Exploring Canine Mammary Cancer through Liquid Biopsy: Proteomic Profiling of Small Extracellular Vesicles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. Liquid Biopsy
2.3. Tissue Samples (Tumor Fragment) and Histopathology
2.4. Exosomes Isolation
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Transmission Electron Microscopy (TEM)
2.7. Proteomics Analysis
2.8. Statistical Analysis
3. Results
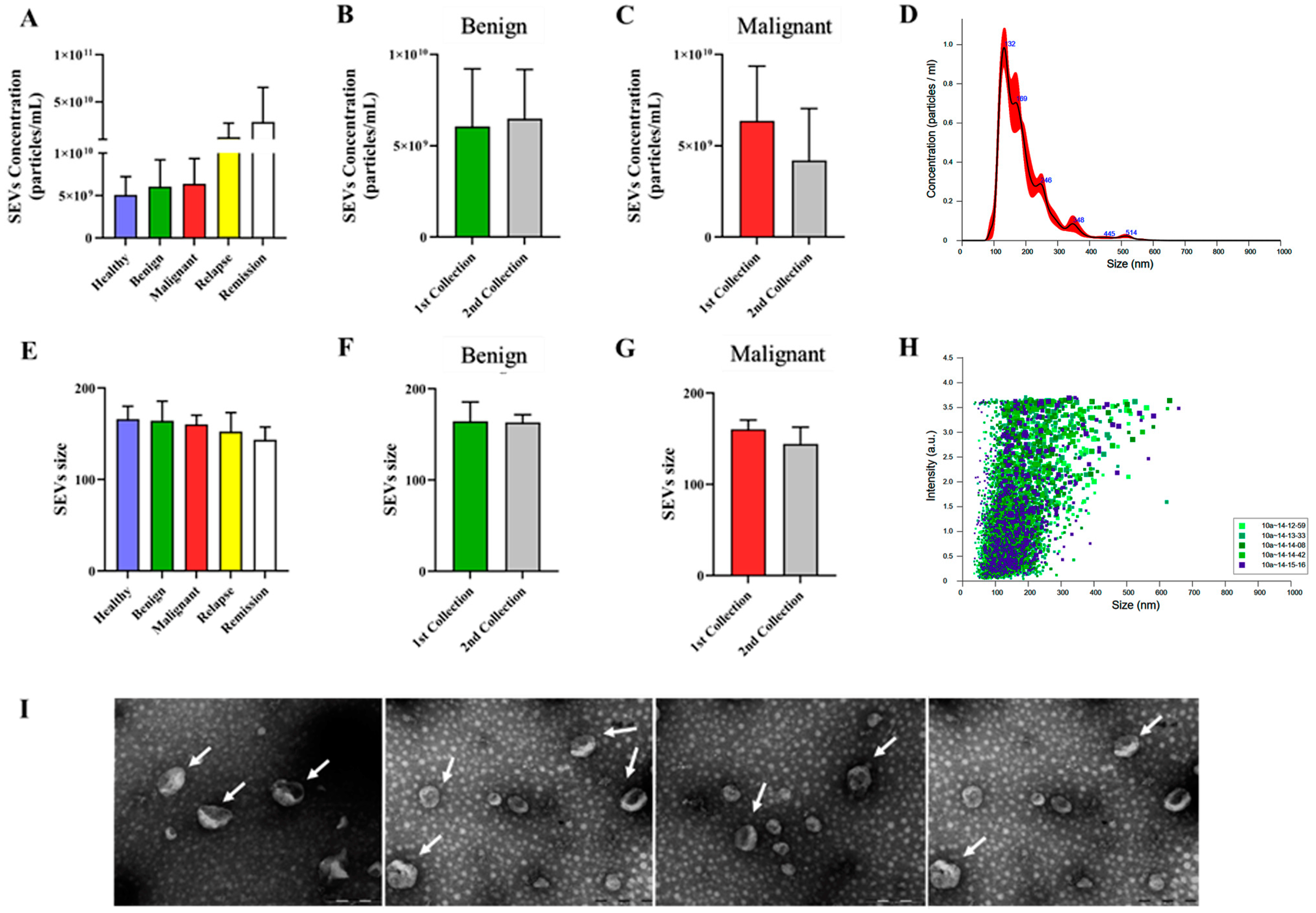
3.1. Extracellular Vesicles Isolated from Canine Plasma Exhibited Characteristics Typical of Small Extracellular Vesicles (SEVs), with Consistent Concentration and Size across Different Groups
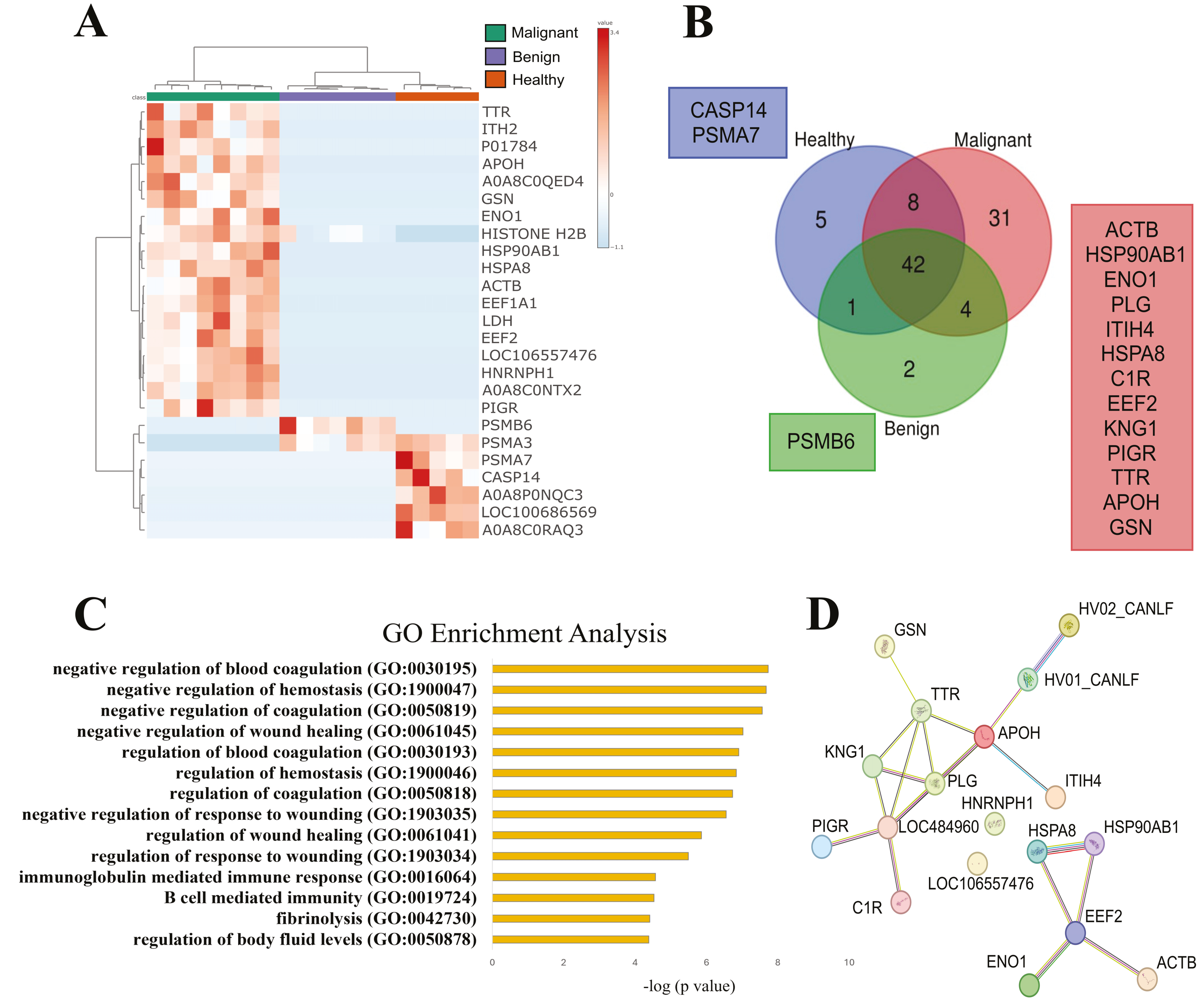
3.2. SEVs’ Proteomic Profile from Dogs with Malignant Tumors Exhibit a Larger Number of Unique Proteins, Most of Them Associated with Immune and Wound Healing Processes
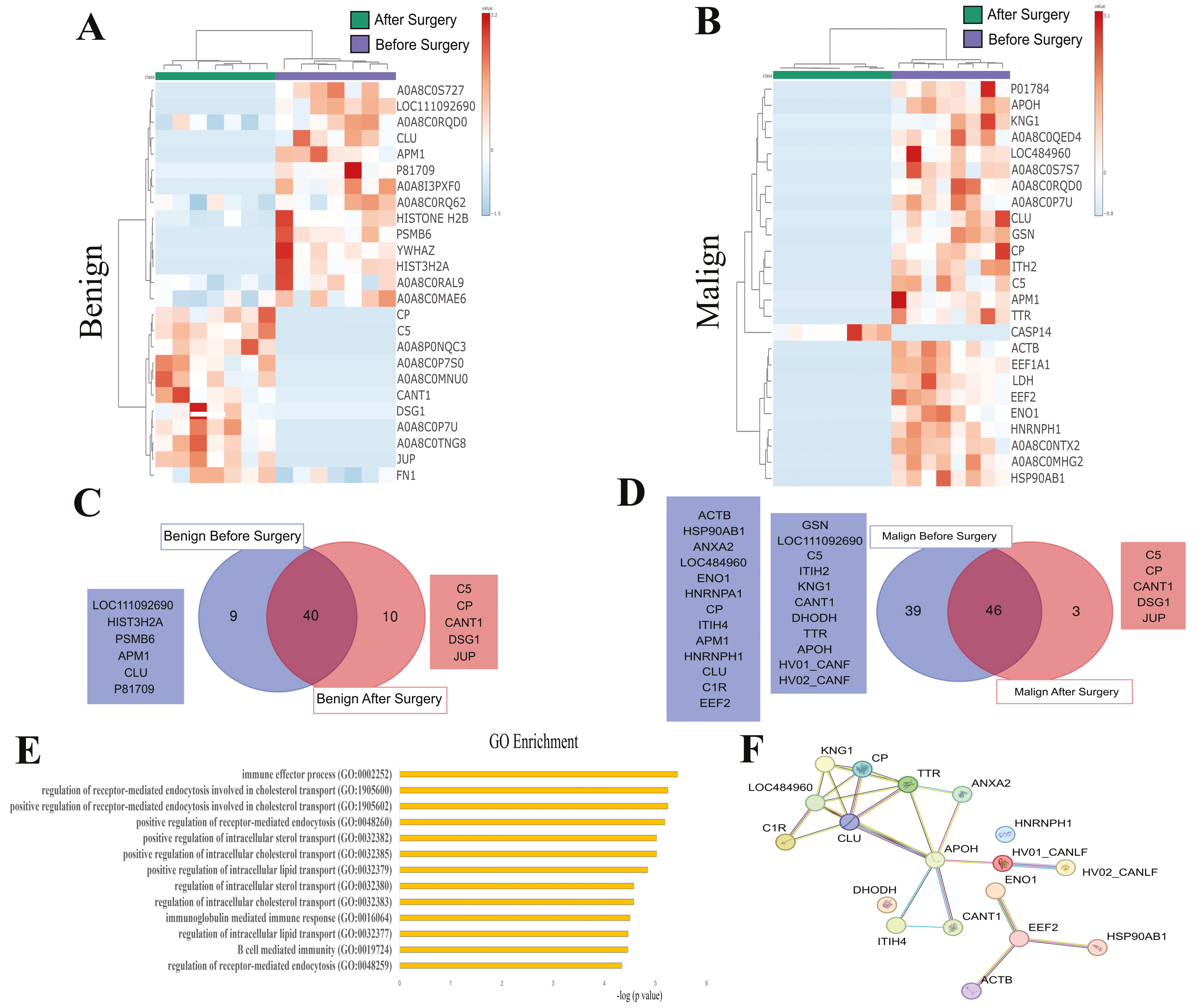
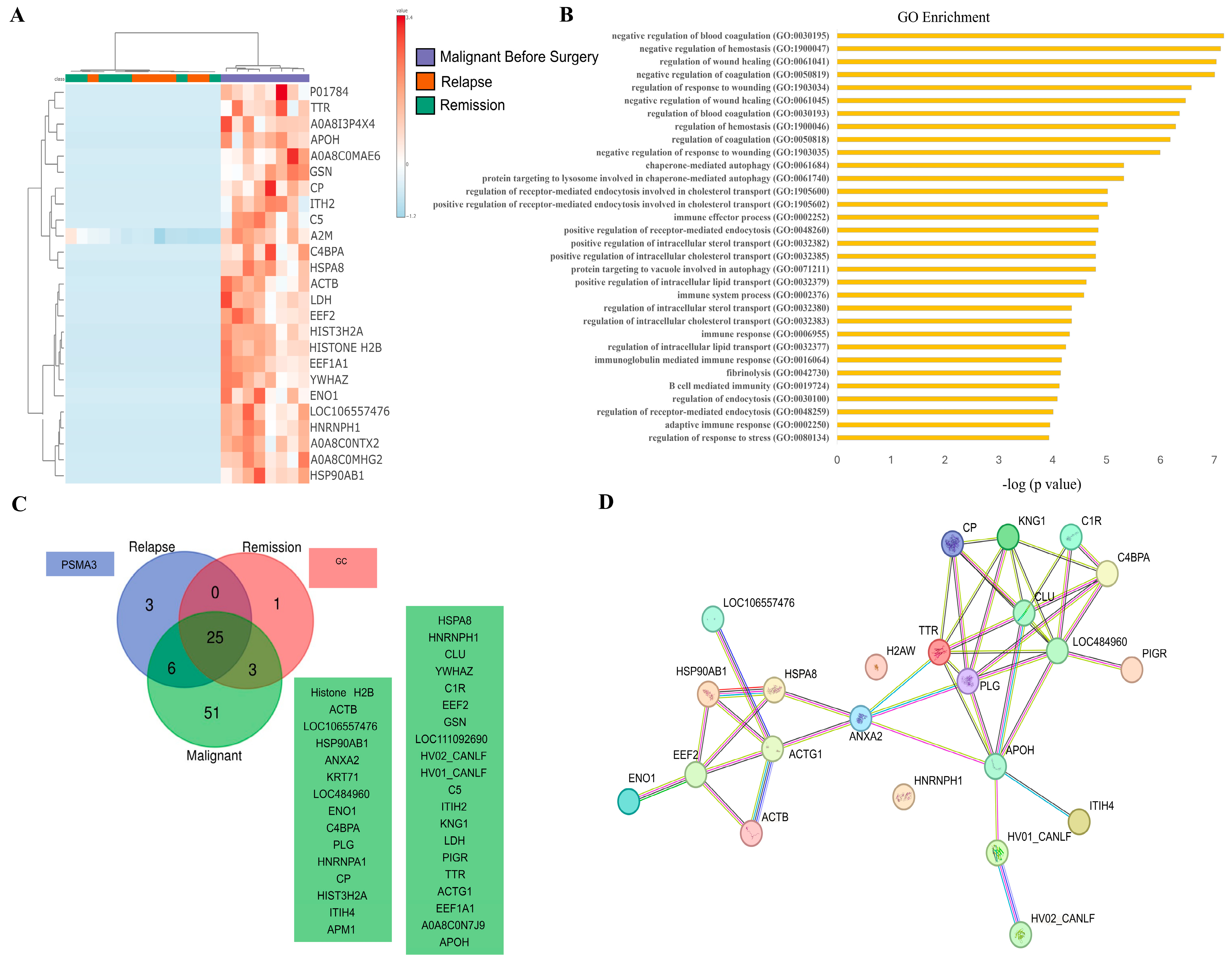
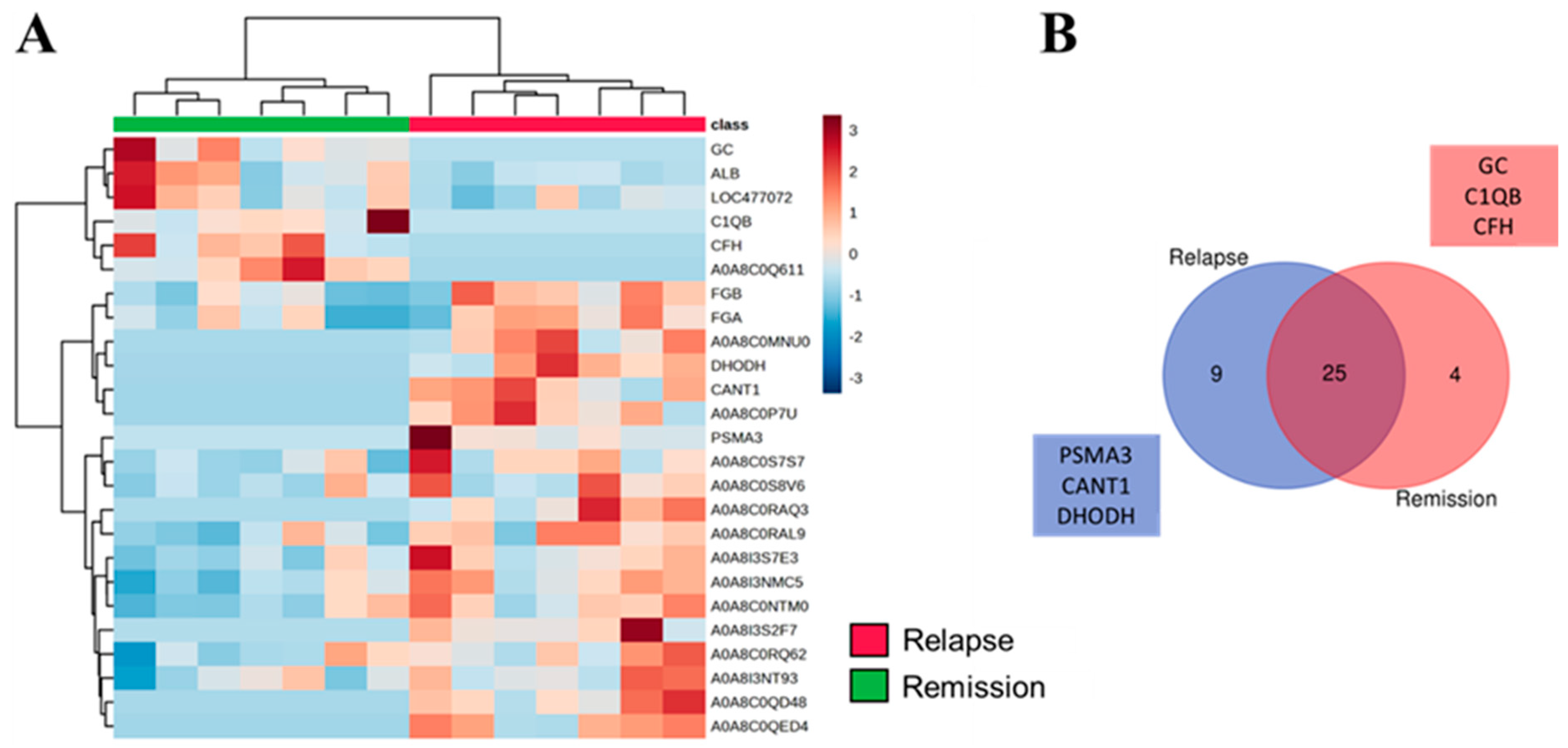
3.3. SEV’s Cargo from Dogs with Malignant Tumor Decreases after Mastectomy, and This Trend Persists Even after Surgery in Cases of Remission or Relapse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Li, W.; Chen, C.-S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Moskwa, N.; Kang, W.; Fan, T.M.; Lee, C. Canine as a comparative and translational model for human mammary tumor. J. Breast Cancer 2023, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Neves Rebello Alves, L.; Dummer Meira, D.; Poppe Merigueti, L.; Correia Casotti, M.; do Prado Ventorim, D.; Ferreira Figueiredo Almeida, J.; Pereira de Sousa, V.; Cindra Sant’Ana, M.; Gonçalves Coutinho da Cruz, R.; Santos Louro, L.; et al. Biomarkers in Breast Cancer: An Old Story with a New End. Genes 2023, 14, 1364. