Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
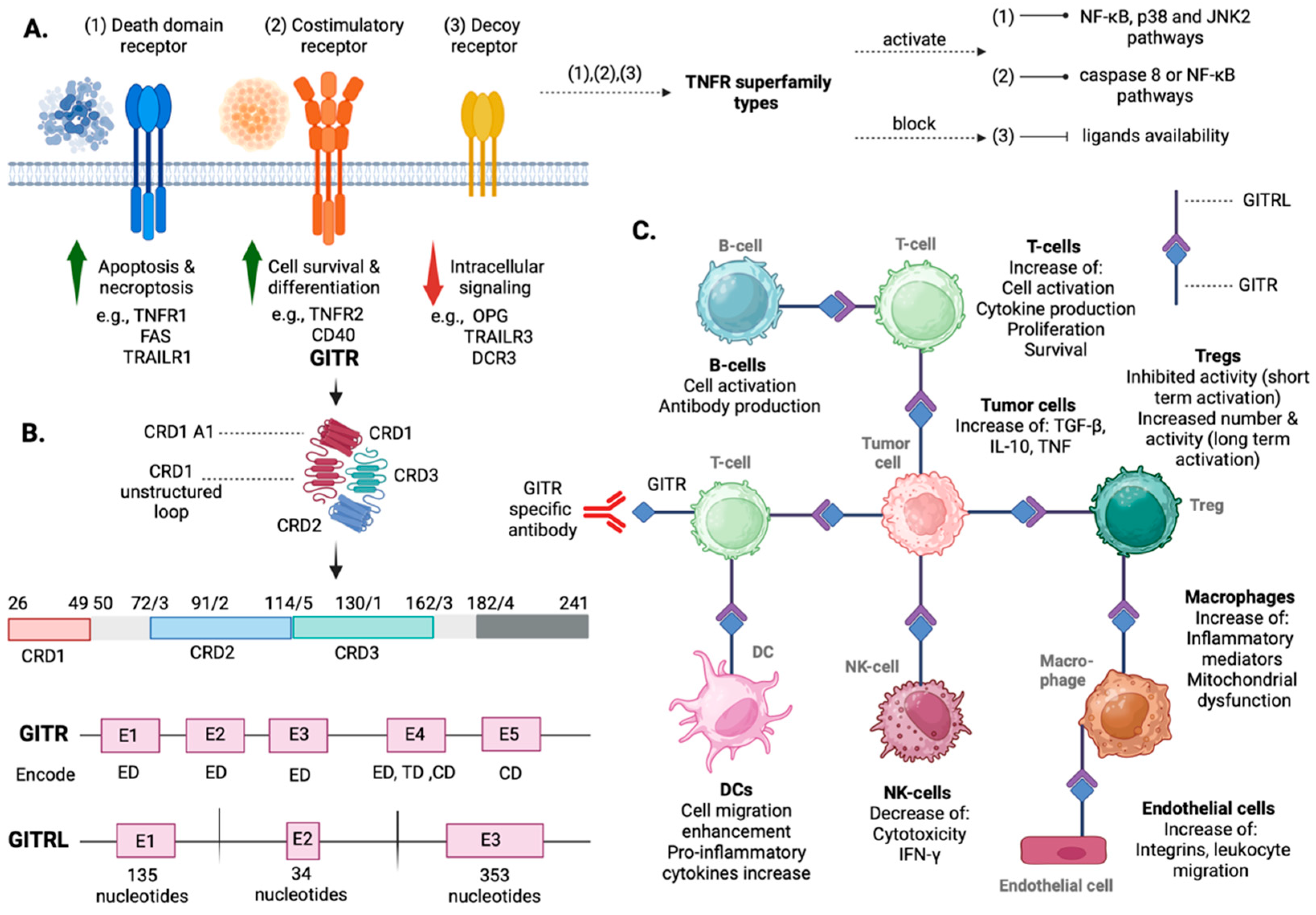
2. Overview of GITR/GITRL: Structure, Tissue Distribution, Signaling Pathways, and Functional Insights
3. Exploring the Role of GITR/GITRL Signaling in Liver Physiology and Disease
3.1. The Role of GITR/GITRL in Fostering Immune Tolerance
3.2. GITR Signaling in Infection and Immune Regulation
3.3. The Role of GITR Signaling in Liver Transplantation
3.4. Exploring the Role of GITR in Liver Cirrhosis
3.5. Insights in the Potential Role of GITR in Autoimmune Hepatitis
4. Investigating the Role of GITR/GITRL Signaling in HCC
4.1. GITR Expression in HCC
4.2. GITR Involvement in Hepatocarcinogenesis Regulation
4.3. GITR as an Individual Target in HCC
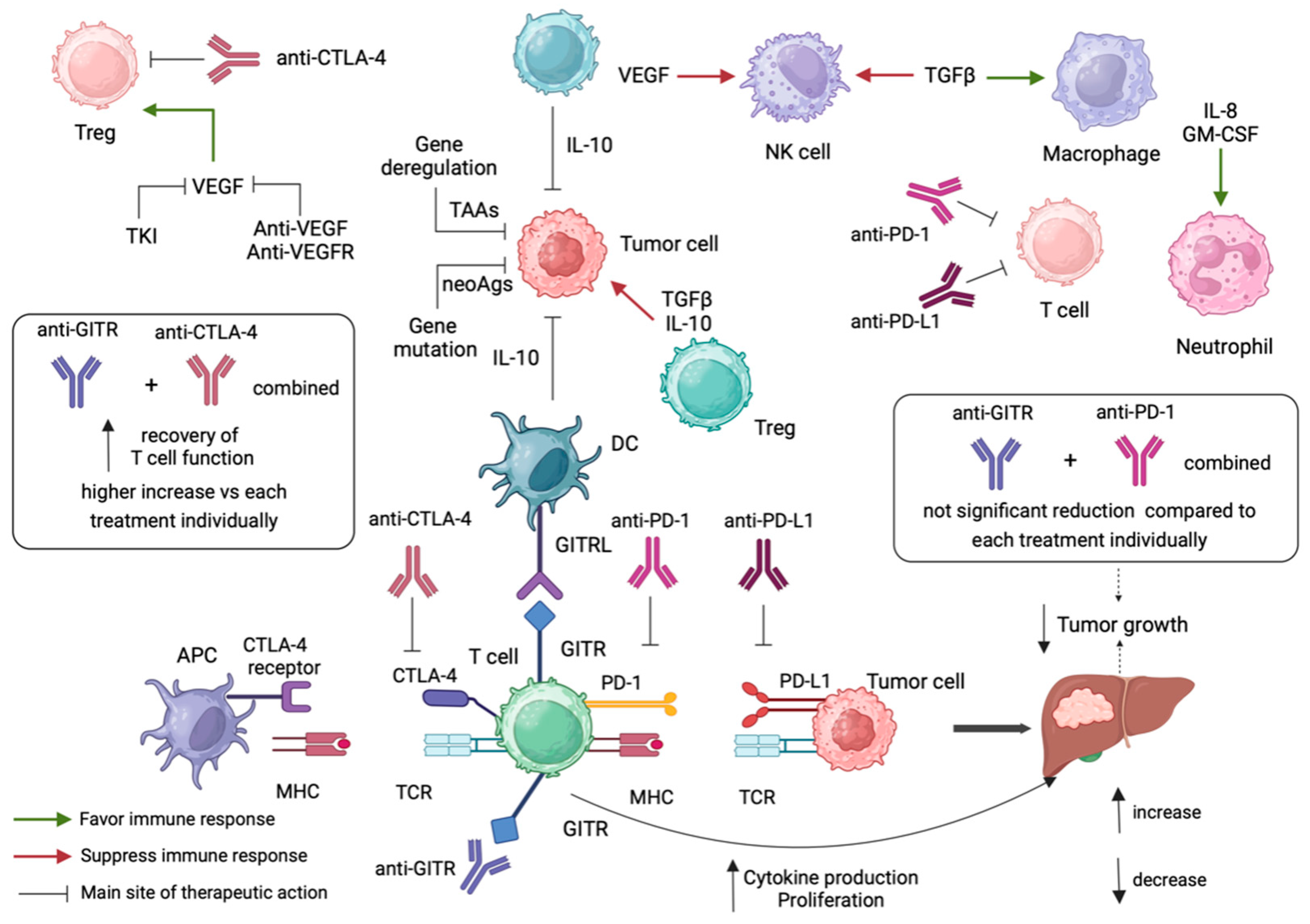
5. Anti-GITR Combination Treatment in Cancer Immunotherapy
5.1. Anti-GITR Combined with Anti-PD-1/Anti-PD-L1
5.2. Anti-GITR Combined with Anti-CLTLA4
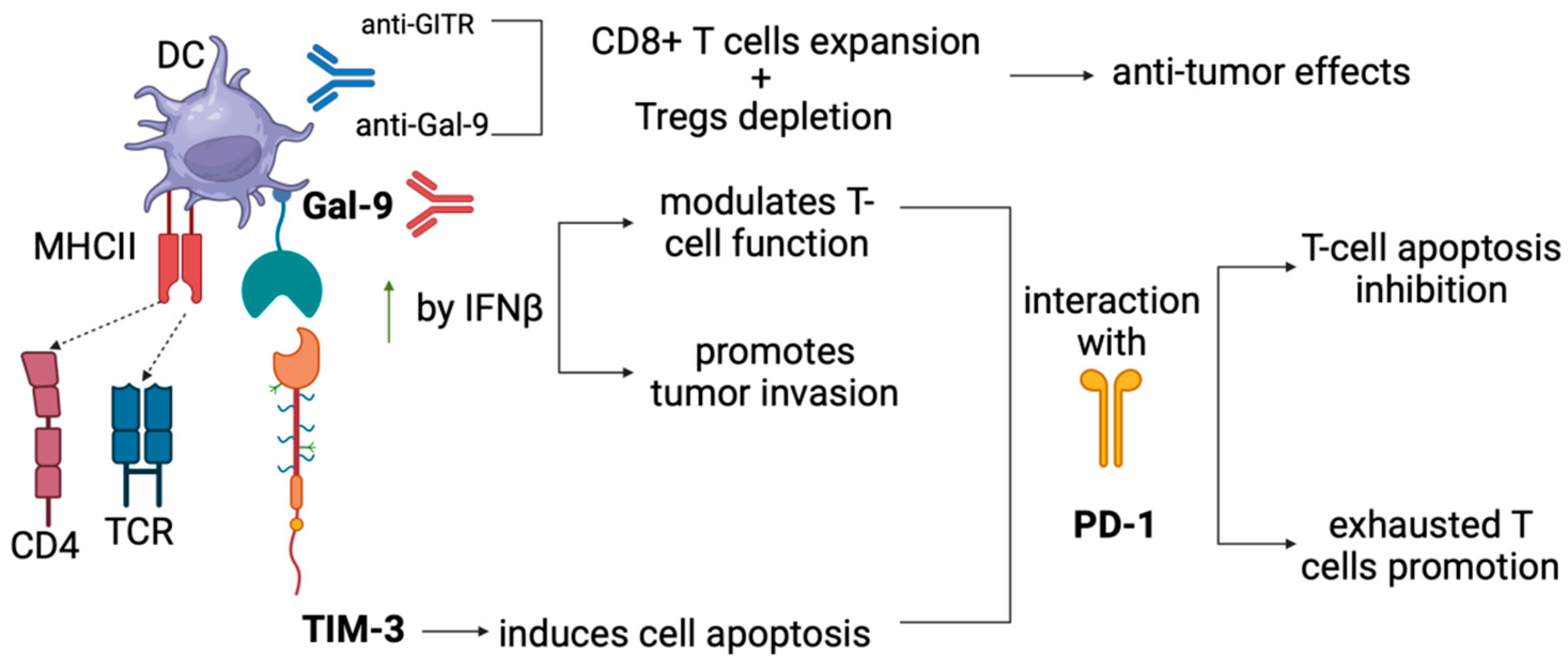
5.3. Anti-GITR Combined with Anti-GAL-9
6. Discussion
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology, and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, A.; Prati, G.M.; Fasani, P.; Ronchi, G.; Romeo, R.; Manini, M.; Del Ninno, E.; Morabito, A.; Colombo, M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006, 43, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, X.; Zhang, X.; Zhao, X.; Zhang, Y.; Shi, Y.; Hua, S. Hepatocellular carcinoma: Signaling pathways, targeted therapy, and immunotherapy. MedComm 2024, 5, 474. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lezana, T.; Lopez-Canovas, J.L.; Villanueva, A. Signaling pathways in hepatocellular carcinoma. Adv. Cancer Res. 2021, 149, 63–101. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Chatzikalil, E.; Arvanitakis, K.; Vakadaris, G.; Stergiou, I.E.; Koutsompina, M.L.; Argyrou, A.; Lekakis, V.; Konstantinidis, I.; Germanidis, G.; et al. Understanding the Role of Connexins in Hepatocellular Carcinoma: Molecular and Prognostic Implications. Cancers 2024, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Y.; Li, W.; Quan, C.; Li, Y. Claudins and hepatocellular carcinoma. Biomed. Pharmacother. 2024, 171, 116109. [Google Scholar] [CrossRef]
- Guizhen, Z.; Guanchang, J.; Liwen, L.; Huifen, W.; Zhigang, R.; Ranran, S.; Zujiang, Y. The tumor microenvironment of hepatocellular carcinoma and its targeting strategy by CAR-T cell immunotherapy. Front. Endocrinol. 2022, 13, 918869. [Google Scholar] [CrossRef]
- Sas, Z.; Cendrowicz, E. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef]
- Saito, A.; Toyoda, H.; Kobayashi, M.; Koiwa, Y.; Fujii, H.; Fujita, K.; Maeda, A.; Kaneoka, Y.; Hazama, S.; Nagano, H.; et al. Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod. Pathol. 2021, 34, 417–425. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef]
- Rimassa, L.; Finn, R.S.; Sangro, B. Combination immunotherapy for hepatocellular carcinoma. J. Hepatol. 2023, 79, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.H.; Shin, E.C.; Kim, Y.Y.; et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Ben Mousa, A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J. Gastroenterol. 2008, 14, 40–42. [Google Scholar] [CrossRef]
- Kudo, M. Combination immunotherapy with anti-VEGF/TKI for hepatocellular carcinoma: Present and future perspective. Hepatobiliary Surg. Nutr. 2021, 10, 241–245. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Stanneart, J.; Nunez, K.G. Imaging Delay Following Liver-Directed Therapy Increases Progression Risk in Early- to Intermediate-Stage Hepatocellular Carcinoma. Cancers 2024, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Buzzatti, G.; Dellepiane, C.; Del Mastro, L. New emerging targets in cancer immunotherapy: The role of GITR. ESMO Open 2020, 4, e000738. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Herbert, P.E.; Warrens, A.N. An introduction to death receptors in apoptosis. Int. J. Surg. 2005, 3, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Wang, Y.; Lee, S.J.; Jin, M.H.; Sun, H.N.; Kwon, T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal. 2023, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Baeyens, A.; Dustin, M.L. Tumor Necrosis Factor Receptor Superfamily in T Cell Priming and Effector Function. Adv. Immunol. 