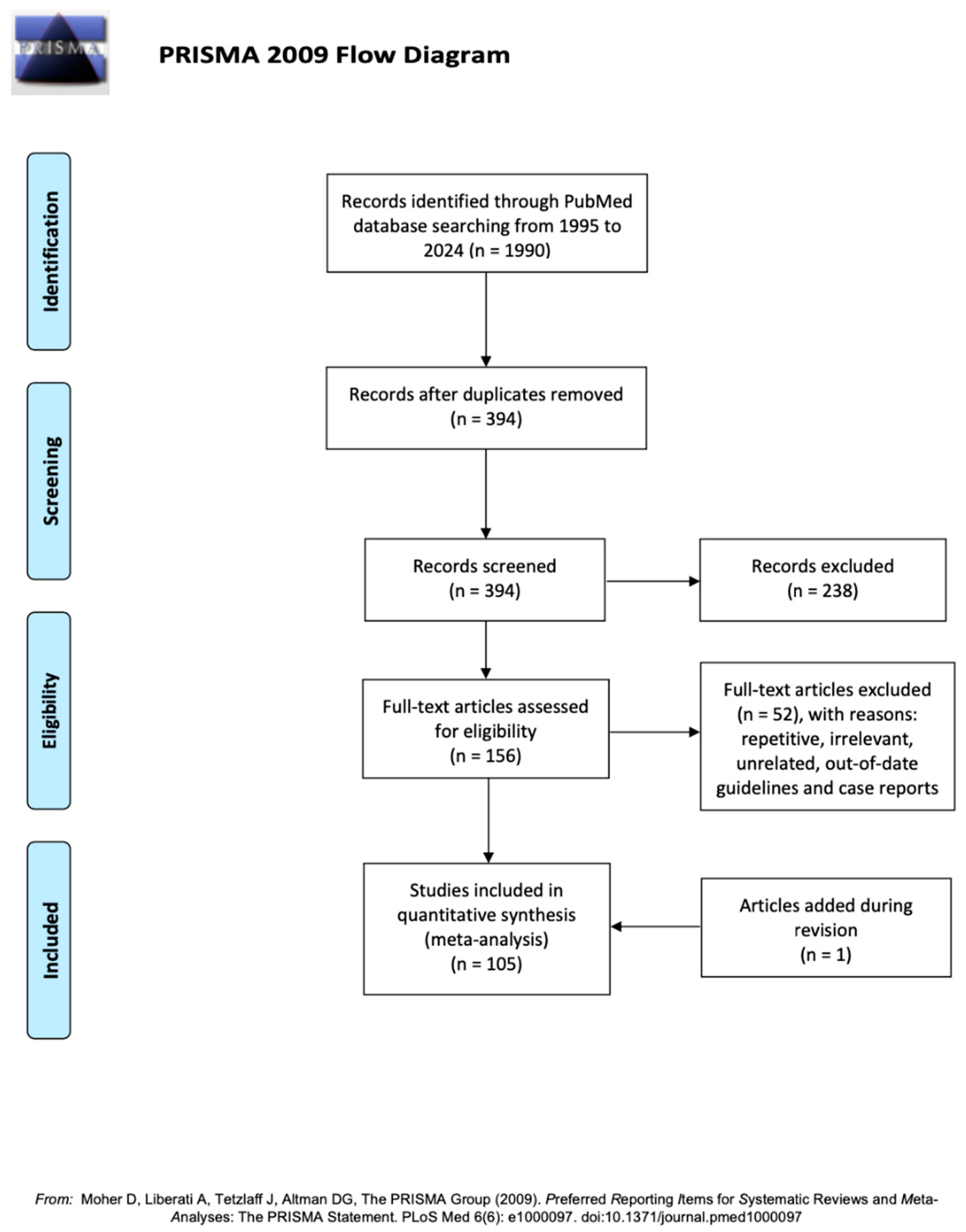
2. Methodology
3. Discussion
3.1. Guidelines in Patients with PC
3.2. History of Whole-Body MRI
3.3. Technique and Protocol of Whole-Body MRI
3.4. Imaging Features of Metastatic PC in WB-MRI
3.4.1. Evaluation of Bone Metastases with WB-MRI
3.4.2. Assessment of Nodal Disease
3.4.3. Assessment of Visceral Disease
3.4.4. Assessing Local Disease and Biochemical Recurrence
3.5. Response Assessment in Metastatic Prostate Cancer
| Morphological Patterns of Response | |||
|---|---|---|---|
| Paper | Technique | Response | Progression |
| Scher et al. J Clin Oncol 2016 (PCWG3) * [16] | CT-scan + Bone Scan |
|
|
| Lecouvet et al. European Radiology 2013 [95] | MRI (bone disease) |
|
|
| Multiparametric Patterns of Response | |||
| Padhani et al Eur Urol 2017 (MET-RADs-P) * [10] | WB-MRI |
|
|
| Messiou et al. Eur Radiol 2011 [102] | Diffusion Weighted MRI | Overall ADC of bone lesions increases both in responders and progressors, but the magnitude of increase if higher for responders. An increase in overall ADC > 25% is 75% sensitive and 66.6% specific for response. ADC alone cannot confidently assess response or progression as the changes in bone marrow composition significantly influence ADC values. | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Pettersson, A.; Graff, R.E.; Giunchi, F.; Ahearn, T.U.; Gonzalez-Feliciano, A.G.; Markt, S.C.; Wilson, K.M.; Stopsack, K.H.; et al. A Prospective Study of the Association between Physical Activity and Risk of Prostate Cancer Defined by Clinical Features and TMPRSS2:ERG. Eur. Urol. 2019, 76, 33–40. [Google Scholar] [CrossRef]
- Aihara, M.; Wheeler, T.M.; Ohori, M.; Scardino, P.T. Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology 1994, 43, 60–66, discussion 66–67. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Conteduca, V.; Poti, G.; Caroli, P.; Russi, S.; Brighi, N.; Lolli, C.; Schepisi, G.; Romeo, A.; Matteucci, F.; Paganelli, G.; et al. Flare phenomenon in prostate cancer: Recent evidence on new drugs and next generation imaging. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987654. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Padhani, A.R.; Lecouvet, F.E.; Tunariu, N.; Koh, D.-M.; De Keyzer, F.; Collins, D.J.; Sala, E.; Schlemmer, H.P.; Petralia, G.; Vargas, H.A.; et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur. Urol. 2017, 71, 81–92. [Google Scholar] [CrossRef]
- Van Nieuwenhove, S.; Van Damme, J.; Padhani, A.R.; Vandecaveye, V.; Tombal, B.; Wuts, J.; Pasoglou, V.; Lecouvet, F.E. Whole-body magnetic resonance imaging for prostate cancer assessment: Current status and future directions. J. Magn. Reson. Imaging JMRI 2022, 55, 653–680. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341.