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Hassan, M.; Ullah, S.; Abbas, H.; Tawakkal, F.; Khan, M.A. Breast cancer; discovery of novel diagnostic biomarkers, drug resistance, and therapeutic implications. Front. Mol. Biosci. 2022, 9, 783450. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.K.Y.; Tan, P.H. Liquid biopsy in breast cancer: A focused review. Arch. Pathol. Lab. Med. 2021, 145, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Diomaiuto, E.; Principe, V.; De Luca, A.; Laperuta, F.; Alterisio, C.; Di Loria, A. Exosomes in Dogs and Cats: An Innovative Approach to Neoplastic and Non-Neoplastic Diseases. Pharmaceuticals 2021, 14, 766. [Google Scholar] [CrossRef]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef]
- Novais, A.A.; Tamarindo, G.H.; Chuffa, L.G.d.A.; Zuccari, D.A.P.d.C. Decoding hidden messengers: Proteomic profiling of exosomes in mammary cancer research. Biomedicines 2023, 11, 2839. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Meier, F.; Brunner, A.-D.; Koch, S.; Koch, H.; Lubeck, M.; Krause, M.; Goedecke, N.; Decker, J.; Kosinski, T.; Park, M.A.; et al. Online Parallel Accumulation-Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef]
- Meier, F.; Beck, S.; Grassl, N.; Lubeck, M.; Park, M.A.; Raether, O.; Mann, M. Parallel Accumulation-Serial Fragmentation (PASEF): Multiplying Sequencing Speed and Sensitivity by Synchronized Scans in a Trapped Ion Mobility Device. J. Proteome Res. 2015, 14, 5378–5387. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Cox, J. Perseus: A bioinformatics platform for integrative analysis of proteomics data in cancer research. Methods Mol. Biol. 2018, 1711, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Szajnik, M.; Derbis, M.; Lach, M.; Patalas, P.; Michalak, M.; Drzewiecka, H.; Szpurek, D.; Nowakowski, A.; Spaczynski, M.; Baranowski, W.; et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol. Obstet. 2013, (Suppl. S4), 3. [Google Scholar] [CrossRef]