2018, 140, 21–57. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, G.; Giunchi, L.; Ronchetti, S.; Krausz, L.T.; Bartoli, A.; Moraca, R.; Migliorati, G.; Riccardi, C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6216–6221. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Farrah, T.; Goodwin, R.G. The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation, and death. Cell 1994, 76, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, F.; Beutler, B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996, 334, 1717–1725. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; O’Rourke, K.; Yu, G.L.; Lyons, R.H.; Garg, M.; Duan, D.R.; Xing, L.; Gentz, R.; Ni, J.; Dixit, V.M. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 1996, 274, 990–992. [Google Scholar] [CrossRef]
- Hintzen, R.Q.; de Jong, R.; Lens, S.M.; van Lier, R.A. CD27: Marker and mediator of T-cell activation? Immunol. Today 1994, 15, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, J.C.; Kim, S.H.; Pollok, K.E.; Lee, Z.H.; Kwon, B.S. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J. Immunol. 1995, 155, 3360–3367. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, G.; Riccardi, C. GITR: A modulator of immune response and inflammation. Adv. Exp. Med. Biol. 2009, 647, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, G.; Ronchetti, S.; Bartoli, A.; Spinicelli, S.; Delfino, D.; Brunetti, L.; Migliorati, G.; Riccardi, C. Identification of three novel mRNA splice variants of GITR. Cell Death Differ. 2000, 7, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, G.; Bartoli, A.; Ronchetti, S.; Giunchi, L.; Cupelli, A.; Delfino, D.; Migliorati, G.; Riccardi, C. Gene structure and chromosomal assignment of mouse GITR, a member of the tumor necrosis factor/nerve growth factor receptor family. DNA Cell Biol. 2000, 19, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, B.K.; Bae, J.S.; Lee, U.H.; Han, I.S.; Lee, H.W.; Youn, B.S.; Vinay, D.S.; Kwon, B.S. Cloning and characterization of GITR ligand. Genes Immun. 2003, 4, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chau, B.; West, S.M.; Kimberlin, C.R.; Cao, F.; Schwarz, F.; Aguilar, B.; Han, M.; Morishige, W.; Bee, C.; et al. Structures of mouse and human GITR-GITRL complexes reveal unique TNF superfamily interactions. Nat. Commun. 2021, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tone, Y.; Song, X.; Furuuchi, K.; Lear, J.D.; Waldmann, H.; Tone, M.; Greene, M.I.; Murali, R. Structural basis for ligand-mediated mouse GITR activation. Proc. Natl. Acad. Sci. USA 2008, 105, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Yu, K.Y.; Ni, J.; Yu, G.L.; Jang, I.K.; Kim, Y.J.; Xing, L.; Liu, D.; Wang, S.X.; Kwon, B.S. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 1999, 274, 6056–6061. [Google Scholar] [CrossRef]
- Tone, M.; Tone, Y.; Adams, E.; Yates, S.F.; Frewin, M.R.; Cobbold, S.P.; Waldmann, H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15059–15064. [Google Scholar] [CrossRef]
- Yu, K.Y.; Kim, H.S.; Song, S.Y.; Min, S.S.; Jeong, J.J.; Youn, B.S. Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem. Biophys. Res. Commun. 2003, 310, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Hanabuchi, S.; Watanabe, N.; Wang, Y.H.; Wang, Y.H.; Ito, T.; Shaw, J.; Cao, W.; Qin, F.X.; Liu, Y.J. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood 2006, 107, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Kim, S.G.; Lee, M.H.; Suh, J.H.; Kwon, B.S.; Choi, H.S. Soluble glucocorticoid-induced TNF receptor (sGITR) induces inflammation in mice. Exp. Mol. Med. 2003, 35, 358–364. [Google Scholar] [CrossRef]
- Nardelli, B.; Zaritskaya, L.; McAuliffe, W.; Ni, Y.; Lincoln, C.; Cho, Y.H.; Birse, C.E.; Halpern, W.; Ullrich, S.; Moore, P.A. Osteostat/tumor necrosis factor superfamily 18 inhibits osteoclastogenesis and is selectively expressed by vascular endothelial cells. Endocrinology 2006, 147, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Sun, S.C. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor κB and Mitogen-Activated Protein Kinase Pathways. Front. Immunol. 2018, 9, 1849. [Google Scholar] [CrossRef]