- Lecouvet, F.E.; El Mouedden, J.; Collette, L.; Coche, E.; Danse, E.; Jamar, F.; Machiels, J.-P.; Berg, B.V.; Omoumi, P.; Tombal, B. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 2012, 62, 68–75. [Google Scholar] [CrossRef]
- Pasoglou, V.; Larbi, A.; Collette, L.; Annet, L.; Jamar, F.; Machiels, J.-P.; Michoux, N.; Berg, B.C.V.; Tombal, B.; Lecouvet, F.E. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): Toward an upfront simplified ‘all-in-one’ imaging approach? Prostate 2014, 74, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Steinborn, M.M.; Heuck, A.F.; Tiling, R.; Bruegel, M.; Gauger, L.; Reiser, M.F. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J. Comput. Assist. Tomogr. 1999, 23, 123–129. [Google Scholar] [CrossRef]
- Eustace, S.; Tello, R.; DeCarvalho, V.; Carey, J.; Melhem, E.; Yucel, E.K. Whole body turbo STIR MRI in unknown primary tumor detection. J. Magn. Reson. Imaging JMRI 1998, 8, 751–753. [Google Scholar] [CrossRef]
- Traill, Z.C.; Talbot, D.; Golding, S.; Gleeson, F.V. Magnetic resonance imaging versus radionuclide scintigraphy in screening for bone metastases. Clin. Radiol. 1999, 54, 448–451. [Google Scholar] [CrossRef]
- Lauenstein, T.C.; Freudenberg, L.S.; Goehde, S.C.; Ruehm, S.G.; Goyen, M.; Bosk, S.; Debatin, J.F.; Barkhausen, J. Whole-body MRI using a rolling table platform for the detection of bone metastases. Eur. Radiol. 2002, 12, 2091–2099. [Google Scholar] [CrossRef]
- Ghanem, N.; Lohrmann, C.; Engelhardt, M.; Pache, G.; Uhl, M.; Saueressig, U.; Kotter, E.; Langer, M. Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur. Radiol. 2006, 16, 1005–1014. [Google Scholar] [CrossRef]
- Engelhard, K.; Hollenbach, H.P.; Wohlfart, K.; von Imhoff, E.; Fellner, F.A. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur. Radiol. 2004, 14, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.-M.; Blackledge, M.; Padhani, A.R.; Takahara, T.; Kwee, T.C.; Leach, M.O.; Collins, D.J. Whole-body diffusion-weighted MRI: Tips, tricks, and pitfalls. AJR Am. J. Roentgenol. 2012, 199, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.; Franson, D.; Seiberlich, N. Recent advances in parallel imaging for MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 71–95. [Google Scholar] [CrossRef]
- Börnert, P.; Aldefeld, B. Principles of whole-body continuously-moving-table MRI. J. Magn. Reson. Imaging JMRI 2008, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.G.; Riederer, S.J.; Grimm, R.C.; Rossman, P.J. Continuously moving table data acquisition method for long FOV contrast-enhanced MRA and whole-body MRI. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med./Soc. Magn. Reson. Med. 2002, 47, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.-M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R.; On behalf of the Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Medica 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Disler, D.G.; McCauley, T.R.; Ratner, L.M.; Kesack, C.D.; Cooper, J.A. In-phase and out-of-phase MR imaging of bone marrow: Prediction of neoplasia based on the detection of coexistent fat and water. AJR Am. J. Roentgenol. 1997, 169, 1439–1447. [Google Scholar] [CrossRef]
- Douis, H.; Davies, A.M.; Jeys, L.; Sian, P. Chemical shift MRI can aid in the diagnosis of indeterminate skeletal lesions of the spine. Eur. Radiol. 2016, 26, 932–940. [Google Scholar] [CrossRef]
- Summers, P.; Saia, G.; Colombo, A.; Pricolo, P.; Zugni, F.; Alessi, S.; Marvaso, G.; Jereczek-Fossa, B.A.; Bellomi, M.; Petralia, G. Whole-body magnetic resonance imaging: Technique, guidelines and key applications. Ecancermedicalscience 2021, 15, 1164. [Google Scholar] [CrossRef]