- Aguilera-Rojas, M.; Badewien-Rentzsch, B.; Plendl, J.; Kohn, B.; Einspanier, R. Exploration of serum- and cell culture-derived exosomes from dogs. BMC Vet. Res. 2018, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Rzepecka, A.; Cywińska, A.; Jagielski, D.; Winnicka, A. Extracellular Vesicles in the Blood of Dogs with Cancer-A Preliminary Study. Animals 2019, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bai, X.-Y.; Li, B.; Li, Y.; Xia, K.; Wang, M.; Li, S.; Wu, H. Plasma Inter-Alpha-Trypsin Inhibitor Heavy Chains H3 and H4 Serve as Novel Diagnostic Biomarkers in Human Colorectal Cancer. Dis. Markers 2019, 2019, 5069614. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.T.; Stepanov, A.A.; Malsagova, K.A.; Soni, D.; Kushlinsky, N.E.; Enikeev, D.V.; Potoldykova, N.V.; Lisitsa, A.V.; Kaysheva, A.L. Revelation of proteomic indicators for colorectal cancer in initial stages of development. Molecules 2020, 25, 619. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, I.; Sparidans, R.W.; van Winden, A.W.J.; Gast, M.-C.W.; van Dulken, E.J.; Schellens, J.H.M.; Beijnen, J.H. The absolute quantification of eight inter-α-trypsin inhibitor heavy chain 4 (ITIH4)-derived peptides in serum from breast cancer patients. Proteom. Clin. Appl. 2010, 4, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Opstal-van Winden, A.W.J.; Krop, E.J.M.; Kåredal, M.H.; Gast, M.