- An, W.; Kim, J.; Roeder, R.G. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell 2004, 117, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.G.; Ronchetti, S.; Ricci, E.; Alunno, A.; Gerli, R.; Nocentini, G.; Riccardi, C. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun. Rev. 2015, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, L.A.; Shami, A.; Atzler, D.; Weber, C.; Gonçalves, I.; Lutgens, E. Glucocorticoid induced TNF receptor family-related protein (GITR)—A novel driver of atherosclerosis. Vasc. Pharmacol. 2021, 139, 106884. [Google Scholar] [CrossRef] [PubMed]
- Hultkrantz, S.; Ostman, S.; Telemo, E. Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration. Immunology 2005, 116, 362–372. [Google Scholar] [CrossRef]
- Liao, G.; Nayak, S.; Regueiro, J.R.; Berger, S.B.; Detre, C.; Romero, X.; de Waal Malefyt, R.; Chatila, T.A.; Herzog, R.W.; Terhorst, C. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int. Immunol. 2010, 22, 259–270. [Google Scholar] [CrossRef]
- Lin, G.H.; Snell, L.M.; Wortzman, M.E.; Clouthier, D.L.; Watts, T.H. GITR-dependent regulation of 4-1BB expression: Implications for T cell memory and anti-4-1BB-induced pathology. J. Immunol. 2013, 190, 4627–4639. [Google Scholar] [CrossRef] [PubMed]
- Cao, O.; Dobrzynski, E.; Wang, L.; Nayak, S.; Mingle, B.; Terhorst, C.; Herzog, R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 2007, 110, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; O’Keeffe, M.S.; Wang, G.; van Driel, B.; de Waal Malefyt, R.; Reinecker, H.C.; Herzog, R.W.; Terhorst, C. Glucocorticoid-Induced TNF Receptor Family-Related Protein Ligand is Requisite for Optimal Functioning of Regulatory CD4(+) T Cells. Front. Immunol. 2014, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Wong, D.K.; Huang, F.Y.; Cheung, K.S.; Mak, L.Y.; Fung, J.; Yuen, M.F.; Seto, W.K. Phenotypic Changes of PD-1 and GITR in T Cells Are Associated with Hepatitis B Surface Antigen Seroclearance. J. Clin. Gastroenterol. 2022, 56, e31–e37. [Google Scholar] [CrossRef] [PubMed]
- Batista, N.V.; Chang, Y.H.; Chu, K.L.; Wang, K.C.; Girard, M.; Watts, T.H. T Cell-Intrinsic CX3CR1 Marks the Most Differentiated Effector CD4(+) T Cells, but Is Largely Dispensable for CD4(+) T Cell Responses during Chronic Viral Infection. Immunohorizons 2020, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Cheever, A.W.; White, S.D.; Thompson, R.W.; Wynn, T.A. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 2011, 7, e1002171. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.J.; Kumar, R.; Bunn, P.T.; Singh, N.; Chauhan, S.B.; Sheel, M.; Amante, F.H.; Montes de Oca, M.; Edwards, C.L.; Ng, S.S. Combined Immune Therapy for the Treatment of Visceral Leishmaniasis. PLoS Neglected Trop. Dis. 2016, 10, e0004415. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Smith, P.; Fallon, P.G. Role for CTLA-4 but not CD25+ T cells during Schistosoma mansoni infection of mice. Parasite Immunol. 2007, 29, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Stanley, A.C.; Amante, F.H.; Rivera Fde, L.; Zhou, Y.; Kuns, R.D.; Yardley, V.; Sakaguchi, S.; Hill, G.R.; Engwerda, C.R. Therapeutic glucocorticoid-induced TNF receptor-mediated amplification of CD4+ T cell responses enhances antiparasitic immunity. J. Immunol. 2010, 184, 2583–2592. [Google Scholar] [CrossRef]
- van der Werf, N.; Redpath, S.A.; Phythian-Adams, A.T.; Azuma, M.; Allen, J.E.; Maizels, R.M.; Macdonald, A.S.; Taylor, M.D. Th2 responses to helminth parasites can be therapeutically enhanced by, but are not dependent upon, GITR-GITR ligand costimulation in vivo. J. Immunol. 2011, 187, 1411–1420. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Bu, H.; Chen, H.; Tong, H.; Liu, D.; Guo, H.; Lin, S.Z. Emodin inhibits the differentiation and maturation of dendritic cells and increases the production of regulatory T cells. Int. J. Mol. Med. 2012, 29, 159–164. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Lian, Z.; Chen, Y.; Liu, Z.; You, H.; Gong, J. Expression of glucocorticoid-induced tumor necrosis factor receptor ligand in rat graft after liver transplantation. Transplant. Proc. 2011, 43, 1971–1975. [Google Scholar] [CrossRef]