- Lee, K.; Park, H.Y.; Kim, K.W.; Lee, A.J.; Yoon, M.A.; Chae, E.J.; Lee, J.H.; Chung, H.W. Advances in whole body MRI for musculoskeletal imaging: Diffusion-weighted imaging. J. Clin. Orthop. Trauma 2019, 10, 680–686. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Kershah, S.M. Advances in imaging of vertebral and spinal cord injury. J. Spinal Cord Med. 2010, 33, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; On behalf of the Italian Working Group on Magnetic Resonance; Padhani, A.R.; Pricolo, P.; Zugni, F.; Martinetti, M.; Summers, P.E.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: Recommendations and key uses. Radiol. Medica 2019, 124, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.P.; Reiser, M.F.; Baur-Melnyk, A. Whole-body MRI for the staging and follow-up of patients with metastasis. Eur. J. Radiol. 2009, 70, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.P.S.; Haslam, P.; Keanie, J.Y.; McCafferty, I.; Padhani, A.R.; Punwani, S.; Richenberg, J.; Rottenberg, G.; Sohaib, A.; Thompson, P.; et al. Prostate MRI: Who, when, and how? Report from a UK consensus meeting. Clin. Radiol. 2013, 68, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kassarjian, A.; Bredella, M.A.; Harris, G.J.; Yoshida, H.; Mautner, V.F.; Wenzel, R.; Plotkin, S.R. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: Determination on whole-body MR images. Radiology 2009, 250, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.L.; Best, A.; Mai, P.L.; Khincha, P.P.; Loud, J.T.; Peters, J.A.; Achatz, M.I.; Chojniak, R.; da Costa, A.B.; Santiago, K.M.; et al. Baseline Surveillance in Li-Fraumeni Syndrome Using Whole-Body Magnetic Resonance Imaging: A Meta-analysis. JAMA Oncol. 2017, 3, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Hillengass, J.; Delorme, S.; Lecouvet, F.E.; Moulopoulos, L.A.; Collins, D.J.; Blackledge, M.D.; Abildgaard, N.; Østergaard, B.; Schlemmer, H.-P.; et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 2019, 291, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Guo, H.; Chen, H.; Guo, D.; Huang, F.; Xu, Q.; Qu, X. Phase-constrained reconstruction of high-resolution multi-shot diffusion weighted image. J. Magn. Reson. 2020, 312, 106690. [Google Scholar] [CrossRef] [PubMed]
- Rata, M.; Blackledge, M.; Scurr, E.; Winfield, J.; Koh, D.-M.; Dragan, A.; Candito, A.; King, A.; Rennie, W.; Gaba, S.; et al. Implementation of Whole-Body MRI (MY-RADS) within the OPTIMUM/MUKnine multi-centre clinical trial for patients with myeloma. Insights Imaging 2022, 13, 123. [Google Scholar] [CrossRef]
- Johnston, E.W.; Latifoltojar, A.; Sidhu, H.S.; Ramachandran, N.; Sokolska, M.; Bainbridge, A.; Moore, C.; Ahmed, H.U.; Punwani, S. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate- and high-risk prostate cancer: Diagnostic accuracy and interobserver agreement for nodal and metastatic staging. Eur. Radiol. 2019, 29, 3159–3169. [Google Scholar] [CrossRef]
- Padhani, A.R.; Tunariu, N. Metastasis Reporting and Data System for Prostate Cancer in Practice. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Prochowski Iamurri, A.; Diano, D.; Oboldi, D.; Sintuzzi, E.; Maurizio, L.; Andalò, A.; Cavallucci, M.; Ferroni, F.; Amadori, E.; et al. Patient centered radiology: Investigating 3 Tesla whole body MRI acceptance in cancer patients. Radiol. Medica 2023, 128, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, I.; Glocker, B.; Kamnitsas, K.; Rueckert, D.; Mair, H.; Sandhu, A.; Taylor, S.A.; Aboagye, E.O.; Rockall, A.G. Fully automatic, multiorgan segmentation in normal whole body magnetic resonance imaging (MRI), using classification forests (CFs), convolutional neural networks (CNNs), and a multi-atlas (MA) approach. Med. Phys. 2017, 44, 5210–5220. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.K.; Park, B.K.; Kwon, G.Y. Comparison of apparent diffusion coefficient calculation between two-point and multipoint B value analyses in prostate cancer and benign prostate tissue at 3 T: Preliminary experience. AJR Am. J. Roentgenol. 2014, 203, W287–W294. [Google Scholar] [CrossRef] [PubMed]