-C.W.; Lindh, C.H.; Jeppsson, M.C.; Jönsson, B.A.G.; Grobbee, D.E.; Peeters, P.H.M.; Beijnen, J.H.; et al. Searching for early breast cancer biomarkers by serum protein profiling of pre-diagnostic serum; a nested case-control study. BMC Cancer 2011, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, X.; Liu, S.; Zhu, J.; Luo, M.; Liu, L.; Zhao, L.; Qin, Y.; Song, T.; Huang, C. Identification of novel serum peptides biomarkers for female breast cancer patients in Western China. Proteomics 2016, 16, 925–934. [Google Scholar] [CrossRef]
- Hoang, A.T.; Vizio, B.; Chiusa, L.; Cimino, A.; Solerio, D.; Do, N.H.; Pileci, S.; Camandona, M.; Bellone, G. Impact of tissue enolase 1 protein overexpression in esophageal cancer progression. Int. J. Med. Sci. 2021, 18, 1406–1414. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Q.; Long, H.; Hu, Z.; Que, T.; Zhang, X.; Li, Z.; Wang, G.; Yi, L.; Liu, Z.; et al. Correction: Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer 2015, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Huang, Z.; Li, B.; Nice, E.C.; Huang, C.; Wei, L.; Zou, B. Targeting glucose metabolism enzymes in cancer treatment: Current and emerging strategies. Cancers 2022, 14, 4568. [Google Scholar] [CrossRef]
- Qiao, G.; Wu, A.; Chen, X.; Tian, Y.; Lin, X. Enolase 1, a moonlighting protein, as a potential target for cancer treatment. Int. J. Biol. Sci. 2021, 17, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Tamarindo, G.H.; Novais, A.A.; Chuffa, L.G.A.; Zuccari, D.A.P.C. Metabolic alterations in canine mammary tumors. Animals 2023, 13, 2757. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Hsu, N.C.; Liao, A.T.; Shih, N.-Y.; Hou, M.-F.; Liu, C.