Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Measurement Methodology
2.3. Statistical Analysis
2.3.1. Descriptive Analysis
2.3.2. Univariable Analysis
2.3.3. Multivariable Analysis
2.3.4. Software
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reagents and Devices | Data of the Manufacturers |
|---|---|
| Vacutainer® System | BD, Plymouth, UK |
| ICP-MS, NexION 350D | PerkinElmer, Concord, ON, Canada |
| Multi-Element Calibration Standard 3 | PerkinElmer Pure Plus, Shelton, CT, USA |
| TMAH | AlfaAesar, Kandel, Germany |
| Triton X-100 | PerkinElmer, Shelton, CT, USA |
| n-butanol | Merck, Darmstadt, Germany |
| EDTA | Merck, Darmstadt, Germany |
| Clincheck Plasmonorm Serum Trace Elements Level 1 | Recipe, Munich, Germany |
| Seronorm Serum Trace Elements | Sero, Hvalstad, Norway |
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Prostate Cancer. 2023. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 24 April 2024).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Darst, B.F.; Saunders, E.; Dadaev, T.; Sheng, X.; Wan, P.; Pooler, L.; Xia, L.Y.; Chanock, S.; Berndt, S.I.; Wang, Y.; et al. Germline Sequencing Analysis to Inform Clinical Gene Panel Testing for Aggressive Prostate Cancer. JAMA Oncol. 2023, 9, 1514–1524. [Google Scholar] [CrossRef]
- Finch, A.; Clark, R.; Vesprini, D.; Lorentz, J.; Kim, R.H.; Thain, E.; Fleshner, N.; Akbari, M.R.; Cybulski, C.; Narod, S.A. An Appraisal of Genetic Testing for Prostate Cancer Susceptibility. NPJ Precis. Oncol. 2022, 6, 43. [Google Scholar] [CrossRef]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-Associated Thrombosis: An Overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Sears, S.P.; Carr, G.; Bime, C. Acute and Chronic Respiratory Failure in Cancer Patients. In Oncologic Critical Care; Springer International Publishing: Cham, Switzerland, 2020; pp. 445–475. [Google Scholar] [CrossRef]
- Jain, M.A.; Leslie, S.W.; Sapra, A. Prostate Cancer Screening; StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Lopez-Valcarcel, M. Liquid Biopsy to Personalize Treatment for Metastatic Prostate Cancer. Am. J. Transl. Res. 2024, 16, 1531–1549. [Google Scholar] [CrossRef]
- Matuszczak, M.; Schalken, J.A.; Salagierski, M. Prostate Cancer Liquid Biopsy Biomarkers’ Clinical Utility in Diagnosis and Prognosis. Cancers 2021, 13, 3373. [Google Scholar] [CrossRef]
- Terracciano, D.; La Civita, E.; Athanasiou, A.; Liotti, A.; Fiorenza, M.; Cennamo, M.; Crocetto, F.; Tennstedt, P.; Schiess, R.; Haese, A.; et al. New Strategy for the Identification of Prostate Cancer: The Combination of Proclarix and the Prostate Health Index. Prostate 2022, 82, 1469–1476. [Google Scholar] [CrossRef]
- Resnick, M.J.; Koyama, T.; Fan, K.-H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-Term Functional Outcomes after Treatment for Localized Prostate Cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef]
- Kotamarti, S.; Polascik, T.J. Focal Cryotherapy for Prostate Cancer: A Contemporary Literature Review. Ann. Transl. Med. 2023, 11, 26. [Google Scholar] [CrossRef]
- Peters, I.; Hensen, B.; Glandorf, J.; Gutberlet, M.; Dohna, M.; Struckmann, S.; Kuczyk, M.A.; Wacker, F.; Hellms, S. First Experiences Using Transurethral Ultrasound Ablation (TULSA) as a Promising Focal Approach to Treat Localized Prostate Cancer: A Monocentric Study. BMC Urol. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Cocci, A.; Pezzoli, M.; Bianco, F.; Blefari, F.; Bove, P.; Cornud, F.; De Rienzo, G.; Destefanis, P.; Di Trapani, D.; Giacobbe, A.; et al. Transperineal Laser Ablation of the Prostate as a Treatment for Benign Prostatic Hyperplasia and Prostate Cancer: The Results of a Delphi Consensus Project. Asian J. Urol. 2024, 11, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xue, K.; Verma, A.; Shi, J.; Wei, Z.; Xia, X.; Wang, K.; Zhang, X. Photothermal Therapy: A Novel Potential Treatment for Prostate Cancer. Biomater. Sci. 2024, 12, 2480–2503. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, J.; Kim, H.J.; Rahmat, J.N.B.; Neoh, K.G.; Zhang, Y. Light-Responsive Smart Nanocarriers for Wirelessly Controlled Photodynamic Therapy for Prostate Cancers. Acta Biomater. 2023, 171, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lan, J.; Zhang, D.; Shen, W. Nanotherapeutics for Prostate Cancer Treatment: A Comprehensive Review. Biomaterials 2024, 305, 122469. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, R.; Schafer, C.; Tan, S.-H. Prostate Cancer Autoantibodies—Applications in Diagnosis, Prognosis, Monitoring Disease Progression and Immunotherapy. Am. J. Clin. Exp. Urol. 2023, 11, 79–102. [Google Scholar]