- Pricolo, P.; Ancona, E.; Summers, P.; Abreu-Gomez, J.; Alessi, S.; Jereczek-Fossa, B.A.; De Cobelli, O.; Nolè, F.; Renne, G.; Bellomi, M.; et al. Whole-body magnetic resonance imaging (WB-MRI) reporting with the METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P): Inter-observer agreement between readers of different expertise levels. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2020, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, J.; Tombal, B.; Collette, L.; Van Nieuwenhove, S.; Pasoglou, V.; Gérard, T.; Jamar, F.; Lhommel, R.; Lecouvet, F.E. Comparison of Ga-Prostate Specific Membrane Antigen (PSMA) Positron Emission Tomography Computed Tomography (PET-CT) and Whole-Body Magnetic Resonance Imaging (WB-MRI) with Diffusion Sequences (DWI) in the Staging of Advanced Prostate Cancer. Cancers 2021, 13, 5286. [Google Scholar] [CrossRef] [PubMed]
- Curcean, A.; Curcean, S.; Rescigno, P.; Dafydd, D.A.; Tree, A.; Reid, A.; Koh, D.-M.; Sohaib, A.; Tunariu, N.; Shur, J. Imaging features of the evolving patterns of metastatic prostate cancer. Clin. Radiol. 2022, 77, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Donners, R.; Yiin, R.S.Z.; Blackledge, M.; Koh, D.-M. Whole-body diffusion-weighted MRI of normal lymph nodes: Prospective apparent diffusion coefficient histogram and nodal distribution analysis in a healthy cohort. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2021, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.H.; Ferraro, D.A.; Muehlematter, U.J.; Laudicella, R.; Hermanns, T.; Rodewald, A.-K.; Moch, H.; Eberli, D.; Burger, I.A.; Rupp, N.J. What’s behind 68Ga-PSMA-11 uptake in primary prostate cancer PET? Investigation of histopathological parameters and immunohistochemical PSMA expression patterns. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4042–4053. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Yang, H.-L.; Liu, T.; Wang, X.-M.; Xu, Y.; Deng, S.-M. Diagnosis of bone metastases: A meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur. Radiol. 2011, 21, 2604–2617. [Google Scholar] [CrossRef]
- Dotan, Z.A. Bone imaging in prostate cancer. Nat. Clin. Pract. Urol. 2008, 5, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, R.; Kato, S.; Koyama, H.; Ishida, M.; Kurokawa, M.; Kuroda, R.; Ushiku, T.; Kume, H.; Abe, O. Osteolytic or mixed bone metastasis is not uncommon in patients with high-risk prostate cancer. Eur. J. Radiol. 2022, 157, 110595. [Google Scholar] [CrossRef]
- Acar, E.; Leblebici, A.; Ellidokuz, B.E.; Başbınar, Y.; Kaya, G.Ç. Machine learning for differentiating metastatic and completely responded sclerotic bone lesion in prostate cancer: A retrospective radiomics study. Br. J. Radiol. 2019, 92, 20190286. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Madewell, J.E.; Podoloff, D.A.; Hortobagyi, G.N.; Ueno, N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2942–2953. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, G.J.; Carty, F.L.; Cronin, C.G. Imaging of bone metastasis: An update. World J. Radiol. 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Jacobsson, H.; Hatschek, T.; Torkzad, M.R.; Bodén, K.; Eriksson-Alm, Y.; Berg, E.; Fujii, H.; Kubo, A.; Blomqvist, L. Radiologic measurements of tumor response to treatment: Practical approaches and limitations. Radiogr. A Rev. Publ. Radiol. Soc. N. Am. Inc 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Heindel, W.; Gübitz, R.; Vieth, V.; Weckesser, M.; Schober, O.; Schäfers, M. The diagnostic imaging of bone metastases. Dtsch. Arztebl. Int. 2014, 111, 741–747. [Google Scholar] [CrossRef]
- Rydh, A.; Lundblad, M.; Ahlström, K.R.; Tavelin, B.; Stattin, P. MRI of the skeleton in prostate cancer staging. Scand. J. Urol. Nephrol. 2003, 37, 222–225. [Google Scholar] [CrossRef]