- Wang, W.; Ji, G.; Chen, Y.; Wang, J.; Sun, J.; Tang, G.; Xie, Z.; Zhao, H.; Liu, G.; Tan, S.; et al. Changes in DNA Methylation of Glucocorticoid-Induced Tumor Necrosis Factor Receptor and Its Ligand in Liver Transplantation. Transplant. Proc. 2017, 49, 1824–1833. [Google Scholar] [CrossRef]
- Rueschenbaum, S.; Ciesek, S.; Queck, A.; Widera, M.; Schwarzkopf, K.; Brüne, B.; Welsch, C.; Wedemeyer, H.; Zeuzem, S.; Weigert, A.; et al. Dysregulated Adaptive Immunity Is an Early Event in Liver Cirrhosis Preceding Acute-on-Chronic Liver Failure. Cell Death Dis. 2020, 11, 534731. [Google Scholar] [CrossRef]
- Fadriquela, A.; Kim, C.S.; Lee, K.J.; Kang, S.H.; Kim, M.Y.; Lee, J.H. Soluble type immune checkpoint regulators using multiplex luminex immunoassay in chronic hepatitis B patients. J. Clin. Pathol. 2021, 74, 780–786. [Google Scholar] [CrossRef]
- Wen, C.; Dong, Z.; Wang, Y.; Ye, G.; Ma, Y.; Yi, X.; Zhou, Y.; Li, X.; Zheng, X.; Hou, J.; et al. CTLA4(+)CD4(+)CXCR5(-)FOXP3(+) T cells associate with unfavorable outcome in patients with chronic HBV infection. BMC Immunol. 2023, 24, 3. [Google Scholar] [CrossRef]
- Wen, C.; Dong, Z.; Wang, Y.; Ye, G.; Ma, Y.; Yi, X.; Zhou, Y.; Li, X.; Zheng, X.; Hou, J.; et al. Immunological and senescence biomarker profiles in patients after spontaneous clearance of hepatitis C virus: Gender implications for long-term health risk. Immun. Ageing 2023, 20, 62. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Virseda-Berdices, A.; Berenguer, J.; González-García, J.; Brochado-Kith, O.; Fernández-Rodríguez, A.; Díez, C.; Hontañon, V.; Resino, S.; Jiménez-Sousa, M.Á. Predictive plasma biomarkers of long-term increase in hepatic steatosis index after HCV eradication in HIV/HCV-coinfected patients. Biomed. Pharmacother. 2023, 164, 114913. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, B.; Rui, K.; Wang, S. The Role of GITR/GITRL Interaction in Autoimmune Diseases. Front. Immunol. 2000, 11, 588682. [Google Scholar] [CrossRef]
- Riccardi, C.; Ronchetti, S.; Nocentini, G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin. Ther. Targets 2018, 22, 783–797. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Ayroldi, E.; Di Paola, R.; Agostini, M.; Mazzon, E.; Bruscoli, S.; Genovese, T.; Ronchetti, S.; Caputi, A.P.; Riccardi, C. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 2005, 19, 1253–1265. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Verhoef, C.; Ijzermans, J.N.; Peppelenbosch, M.P.; Kwekkeboom, J.; Verheij, J.; Janssen, H.L.; Sprengers, D. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology 2013, 57, 183–194. [Google Scholar] [CrossRef]
- Cao, Μ.; Cabrera, R.; Xu, Y.; Firpi, R.; Zhu, H.; Liu, C.; Nelson, D.R. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab. Investig. A J. Tech. Methods Pathol. 2007, 87, 582–590. [Google Scholar] [CrossRef]
- Zhang, H.H.; Mei, M.H.; Fei, R.; Liao, W.J.; Wang, X.Y.; Qin, L.L.; Wang, J.H.; Wei, L.; Chen, H.S. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int. J. Oncol. 2010, 36, 841–848. [Google Scholar] [CrossRef]
- Ormandy, L.A.; Hillemann, T.; Wedemeyer, H.; Manns, M.P.; Greten, T.F.; Korangy, F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005, 65, 2457–2464. [Google Scholar] [CrossRef]
- Zhang, H.H.; Mei, M.H.; Fei, R.; Liu, F.; Wang, J.H.; Liao, W.J.; Qin, L.L.; Wei, L.; Chen, H.S. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J. Viral Hepat. 2010, 17, 34–43. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Zhang, J.P.; Liang, J.; Li, L.; Zheng, L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE 2013, 8, e58006. [Google Scholar] [CrossRef]
- He, Y.; Pei, Y.; Liu, K.; Liu, L.; Tian, Y.; Li, H.; Cong, M.; Liu, T.; Ma, H.; You, H.; et al. GITR/GITRL reverse signalling modulates the proliferation of hepatic progenitor cells by recruiting ANXA2 to phosphorylate ERK1/2 and Akt. Cell Death Dis. 2022, 13, 297. [Google Scholar] [CrossRef]
- Tang, C.; Welsh, J.W.; de Groot, P.; Massarelli, E.; Chang, J.Y.; Hess, K.R.; Basu, S.; Curran, M.A.; Cabanillas, M.E.; Subbiah, V.; et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin. Cancer Res. 2017, 23, 1388–1396. [Google Scholar] [CrossRef]