-H. Overexpression of α-enolase correlates with poor survival in canine mammary carcinoma. BMC Vet. Res. 2011, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Ahmadmahmudi, M.; Nassiri, S.M.; Rahbarghazi, R. Serological proteome analysis of dogs with breast cancer unveils common serum biomarkers with human counterparts. Electrophoresis 2014, 35, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Liu, J.; Zhou, L.; Zhang, C.; Xu, L.; Qin, Q.; Zhan, L.; Lu, J.; Cheng, H.; et al. Eukaryotic elongation factor 2 kinase confers tolerance to stress conditions in cancer cells. Cell Stress Chaperones 2015, 20, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zou, J.; Zhang, Q.; Wang, Z.; Wang, N.; He, S.; Zhao, Y.; Naman, C.B. Progress in the development of eukaryotic elongation factor 2 kinase (eef2k) natural product and synthetic small molecule inhibitors for cancer chemotherapy. Int. J. Mol. Sci. 2021, 22, 2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-X.; Xu, X.-E.; Huang, L.; Chen, S.; Shao, Z.-M. eEF2 kinase mediated autophagy as a potential therapeutic target for paclitaxel-resistant triple-negative breast cancer. Ann. Transl. Med. 2019, 7, 783. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, S.; Wang, J.; Sun, M.-Z.; Greenaway, F.T. ACTB in cancer. Clin. Chim. Acta 2013, 417, 39–44. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.-J.; Deng, T.; Li, B.-H.; Geng, P.-L.; Zeng, X.-T. Actinin-4 as a Diagnostic Biomarker in Serum of Breast Cancer Patients. Med. Sci. Monit. 2019, 25, 3298–3302. [Google Scholar] [CrossRef]
- Procházková, I.; Lenčo, J.; Fučíková, A.; Dresler, J.; Čápková, L.; Hrstka, R.; Nenutil, R.; Bouchal, P. Targeted proteomics driven verification of biomarker candidates associated with breast cancer aggressiveness. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Tentler, D.; Lomert, E.; Novitskaya, K.; Barlev, N.A. Role of ACTN4 in tumorigenesis, metastasis, and EMT. Cells 2019, 8, 1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Q.; Tang, H.; Zhang, F.; Zheng, Y.; Wang, S.; Zhang, J.; Wang, Z.; Xie, X. Direct inhibition of ACTN4 by ellagic acid limits breast cancer metastasis via regulation of β-catenin stabilization in cancer stem cells. J. Exp. Clin. Cancer Res. 2017, 36, 172. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Moore, K.; Phillips, L.; Boyle, F.M.; Marsh, D.J.; Baxter, R.C. Novel serum protein biomarker panel revealed by mass spectrometry and its prognostic value in breast cancer. Breast Cancer Res. 2014, 16, R63. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.-U.-H.; Ejaz, S.; Ashraf, M.; Asif, A.R.; Oellerich, M.; Ahmad, G.; Malik, G.A. Attiq-Ur-Rehman Potential biomarkers in the sera of breast cancer patients from bahawalpur, pakistan. Biomark. Cancer 2012, 4, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Malhotra, L.; Mukherjee, P.; Kaur, N.; Krishanlata, T.; Srikanth, C.V.; Mishra, V.; Banerjee, B.D.; Ethayathulla, A.S.; Sharma, R.S. Putative interactions between transthyretin and endosulfan II and its relevance in breast cancer. Int. J. Biol. Macromol. 2023, 235, 123670. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; van der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. IDEAL Study Group Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Ma, Y.; Chen, H. Apolipoproteins: New players in cancers. Front. Pharmacol. 2022, 13, 1051280. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Tang, C.-T.; Kan, J.-Y.; Chiang, C.-P.; Li, H.-T.; Sung, J.-N.; Chiu, W.-C. Correlation of Beta2-Glycoprotein I With Tumor Prognosis in Breast Cancer Patients. Anticancer Res. 2023, 43, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hong, S.; Huang, J.; Chen, Y.; Chen, Y.; Wu, Z. Plasma apolipoprotein A1 levels at diagnosis are independent prognostic factors in invasive ductal breast cancer. Discov. Med. 2017, 23, 247–258. [Google Scholar] [PubMed]