- Koh, D.-M.; Takahara, T.; Imai, Y.; Collins, D.J. Practical aspects of assessing tumors using clinical diffusion-weighted imaging in the body. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2007, 6, 211–224. [Google Scholar] [CrossRef]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Koh, D.-M.; Collins, D.J. Whole-body diffusion-weighted MR imaging in cancer: Current status and research directions. Radiology 2011, 261, 700–718. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.A.; Macura, K.J.; Zaheer, A.; Antonarakis, E.S.; Stearns, V.; Wolff, A.C.; Feiweier, T.; Kamel, I.R.; Wahl, R.L.; Pan, L. Multiparametric Whole-body MRI with Diffusion-weighted Imaging and ADC Mapping for the Identification of Visceral and Osseous Metastases from Solid Tumors. Acad. Radiol. 2018, 25, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Donners, R.; Figueiredo, I.; Tunariu, N.; Blackledge, M.; Koh, D.-M.; de los, M.; de la Maza, D.F.; Chandran, K.; de Bono, J.S.; Fotiadis, N. Multiparametric bone MRI can improve CT-guided bone biopsy target selection in cancer patients and increase diagnostic yield and feasibility of next-generation tumour sequencing. Eur. Radiol. 2022, 32, 4647–4656. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yoshida, S.; Ishii, C.; Takahara, T.; Arita, Y.; Fukushima, H.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; Fujii, Y. Metastatic Diffusion Volume Based on Apparent Diffusion Coefficient as a Prognostic Factor in Castration-Resistant Prostate Cancer. J. Magn. Reson. Imaging JMRI 2021, 54, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, F.; Donners, R.; Tunariu, N.; Messiou, C.; Koh, D.-M. Relative fat fraction of malignant bone lesions from breast cancer, prostate cancer and myeloma are significantly lower than normal bone marrow and shows excellent interobserver agreement. Br. J. Radiol. 2023, 96, 20230240. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Hong, S.H.; Kim, D.H.; Choi, J.-Y.; Chae, H.D.; Jeong, B.M.; Ahn, J.M.; Kang, H.S. Measurement of fat content in vertebral marrow using a modified dixon sequence to differentiate benign from malignant processes. J. Magn. Reson. Imaging JMRI 2017, 45, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Donners, R.; Obmann, M.M.; Boll, D.; Gutzeit, A.; Harder, D. Dixon or DWI—Comparing the utility of fat fraction and apparent diffusion coefficient to distinguish between malignant and acute osteoporotic vertebral fractures. Eur. J. Radiol. 2020, 132, 109342. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Morbelli, S.; Albano, D.; Fornarini, G.; Cioffi, M.; Laudicella, R.; Dondi, F.; Grimaldi, S.; Bertagna, F.; Racca, M.; et al. The Homunculus of unspecific bone uptakes associated with PSMA-targeted tracers: A systematic review-based definition. Eur. J. Nucl. Med. Mol. Imaging 2021. ahead of printing. [Google Scholar] [PubMed]
- Farolfi, A.; Hadaschik, B.; Hamdy, F.C.; Herrmann, K.; Hofman, M.S.; Murphy, D.G.; Ost, P.; Padhani, A.R.; Fanti, S. Positron Emission Tomography and Whole-body Magnetic Resonance Imaging for Metastasis-directed Therapy in Hormone-sensitive Oligometastatic Prostate Cancer after Primary Radical Treatment: A Systematic Review. Eur. Urol. Oncol. 2021, 4, 714–730. [Google Scholar] [CrossRef]
- Schiavina, R.; Bianchi, L.; Mineo Bianchi, F.; Borghesi, M.; Pultrone, C.V.; Dababneh, H.; Castellucci, P.; Ceci, F.; Nanni, C.; Gaudiano, C.; et al. Preoperative Staging with C-Choline PET/CT Is Adequately Accurate in Patients with Very High-Risk Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, 305–312.e1. [Google Scholar] [CrossRef]
- Zanoni, L.; Bianchi, L.; Nanni, C.; Pultrone, C.; Giunchi, F.; Bossert, I.; Matti, A.; Schiavina, R.; Fiorentino, M.; Romagnoli, D.; et al. [18F]-Fluciclovine PET/CT for preoperative nodal staging in high-risk primary prostate cancer: Final results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 390–409. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, S.; Jochumsen, M.R.; Ulhøi, B.P.; Fredsøe, J.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. Ga-PSMA PET/CT for Primary Lymph Node and Distant Metastasis NM Staging of High-Risk Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 214–220. [Google Scholar]