- Yamaguchi, Y.; Gibson, J.; Ou, K.; Lopez, L.S.; Ng, R.H.; Leggett, N.; Jonsson, V.D.; Zarif, J.C.; Lee, P.P.; Wang, X.; et al. PD-L1 Blockade Restores CAR T Cell Activity through IFN-γ-Regulation of CD163+ M2 Macrophages. J. Immunother. Cancer 2022, 10, e004400. [Google Scholar] [CrossRef]
- Schatten, H. Immunodiagnostics and Immunotherapy Possibilities for Prostate Cancer. In Molecular & Diagnostic Imaging in Prostate Cancer; Springer: Cham, Switzerland, 2018; pp. 185–194. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Yan, B.; Zhang, S.; Xu, B.; Lin, M.; Shuai, X.; Huang, J.; Pang, J. Tumor Acidity-Activatable Macromolecule Autophagy Inhibitor and Immune Checkpoint Blockade for Robust Treatment of Prostate Cancer. Acta Biomater. 2023, 168, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.; Simko, J.P.; Carroll, P.R.; Noworolski, S.M. Prostate Cancer Lesion Detection, Volume Quantification and High-Grade Cancer Differentiation Using Cancer Risk Maps Derived from Multiparametric MRI with Histopathology as the Reference Standard. Magn. Reson. Imaging 2023, 99, 48–57. [Google Scholar] [CrossRef]
- Wang, B.; Gao, J.; Zhang, Q.; Zhang, C.; Liu, G.; Wei, W.; Huang, H.; Fu, Y.; Li, D.; Zhang, B.; et al. Investigating the Equivalent Performance of Biparametric Compared to Multiparametric MRI in Detection of Clinically Significant Prostate Cancer. Abdom. Radiol. 2020, 45, 547–555. [Google Scholar] [CrossRef]
- Roberts, M.J.; Maurer, T.; Perera, M.; Eiber, M.; Hope, T.A.; Ost, P.; Siva, S.; Hofman, M.S.; Murphy, D.G.; Emmett, L.; et al. Using PSMA Imaging for Prognostication in Localized and Advanced Prostate Cancer. Nat. Rev. Urol. 2023, 20, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, C.; Zhang, Z.; Zhang, N.; Guo, X.; Xia, L.; Jiang, J.; Xie, Q.; Yan, K.; Rowe, S.P.; et al. 64Cu-PSMA-BCH: A New Radiotracer for Delayed PET Imaging of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Neumann, C.; Eiber, M.; Wester, H.J.; Schottelius, M. [99cmTc]Tc-PSMA-I&S-SPECT/CT: Experience in Prostate Cancer Imaging in an Outpatient Center. EJNMMI Res. 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Groener, D.; Schneider, S.; Baumgarten, J.; Happel, C.; Klimek, K.; Mader, N.; Nguyen Ngoc, C.; Wichert, J.; Mandel, P.; Tselis, N.; et al. Baseline [68Ga]Ga-PSMA-11 PET/CT before [177Lu]Lu-PSMA-617 Radioligand Therapy: Value of PSMA-Uptake Thresholds in Predicting Targetable Lesions. Cancers 2023, 15, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Son, M.H.; Ha, L.N.; Lan, X. PSMA-Based 18F-DCFPyL PET: A Better Choice than Multiparametric MRI for Prostate Cancer Diagnosis? Am. J. Nucl. Med. Mol. Imaging 2022, 12, 195–200. [Google Scholar]
- Zhao, J.; Mangarova, D.B.; Brangsch, J.; Kader, A.; Hamm, B.; Brenner, W.; Makowski, M.R. Correlation between Intraprostatic PSMA Uptake and MRI PI-RADS of [68Ga]Ga-PSMA-11 PET/MRI in Patients with Prostate Cancer: Comparison of PI-RADS Version 2.0 and PI-RADS Version 2.1. Cancers 2020, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Venclovas, Z.; Sasnauskas, G.; Ruzgas, T. The Significance of Prostate Specific Antigen Persistence in Prostate Cancer Risk Groups on Long-Term Oncological Outcomes. Cancers 2021, 13, 2453. [Google Scholar] [CrossRef] [PubMed]
- von Deimling, M.; Rajwa, P.; Tilki, D.; Heidenreich, A.; Pallauf, M.; Bianchi, A.; Yanagisawa, T.; Kawada, T.; Karakiewicz, P.I.; Gontero, P.; et al. The Current Role of Precision Surgery in Oligometastatic Prostate Cancer. ESMO Open 2022, 7, 100597. [Google Scholar] [CrossRef]
- Matuszczak, M.; Salagierski, M. Oligometastatic Disease in Prostate Cancer. Evolving Paradigm: Current Knowledge, Diagnostic Techniques and Treatment Strategies. Arch. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients 2024, 16, 527. [Google Scholar] [CrossRef]