- Baig, R.M.; Mahjabeen, I.; Sabir, M.; Masood, N.; Ali, K.; Malik, F.A.; Kayani, M.A. Mutational spectrum of Gelsolin and its down regulation is associated with breast cancer. Dis. Markers 2013, 34, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mielnicki, L.M.; Ying, A.M.; Head, K.L.; Asch, H.L.; Asch, B.B. Epigenetic regulation of gelsolin expression in human breast cancer cells. Exp. Cell Res. 1999, 249, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.S.; Asch, H.L.; Zhang, P.J.; Edge, S.B.; Hyland, A.; Asch, B.B. Downregulation of gelsolin correlates with the progression to breast carcinoma. Breast Cancer Res. Treat. 2001, 65, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.-M.; Klee, F.; Edlund, K.; Grinberg, M.; Hammad, S.; Marchan, R.; Cadenas, C.; Niggemann, B.; Zänker, K.S.; Rahnenführer, J.; et al. Gelsolin Is Associated with Longer Metastasis-free Survival and Reduced Cell Migration in Estrogen Receptor-positive Breast Cancer. Anticancer Res. 2015, 35, 5277–5285. [Google Scholar] [PubMed]
- Rao, J.; Seligson, D.; Visapaa, H.; Horvath, S.; Eeva, M.; Michel, K.; Pantuck, A.; Belldegrun, A.; Palotie, A. Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer 2002, 95, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, A.; De Corte, V.; Van Impe, K.; Bruyneel, E.; Boucherie, C.; Bracke, M.; Vandekerckhove, J.; Gettemans, J. Downregulation of gelsolin family proteins counteracts cancer cell invasion in vitro. Cancer Lett. 2007, 255, 57–70. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, P.