- Esen, T.; Falay, O.; Tarim, K.; Armutlu, A.; Koseoglu, E.; Kilic, M.; Seymen, H.; Sarikaya, A.F.; Kiremit, M.C.; Balbay, M.D.; et al. Ga-PSMA-11 Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging before Radical Prostatectomy: Central Review of Imaging and Comparison with Histopathology of Extended Lymphadenectomy. Eur. Urol. Focus 2021, 7, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Mytsyk, Y.O.; Pasichnyk, S.M.; Kobilnyk, Y.S.; Borzhiievskyi, O.A.; Lychkovskyy, O.E.; Kowal, P.; Pietrus, M.; Matskevych, V.M.; I Dats, R.; Blavatska, O.M.; et al. SIGNIFICANCE OF ADC MEASUREMENTS AS RADIOLOGICAL MRI MARKER IN DETECTION OF METASTATIC LYMPH NODE INVOLVEMENT IN PATIENTS WITH PROSTATE CANCER. Exp. Oncol. 2022, 44, 142–147. [Google Scholar] [CrossRef]
- Yoshida, S.; Takahara, T.; Arita, Y.; Sakaino, S.; Katahira, K.; Fujii, Y. Whole-body diffusion-weighted magnetic resonance imaging: Diagnosis and follow up of prostate cancer and beyond. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2021, 28, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Beer, A.J.; Holzapfel, K.; Tauber, R.; Ganter, C.; Weirich, G.; Krause, B.J.; Rummeny, E.J.; Gaa, J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Investig. Radiol. 2010, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.J.; Eiber, M.; Souvatzoglou, M.; Holzapfel, K.; Ganter, C.; Weirich, G.; Maurer, T.; Kübler, H.; Wester, H.-J.; Gaa, J.; et al. Restricted water diffusibility as measured by diffusion-weighted MR imaging and choline uptake in (11)C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2011, 13, 352–361. [Google Scholar]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men with Castration-Resistant Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, S.; Anttinen, M.; Taimen, P.; Jambor, I.; Sandell, M.; Rinta-Kiikka, I.; Kajander, S.; Schildt, J.; Saukko, E.; Noponen, T.; et al. Prospective comparison of F-PSMA-1007 PET/CT, whole-body MRI and CT in primary nodal staging of unfavourable intermediate- and high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2951–2959. [Google Scholar] [CrossRef]
- Adeleke, S.; Latifoltojar, A.; Sidhu, H.; Galazi, M.; Shah, T.T.; Clemente, J.; Davda, R.; Payne, H.A.; Chouhan, M.D.; Lioumi, M.; et al. Localising occult prostate cancer metastasis with advanced imaging techniques (LOCATE trial): A prospective cohort, observational diagnostic accuracy trial investigating whole-body magnetic resonance imaging in radio-recurrent prostate cancer. BMC Med. Imaging 2019, 19, 90. [Google Scholar] [CrossRef]
- Ceci, F.; Bianchi, L.; Borghesi, M.; Polverari, G.; Farolfi, A.; Briganti, A.; Schiavina, R.; Brunocilla, E.; Castellucci, P.; Fanti, S. Prediction nomogram for Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Castellucci, P.; Farolfi, A.; Droghetti, M.; Artigas, C.; Leite, J.; Corona, P.; Shagera, Q.A.; Moreira, R.; González, C.; et al. Multicenter External Validation of a Nomogram for Predicting Positive Prostate-specific Membrane Antigen/Positron Emission Tomography Scan in Patients with Prostate Cancer Recurrence. Eur. Urol. Oncol. 2023, 6, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Kirchner, J.; Buddensieck, C.; Antke, C.; Ullrich, T.; Schimmöller, L.; Boos, J.; Schleich, C.; Schaarschmidt, B.M.; Buchbender, C.; et al. Prospective comparison of whole-body MRI and Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Chua, S.; Ho, B.; Punwani, S.; Johnston, E.; Pouliot, F.; Tau, N.; Hawsawy, A.; Anconina, R.; Bauman, G.; et al. The Contribution of Multiparametric Pelvic and Whole-Body MRI to Interpretation of F-Fluoromethylcholine or Ga-HBED-CC PSMA-11 PET/CT in Patients with Biochemical Failure After Radical Prostatectomy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 1253–1258. [Google Scholar]