- Cari, L.; Nocentini, G.; Migliorati, G.; Riccardi, C. Potential effect of tumor-specific Treg-targeted antibodies in the treatment of human cancers: A bioinformatics analysis. Oncoimmunology 2018, 7, e1387705. [Google Scholar] [CrossRef]
- Nagayama, Y.; Hase, W.; Motoyoshi, Y.; Saitoh, O.; Sogawa, R.; Nakao, K. Distinct responses of two hepatocellular carcinoma cell lines of a similar origin to immunotherapies targeting regulatory or effector T cells. Oncol. Rep. 2007, 17, 1269–1273. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Martin, S.; Saha, B. Levels of CD40 expression on dendritic cells dictate tumour growth or regression. Clin. Exp. Immunol. 2007, 149, 194–202. [Google Scholar] [CrossRef]
- Wei, R.; Hu, Y.; Dong, F.; Xu, X.; Hu, A.; Gao, G. Hepatoma cell-derived leptin downregulates the immunosuppressive function of regulatory T-cells to enhance the anti-tumor activity of CD8+ T-cells. Immunol. Cell Biol. 2016, 94, 388–399. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Kwekkeboom, J.; Sprengers, D. T-cell suppression mediated by regulatory T cells infiltrating hepatic tumors can be overcome by GITRL treatment. Oncoimmunology 2013, 2, e22450. [Google Scholar] [CrossRef]
- Pan, C.; Wu, Q.; Wang, S.; Mei, Z.; Zhang, L.; Gao, X.; Qian, J.; Xu, Z.; Zhang, K.; Su, R.; et al. Combination with Toll-like receptor 4 (TLR4) agonist reverses GITR agonism mediated M2 polarization of macrophage in Hepatocellular carcinoma. Oncoimmunology 2022, 11, 2073010. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- van Beek, A.A.; Zhou, G.; Doukas, M.; Boor, P.P.C.; Noordam, L.; Mancham, S.; Campos Carrascosa, L.; van der Heide-Mulder, M.; Polak, W.G.; Ijzermans, J.N.M.; et al. GITR ligation enhances functionality of tumor-infiltrating T cells in hepatocellular carcinoma. Int. J. Cancer 2019, 145, 1111–1124. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Zhou, G.; Singh, S.P.; Boor, P.P.; Pan, Q.; Grunhagen, D.; de Jonge, J.; Tran, T.K.; Verhoef, C.; IJzermans, J.N.; et al. GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumor-derived regulatory T cells ex vivo. Oncoimmunology 2015, 4, e1051297. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Carloni, R.; Sabbioni, S. Immune-Based Combination Therapies for Advanced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 1445–1463. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Papadakos, S.P.; Vakadaris, G.; Chatzikalil, E.; Stergiou, I.E.; Kalopitas, G.; Theocharis, S.; Germanidis, G. Shedding light on the role of LAG-3 in hepatocellular carcinoma: Unraveling immunomodulatory pathways. Hepatoma Res. 2024, 10, 20. [Google Scholar] [CrossRef]
- Ganjalikhani Hakemi, M.; Jafarinia, M.; Azizi, M.; Rezaeepoor, M.; Isayev, O.; Bazhin, A.V. The Role of TIM-3 in Hepatocellular Carcinoma: A Promising Target for Immunotherapy? Front. Oncol. 2020, 10, 601661. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, Q.; Wang, W. Intimate communications within the tumor microenvironment: Stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Yao, J.; Long, J.; Mao, Y.; Wu, D.; Zang, A.; Zhao, J.; Liu, Z.; Meng, R.; et al. A phase Ib study evaluating the safety and efficacy of IBI310 plus sintilimab in patients with advanced non-small-cell lung cancer who have progressed after anti-PD-1/L1 therapy. Cancer Med. 2024, 13, e6855. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, R.; Zhang, Y.; Guo, Y.; Hui, Z.; Wang, P.; Sun, Y. Galectin-9 expression clinically associated with mature dendritic cells infiltration and T cell immune response in colorectal cancer. BMC Cancer 2022, 22, 1319. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, X.; Ma, Y.; Du, Y.; Feng, J. A new emerging target in cancer immunotherapy: Galectin-9 (LGALS9). Genes Dis. 2023, 10, 2366–2382. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role Of PD-1/PD-L1 Axis in Treg Development and Function: Implications for Cancer Immunotherapy. OncoTargets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef]