-W.; Shieh, D.-B.; Chiu, K.-Y.; Liou, Y.-M. Involvement of gelsolin in TGF-beta 1 induced epithelial to mesenchymal transition in breast cancer cells. J. Biomed. Sci. 2015, 22, 90. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Lin, J.; Fu, S.; Feng, P.; Su, H.; He, X.; Liang, X.; Liu, K.; Deng, W. Gelsolin Promotes Cancer Progression by Regulating Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma and Correlates with a Poor Prognosis. J. Oncol. 2020, 2020, 1980368. [Google Scholar] [CrossRef]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1758–1765. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, A.A.; Tamarindo, G.H.; Melo, L.M.M.; Balieiro, B.C.; Nóbrega, D.; dos Santos, G.; Saldanha, S.F.; de Souza, F.F.; Chuffa, L.G.d.A.; Bracha, S.; et al. Exploring Canine Mammary Cancer through Liquid Biopsy: Proteomic Profiling of Small Extracellular Vesicles. Cancers 2024, 16, 2562. https://doi.org/10.3390/cancers16142562
Novais AA, Tamarindo GH, Melo LMM, Balieiro BC, Nóbrega D, dos Santos G, Saldanha SF, de Souza FF, Chuffa LGdA, Bracha S, et al. Exploring Canine Mammary Cancer through Liquid Biopsy: Proteomic Profiling of Small Extracellular Vesicles. Cancers. 2024; 16(14):2562. https://doi.org/10.3390/cancers16142562
Chicago/Turabian StyleNovais, Adriana Alonso, Guilherme Henrique Tamarindo, Luryan Mikaelly Minotti Melo, Beatriz Castilho Balieiro, Daniela Nóbrega, Gislaine dos Santos, Schaienni Fontoura Saldanha, Fabiana Ferreira de Souza, Luiz Gustavo de Almeida Chuffa, Shay Bracha, and et al. 2024. "Exploring Canine Mammary Cancer through Liquid Biopsy: Proteomic Profiling of Small Extracellular Vesicles" Cancers 16, no. 14: 2562. https://doi.org/10.3390/cancers16142562