- Robertson, N.L.; Sala, E.; Benz, M.; Landa, J.; Scardino, P.; Scher, H.I.; Hricak, H.; Vargas, H.A. Combined Whole Body and Multiparametric Prostate Magnetic Resonance Imaging as a 1-Step Approach to the Simultaneous Assessment of Local Recurrence and Metastatic Disease after Radical Prostatectomy. J. Urol. 2017, 198, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Jannusch, K.; Bruckmann, N.M.; Morawitz, J.; Boschheidgen, M.; Quick, H.H.; Herrmann, K.; Fendler, W.P.; Umutlu, L.; Stuschke, M.; Hadaschik, B.; et al. Recurrent prostate cancer: Combined role for MRI and PSMA-PET in 68Ga-PSMA-11 PET/MRI. Eur Radiol. 2024, 34, 4789–4800. [Google Scholar] [CrossRef]
- Eiber, M.; Holzapfel, K.; Ganter, C.; Epple, K.; Metz, S.; Geinitz, H.; Kübler, H.; Gaa, J.; Rummeny, E.J.; Beer, A.J. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: Technical feasibility and assessment of lesion conspicuity in DWI. J. Magn. Reson. Imaging JMRI 2011, 33, 1160–1170. [Google Scholar] [CrossRef]
- Jambor, I.; Kuisma, A.; Ramadan, S.; Huovinen, R.; Sandell, M.; Kajander, S.; Kemppainen, J.; Kauppila, E.; Auren, J.; Merisaari, H.; et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016, 55, 59–67. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; de Wit, R.; Eisenberger, M.; Tannock, A.I.F. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef]
- Halabi, S.; Armstrong, A.J.; Sartor, O.; de Bono, J.; Kaplan, E.; Lin, C.-Y.; Solomon, N.C.; Small, E.J. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3944–3950. [Google Scholar] [CrossRef]
- Lecouvet, F.E.; Larbi, A.; Pasoglou, V.; Omoumi, P.; Tombal, B.; Michoux, N.; Malghem, J.; Lhommel, R.; Berg, B.C.V. MRI for response assessment in metastatic bone disease. Eur. Radiol. 2013, 23, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Vande Berg, B.C.; Lecouvet, F.E.; Galant, C.; Maldague, B.E.; Malghem, J. Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging. Radiol. Clin. N. Am. 2005. [CrossRef] [PubMed]
- Ryan, S.P.; Weinberger, E.; White, K.S.; Shaw, D.W.; Patterson, K.; Nazar-Stewart, V.; Miser, J.; Ryan, E.W.S.P.; Hartman, R.P.; Sundaram, M.; et al. MR imaging of bone marrow in children with osteosarcoma: Effect of granulocyte colony-stimulating factor. AJR Am. J. Roentgenol. 1995, 165, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-S.; Li, W.-H.; Li, M.-H.; Meng, X.; Kong, L.I.; Yu, J.-M. False-positive diagnosis of disease progression by magnetic resonance imaging for response assessment in prostate cancer with bone metastases: A case report and review of the pitfalls of images in the literature. Oncol. Lett. 2015, 10, 3585–3590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Padhani, A.R.; Koh, D.-M. Diffusion MR imaging for monitoring of treatment response. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Charles-Edwards, E.M.; deSouza, N.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2006, 6, 135–143. [Google Scholar] [CrossRef]
- Reischauer, C.; Froehlich, J.M.; Koh, D.-M.; Graf, N.; Padevit, C.; John, H.; Binkert, C.A.; Boesiger, P.; Gutzeit, A. Bone metastases from prostate cancer: Assessing treatment response by using diffusion-weighted imaging and functional diffusion maps--initial observations. Radiology 2010, 257, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Collins, D.J.; Giles, S.; de Bono, J.S.; Bianchini, D.; de Souza, N.M. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur. Radiol. 2011, 21, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, R.; Mateo, J.; Mossop, H.; Blackledge, M.D.; Collins, D.J.; Rata, M.; Morgan, V.A.; Macdonald, A.; Sandhu, S.; Lorente, D.; et al. Diffusion-weighted Imaging as a Treatment Response Biomarker for Evaluating Bone Metastases in Prostate Cancer: A Pilot Study. Radiology 2017, 283, 168–177. [Google Scholar] [CrossRef]