- Li, Q.; Han, J.; Yang, Y.; Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol. 2022, 13, 1070961. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Fujita, K.; Iwama, H.; Sakamoto, T.; Okura, R.; Kobayashi, K.; Takano, J.; Katsura, A.; Tatsuta, M.; Maeda, E.; Mimura, S.; et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int. J. Oncol. 2015, 46, 2419–2430. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, H.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.T.; Burey, A.P.; Beebe, A.M.; Gu, D.; Presta, L.G.; Merghoub, T.; Wolchok, J.D. Anaphylaxis caused by repetitive doses of a GITR agonist monoclonal antibody in mice. Blood 2014, 123, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Liu, H.; Qi, Q.; Dai, C.; Yang, T.; Qian, F. Development of a fully human anti-GITR antibody with potent antitumor activity using H2L2 mice. FEBS Open Bio 2022, 12, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Durham, N.M.; Holoweckyj, N.; MacGill, R.S.; McGlinchey, K.; Leow, C.C.; Robbins, S.H. GITR ligand fusion protein agonist enhances the tumor antigen-specific CD8 T-cell response and leads to long-lasting memory. J. Immunother. Cancer 2017, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Balmanoukian, A.S.; Infante, J.R.; Aljumaily, R.; Naing, A.; Chintakuntlawar, A.V.; Rizvi, N.A.; Ross, H.J.; Gordon, M.; Mallinder, P.R.; Elgeioushi, N.; et al. Safety and Clinical Activity of MEDI1873, a Novel GITR Agonist, in Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Zappasodi, R.; Wang, H.; Naik, G.S.; Sato, T.; Bauer, T.; Bajor, D.; Rixe, O.; Newman, W.; Qi, J.; et al. Phase IB Study of GITR Agonist Antibody TRX518 Singly and in Combination with Gemcitabine, Pembrolizumab, or Nivolumab in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 3990–4002. [Google Scholar] [CrossRef]
- Davar, D.; Zappasodi, R. Targeting GITR in cancer immunotherapy—There is no perfect knowledge. Oncotarget 2023, 14, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Autio, K.; Golan, T.; Dobrenkov, K.; Chartash, E.; Chen, Q.; Wnek, R.; Long, G.V. Phase I Study of MK-4166, an Anti-human Glucocorticoid-Induced TNF Receptor Antibody, Alone or with Pembrolizumab in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 1904–1911. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K.; Choudhary, H.B. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J. Gastroenterol. 2023, 29, 1054–1075. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. Immunotherapy for hepatocellular carcinoma: Recent advances and future targets. Pharmacol. Ther. 2023, 244, 108387. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Nobuoka, D.; Shirakawa, H.; Kuronuma, T.; Motomura, Y.; Mizuno, S.; Ishii, H.; Nakachi, K.; Konishi, M.; et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: Immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012, 18, 3686–3696. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Koutsompina, M.-L.; Germanidis, G.; Theocharis, S. γδ T Cells: A Game Changer in the Future of Hepatocellular Carcinoma Immunotherapy. Int. J. Mol. Sci. 2024, 25, 1381. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Machairas, N.; Stergiou, I.E.; Arvanitakis, K.; Germanidis, G.; Frampton, A.E.; Theocharis, S. Unveiling the Yin-Yang Balance of M1 and M2 Macrophages in Hepatocellular Carcinoma: Role of Exosomes in Tumor Microenvironment and Immune Modulation. Cells 2023, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Vallilas, C.; Sougioultzis, S.; Germanidis, G.; Theocharis, S. Interplay of Extracellular Vesicles and TLR4 Signaling in Hepatocellular Carcinoma Pathophysiology and Therapeutics. Pharmaceutics 2023, 15, 2460. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Lekakis, V.; Davakis, S.; Christodoulou, M.-I.; Germanidis, G.; Theocharis, S. The Role of TLR4 in the Immunotherapy of Hepatocellular Carcinoma: Can We Teach an Old Dog New Tricks? Cancers 2023, 15, 2795. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, H.; Jiang, R.; Lu, L.; Liu, T.; He, X. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumour Biol. 2016, 37, 799–806. [Google Scholar] [CrossRef]
- Gerry, A.B.; Pumphrey, N.J.; Docta, R.Y.; Brewer, J.E.; Bennett, A.D.; Jakobsen, B.K. Improved affinity AFP-specific T cell receptor for hepatocellular carcinoma. J. Immuno Ther. Cancer 2013, 1, P10. [Google Scholar] [CrossRef]