- Blackledge, M.D.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Leach, M.O.; Koh, D.-M. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: A feasibility study. PLoS ONE 2014, 9, e91779. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Debus, N.; Uhrig, M.; Hope, T.A.; Evans, M.J.; Holland-Letz, T.; Giesel, F.L.; Kopka, K.; Hadaschik, B.; Kratochwil, C.; et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Truillet, C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef] [PubMed]

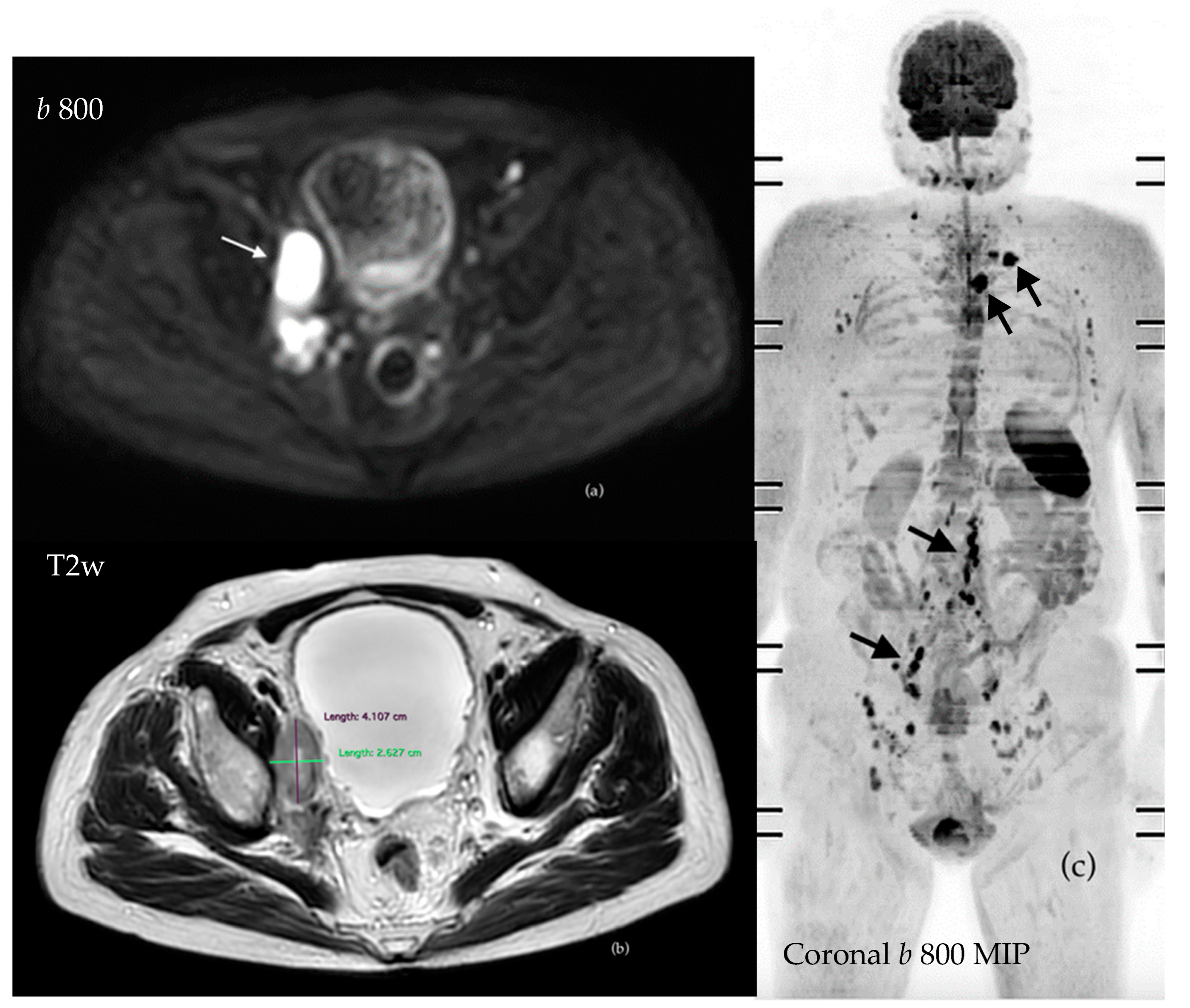
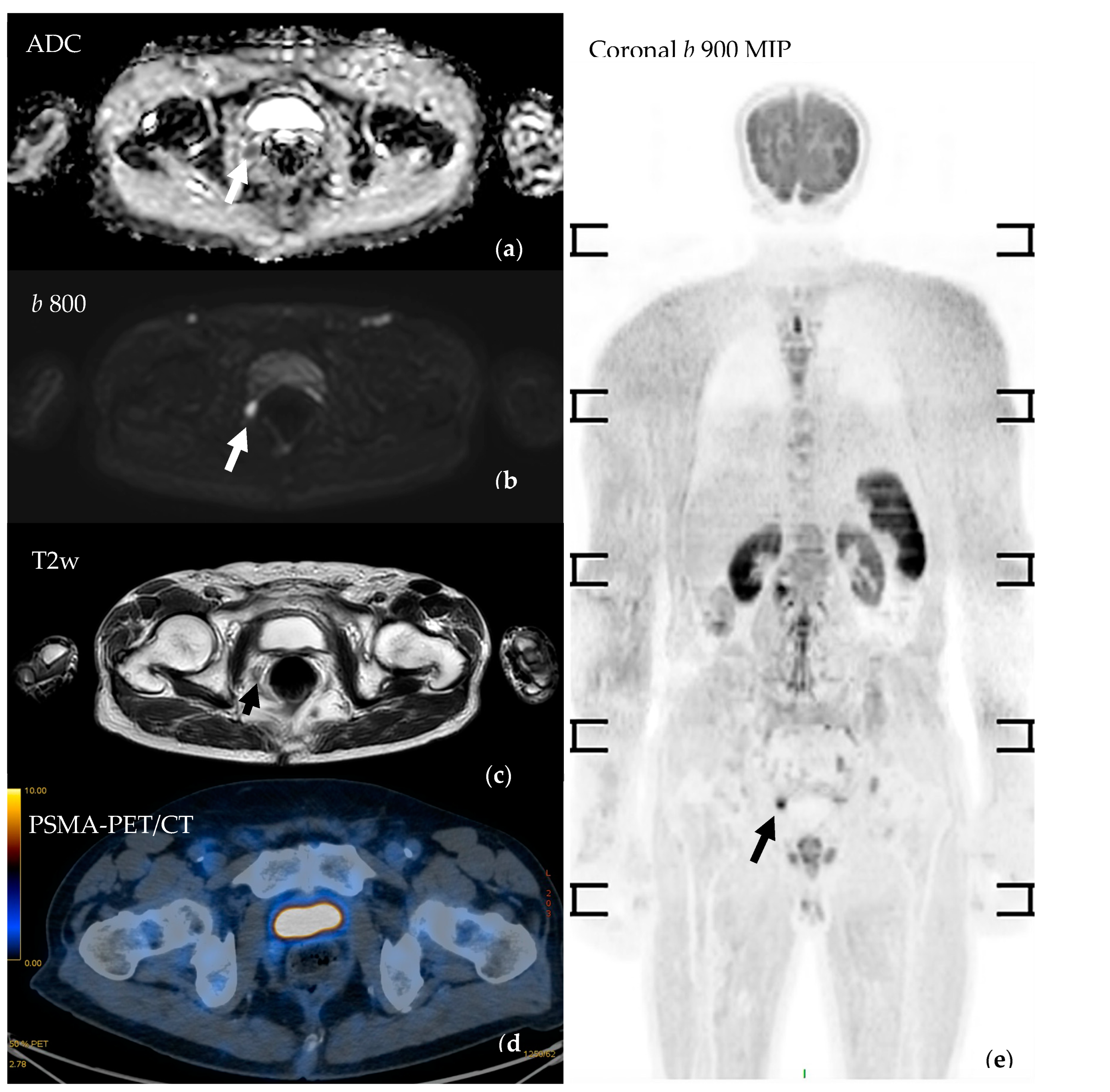
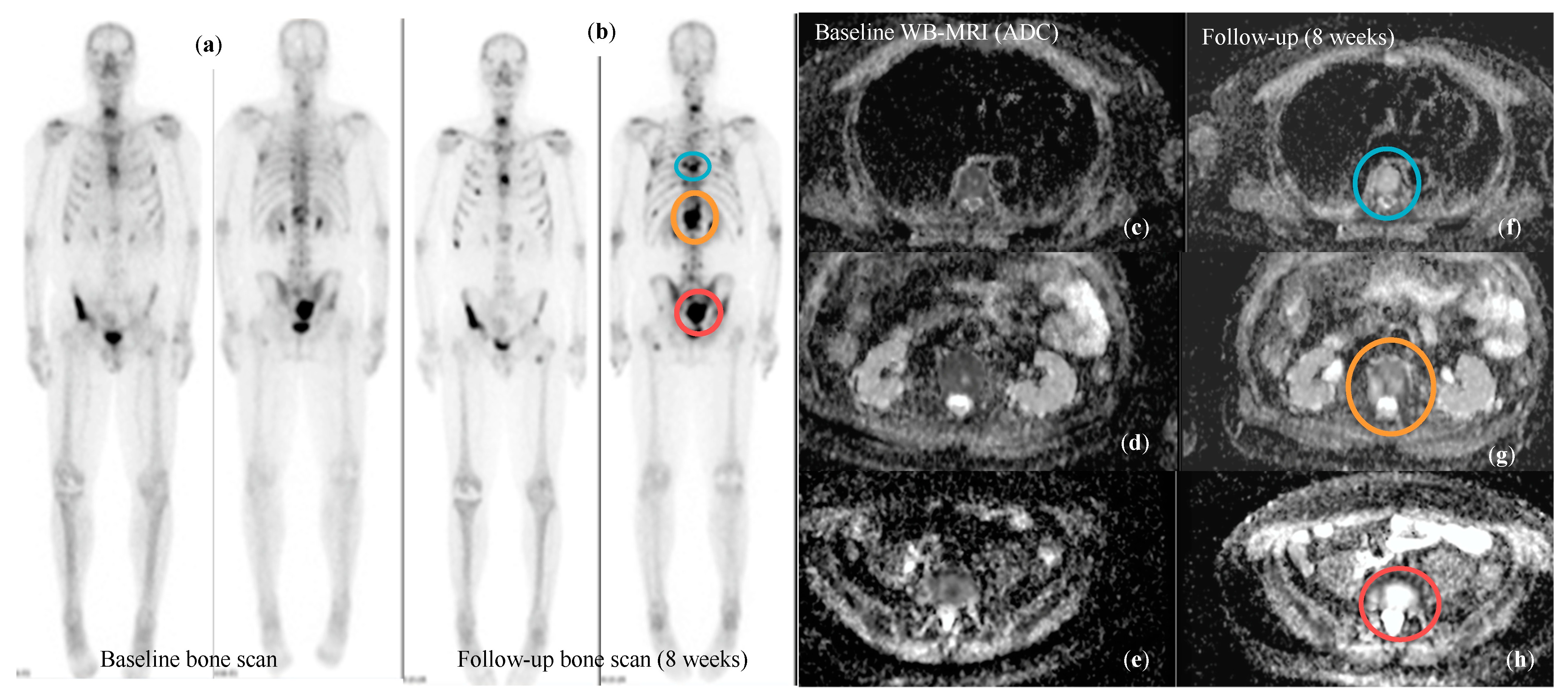
| Study | Population | WB-MRI | Standard Techniques | Comments/Conclusions | |||
|---|---|---|---|---|---|---|---|
| Sens % | Spec % | Technique | Sens % | Spec % | |||
| Lecouvet et al. European Radiology 2012 [14] | 100 high risk PCa patients | 98–100 | 98–100 | BS ± X-ray | 86 | 98 | WB-MRI shows higher sensitivity and similar specificity compared to the combination of BS ± X-ray for the detection of bone metastases |
| Pasoglou et al. The Prostate 2014 [15] | 30 high risk PCa patients | 100 | 100 | BS ± X-ray | 89 | 90 | Comparison of AUC shows no significant difference between WB-MRI & BS ± X-ray. However, WB-MRI is significantly better for Global Metastatic Status (Bone + Node) Sens% = 100 vs. 85 |
| Johnston et al. Eur Radiol 2019 [43] | 56 PCa patients only 33 18F-choline PET/CT | 90 | 88 | BS | 60 | 100 | WB-MRI outperforms BS for detecting bone lesions, while is comparable to 18F-choline PET/CT. |
| 18F-choline PET/CT | 80 | 92 | |||||
| Study | Patients Enrolled | WB-MRI | Compared Technique | Comments/Conclusions | |||
|---|---|---|---|---|---|---|---|
| Sens % | Spec % | Technique | Sens % | Spec % | |||
| Lecouvet et al. European Radiology 2012 [14] | 100 high risk PCa patients | 77–82 | 96–98 | CT-scan | 77–82 | 95–96 | WB-MRI and CT-scan show similar performancesfor detecting of enlarged lymph nodes |
| Pasoglou et al. The Prostate 2014 [15] | 30 high risk PCa patients | 100 | 100% | CT-scan | 82 | 100 | Comparison of AUC shows no significant difference between WB-MRI & CT-scan. However, WB-MRI is significantly better for Global Metastatic Status (Bone + Node) Sens = 100% vs. 85% |
| Malaspina et al. Eur J Nucl Med Mol Imaging 2021 [83] | 78 PCa patients | 40–50 | 96–91 | PSMA-PET/CT | 84–90 | 94–96 | PSMA-PET/CT outperforms WB-MRI for the detection of pathologic lymph nodes. 74% of metastatic lymph nodes, confirmed by histological examination, had a short axis < 8 mm |
| Johnston et al. Eur Radiol 2019 [43] | 33 PCa patients | 100 | 96 | 18F-choline PET/CT | 100 | 82 | WB-MRI & 18F-choline PET/CT have similar diagnostic performance for detecting nodal disease (however small cohort) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattabriga, A.; Renzetti, B.; Galuppi, F.; Bartalena, L.; Gaudiano, C.; Brocchi, S.; Rossi, A.; Schiavina, R.; Bianchi, L.; Brunocilla, E.; et al. Multiparametric Whole-Body MRI: A Game Changer in Metastatic Prostate Cancer. Cancers 2024, 16, 2531. https://doi.org/10.3390/cancers16142531
Cattabriga A, Renzetti B, Galuppi F, Bartalena L, Gaudiano C, Brocchi S, Rossi A, Schiavina R, Bianchi L, Brunocilla E, et al. Multiparametric Whole-Body MRI: A Game Changer in Metastatic Prostate Cancer. Cancers. 2024; 16(14):2531. https://doi.org/10.3390/cancers16142531
Chicago/Turabian StyleCattabriga, Arrigo, Benedetta Renzetti, Francesco Galuppi, Laura Bartalena, Caterina Gaudiano, Stefano Brocchi, Alice Rossi, Riccardo Schiavina, Lorenzo Bianchi, Eugenio Brunocilla, and et al. 2024. "Multiparametric Whole-Body MRI: A Game Changer in Metastatic Prostate Cancer" Cancers 16, no. 14: 2531. https://doi.org/10.3390/cancers16142531
APA StyleCattabriga, A., Renzetti, B., Galuppi, F., Bartalena, L., Gaudiano, C., Brocchi, S., Rossi, A., Schiavina, R., Bianchi, L., Brunocilla, E., Spinozzi, L., Catanzaro, C., Castellucci, P., Farolfi, A., Fanti, S., Tunariu, N., & Mosconi, C. (2024). Multiparametric Whole-Body MRI: A Game Changer in Metastatic Prostate Cancer. Cancers, 16(14), 2531. https://doi.org/10.3390/cancers16142531