- Gao, H.; Li, K.; Tu, H.; Pan, X.; Jiang, H.; Shi, B.; Kong, J.; Wang, H.; Yang, S.; Gu, J.; et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6418–6428. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, X.; Chen, S.; Lai, Y.; Wei, X.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front. Immunol. 2016, 7, 690. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cabibbo, G.; Celsa, C.; Rimassa, L.; Torres, F.; Rimola, J.; Kloeckner, R.; Bruix, J.; Cammà, C.; Reig, M. Navigating the landscape of liver cancer management: Study designs in clinical trials and clinical practice. J. Hepatol. 2024, 80, 957–966. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab Versus Placebo as Second-Line Therapy in Patients from Asia with Advanced Hepatocellular Carcinoma: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2023, 41, 1434–1443. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Yamashita, T.; Kudo, M.; Ikeda, K.; Izumi, N.; Tateishi, R.; Ikeda, M.; Aikata, H.; Kawaguchi, Y.; Wada, Y.; Numata, K.; et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: An analysis of Japanese subset. J. Gastroenterol. 2020, 55, 113–122. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Plain language summary of the HIMALAYA study: Tremelimumab and durvalumab for unresectable hepatocellular carcinoma (liver cancer). Future Oncol. 2023, 19, 2505–2516. [Google Scholar] [CrossRef]
- Jones, B.; Jarvis, P.; Lewis, J.A.; Ebbutt, A.F. Trials to assess equivalence: The importance of rigorous methods. BMJ 1996, 313, 36–39. [Google Scholar] [CrossRef]
- Cappuzzello, E.; Sommaggio, R.; Zanovello, P.; Rosato, A. Cytokines for the induction of antitumor effectors: The paradigm of Cytokine-Induced Killer (CIK) cells. Cytokine Growth Factor Rev. 2017, 36, 99–105. [Google Scholar] [CrossRef]
- Jiang, S.S.; Tang, Y.; Zhang, Y.J.; Weng, D.S.; Zhou, Z.G.; Pan, K.; Pan, Q.Z.; Wang, Q.J.; Liu, Q.; He, J.; et al. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget 2015, 6, 41339–41349. [Google Scholar] [CrossRef]
- Hammerich, L.; Marron, T.U.; Upadhyay, R.; Svensson-Arvelund, J.; Dhainaut, M.; Hussein, S.; Zhan, Y.; Ostrowski, D.; Yellin, M.; Marsh, H.; et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019, 25, 814–824. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Dedes, N.; Kouroumalis, E.; Theocharis, S. The Role of the NLRP3 Inflammasome in HCC Carcinogenesis and Treatment: Harnessing Innate Immunity. Cancers 2022, 14, 3105. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Bruss, C.; Kellner, K.; Albert, V.; Hutchinson, J.A.; Seitz, S.; Ortmann, O.; Brockhoff, G.; Wege, A.K. Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion. Cancers 2023, 15, 2615. [Google Scholar] [CrossRef]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Voth Park, J.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, U.; He, H.; Messer, R.J.; Schimmer, S.; Olbrich, A.R.; Ohlen, C.; Greenberg, P.D.; Stromnes, I.M.; Iwashiro, M.; Sakaguchi, S.; et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity 2004, 20, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Yamazaki, S.; Nakamura, K.; Nishioka, T.; Hirota, K.; Yamaguchi, T.; Shimizu, J.; Nomura, T.; Chiba, T.; Sakaguchi, S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 2005, 202, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; Stephens, G.L. The GITR-GITRL interaction: Co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 2005, 6, 613–618. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) | Primary Outcome | Secondary Outcome | Prognostic Value |
|---|---|---|---|
| Gonzalez et al. (2012) [82] | Treatment with GITRL increased the proliferation of CD4+/CD8+ T cells and cytokine production while it decreased Tregs suppression | Higher GITR expression was observed on Tregs in tumor tissue | Treatment of GITRL improves HCC prognosis, enhancing anti-tumor immunity (mainly via upregulation in CD4+/CD8+ and cytokines levels) |
| Cao et al. (2007) [83] | GITR expression was up-reregulated in T cells when co-cultured with HCC cells | Increased proliferation of CD4+/CD25+ T cells was observed when PBMCs and HCC cells were co-cultured | GITR upregulation improves HCC prognosis via enhanced immune response due to CD4+/CD25+ T cell proliferation |
| Zhang et al. (2010) [84] | Higher GITR expression on Tregs in tumor-infiltrating lymphocytes | Increased number of Tregs in HCC patients | GITR modulating Tregs depletion strategies may improve HCC prognosis |
| Ormandy et al. (2015) [85] | Higher GITR expression on CD4+/CD25+ T cells in HCC patients | Increased proliferation of CD4+/CD25+ T cells in HCC patients | CD4+/CD25+ T cell activity promotes tumor growth, and targeting them with immunotherapeutic agents may improve HCC prognosis |
| Zhang et al. (2009) [86] | Higher GITR expression on CD4+/CD25+ T cells and Tregs in tumor tissue | Increased proliferation of CD4+/CD25+ T cells in HCC patients | CD4+/CD25+ T cell activity promotes tumor growth, and targeting them with immunotherapeutic agents may improve HCC prognosis |
| He et al. (2022) [88] | The high GITRL expression group had a lower overall survival | Higher GITR and GITRL transcription in tumor tissue | GITRL is associated with lower survival rates, and blocking its activity may improve HCC prognosis |
| Cari et al. (2018) [90] | GITR did not satisfy the overexpression criterion in HCC | GITR was overexpressed in other types of malignancies | n/a |
| Murugaiyan et al. (2007) [92] | Lower GITR expression on T cells with higher CD40 expression | Higher CD40 expression on T cells promotes tumor growth | GITR expression is negatively correlated with negative HCC prognostic factors (CD40 expression) on Tregs |
| Wei et al. (2016) [93] | Lower GITR expression on Tregs after Leptin/LEPR inhibition | Upregulation of Leptin/LEPR on Tregs in HCC cells | Hepatoma cells enhance anti-HCC immune response via secreting leptin to decrease Tregs activity, promoting CD8+ T-cell response and improving HCC prognosis. |
| Pan et al. (2022) [95] | Higher GITR expression patients had higher survival prognoses. DTA-1 treatment decreased tumor size only when combined with anti-IL-4 | Higher GITR expression on Tregs in tumor tissue | The increased GITR expression in tumor Tregs makes them a potential target for DTA-1 treatment, which may improve HCC prognosis. |
| Beek et al. (2019) [97] | The combination of GITRL and nivolumab increased the proliferation of CD4+/CD8+ T cells | Higher GITR expression on CD4+ and Treg in tumor tissue | GITRL/Nivolumab combination improves HCC prognosis, enhancing anti-tumor immunity via Tregs depletion |
| Gonzalez et al. (2015) [98] | GITRL and anti-CTLA-4 increased the proliferation of T cells and cytokine production | Higher GITR expression on Tregs in tumor tissue. GITRL decreased Tregs suppression | The increased GITR expression in tumor immune cells makes them a potential target for combination immunotherapy (targeting GITR and CTLA-4), with promising results in HCC prognosis. |
| Langhans et al. (2019) [99] | Anti-GITR did not decrease IFN-γ secretion in CD8+ T cells | GITR expression does not differ between patients and HCs | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakos, S.P.; Chatzikalil, E.; Vakadaris, G.; Reppas, L.; Arvanitakis, K.; Koufakis, T.; Siakavellas, S.I.; Manolakopoulos, S.; Germanidis, G.; Theocharis, S. Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma. Cancers 2024, 16, 2609. https://doi.org/10.3390/cancers16142609
Papadakos SP, Chatzikalil E, Vakadaris G, Reppas L, Arvanitakis K, Koufakis T, Siakavellas SI, Manolakopoulos S, Germanidis G, Theocharis S. Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma. Cancers. 2024; 16(14):2609. https://doi.org/10.3390/cancers16142609
Chicago/Turabian StylePapadakos, Stavros P., Elena Chatzikalil, Georgios Vakadaris, Lampros Reppas, Konstantinos Arvanitakis, Theocharis Koufakis, Spyros I. Siakavellas, Spilios Manolakopoulos, Georgios Germanidis, and Stamatios Theocharis. 2024. "Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma" Cancers 16, no. 14: 2609. https://doi.org/10.3390/cancers16142609
APA StylePapadakos, S. P., Chatzikalil, E., Vakadaris, G., Reppas, L., Arvanitakis, K., Koufakis, T., Siakavellas, S. I., Manolakopoulos, S., Germanidis, G., & Theocharis, S. (2024). Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma. Cancers, 16(14), 2609. https://doi.org/10.3390/cancers16142609