- Karagas, M.R.; Wang, A.; Dorman, D.C.; Hall, A.L.; Pi, J.; Sergi, C.M.; Symanski, E.; Ward, E.M.; Arrandale, V.H.; Azuma, K.; et al. Carcinogenicity of Cobalt, Antimony Compounds, and Weapons-Grade Tungsten Alloy. Lancet Oncol. 2022, 23, 577–578. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Chapter 3. Toxicokinetics, susceptible populations, biomarkers, chemical interactions. In Toxicological Profile for Cobalt; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2023. [Google Scholar]
- Alexandersson, R. Blood and Urinary Concentrations as Estimators of Cobalt Exposure. Arch. Environ. Health Int. J. 1988, 43, 299–303. [Google Scholar] [CrossRef]
- Tvermoes, B.E.; Unice, K.M.; Paustenbach, D.J.; Finley, B.L.; Otani, J.M.; Galbraith, D.A. Effects and Blood Concentrations of Cobalt after Ingestion of 1 Mg/d by Human Volunteers for 90 d. Am. J. Clin. Nutr. 2014, 99, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lee, V.R. Cobalt Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Leonard, S.; Gannett, P.M.; Rojanasakul, Y.; Schwegler-Berry, D.; Castranova, V.; Vallyathan, V.; Shi, X. Cobalt-Mediated Generation of Reactive Oxygen Species and Its Possible Mechanism. J. Inorg. Biochem. 1998, 70, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Angelé-Martínez, C.; Murray, J.; Stewart, P.A.; Haines, J.; Gaertner, A.A.E.; Brumaghim, J.L. Cobalt-Mediated Oxidative DNA Damage and Its Prevention by Polyphenol Antioxidants. J. Inorg. Biochem. 2023, 238, 112024. [Google Scholar] [CrossRef] [PubMed]
- Valberg, L.S.; Ludwig, J.; Olatunbosun, D. Alteration in Cobalt Absorption in Patients with Disorders of Iron Metabolism. Gastroenterology 1969, 56, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Wu, L.; Yang, L.; Yang, L.; Wang, J. The Diversified Role of Mitochondria in Ferroptosis in Cancer. Cell Death Dis. 2023, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W. The Role of Chelation in the Treatment of Other Metal Poisonings. J. Med. Toxicol. 2013, 9, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Kusaka, Y.; Goto, S. Biological Monitoring of Cobalt Exposure, Based on Cobalt Concentrations in Blood and Urine. Int. Arch. Occup. Environ. Health 1985, 55, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Stuckert, J.; Nedorost, S. Low-cobalt Diet for Dyshidrotic Eczema Patients. Contact Dermat. 2008, 59, 361–365. [Google Scholar] [CrossRef]
- Hokin, B.; Adams, M.; Ashton, J.; Louie, H. Comparison of the Dietary Cobalt Intake in Three Different Australian Diets. Asia Pac. J. Clin. Nutr. 2004, 13, 289–291. [Google Scholar]
- Biego, G.; Joyeux, M.; Hartemann, P.; Debry, G. Daily Intake of Essential Minerals and Metallic Micropollutants from Foods in France. Sci. Total Environ. 1998, 217, 27–36. [Google Scholar] [CrossRef]
- Arnich, N.; Sirot, V.; Rivière, G.; Jean, J.; Noël, L.; Guérin, T.; Leblanc, J.-C. Dietary Exposure to Trace Elements and Health Risk Assessment in the 2nd French Total Diet Study. Food Chem. Toxicol. 2012, 50, 2432–2449. [Google Scholar] [CrossRef]
- Gál, J.; Hursthouse, A.; Tatner, P.; Stewart, F.; Welton, R. Cobalt and Secondary Poisoning in the Terrestrial Food Chain: Data Review and Research Gaps to Support Risk Assessment. Environ. Int. 2008, 34, 821–838. [Google Scholar] [CrossRef]
- Marín, S.; Pardo, O.; Sánchez, A.; Sanchis, Y.; Vélez, D.; Devesa, V.; Font, G.; Yusà, V. Assessment of Metal Levels in Foodstuffs from the Region of Valencia (Spain). Toxicol. Rep. 2018, 5, 654–670. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the Use of Cobalt Compounds as Additives in Animal Nutrition. EFSA J. 2009, 7, 1383. [Google Scholar] [CrossRef]
- Tvermoes, B.E.; Finley, B.L.; Unice, K.M.; Otani, J.M.; Paustenbach, D.J.; Galbraith, D.A. Cobalt Whole Blood Concentrations in Healthy Adult Male Volunteers Following Two-Weeks of Ingesting a Cobalt Supplement. Food Chem. Toxicol. 2013, 53, 432–439. [Google Scholar] [CrossRef]
- Lippi, G.; Franchini, M.; Guidi, G.C. Blood Doping by Cobalt. Should We Measure Cobalt in Athletes? J. Occup. Med. Toxicol. 2006, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W.; Lundby, C. Blood Doping and Its Detection. Blood 2011, 118, 2395–2404. [Google Scholar] [CrossRef]
- National Academies Press. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy Beverages: Content and Safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.M.; Poulsen, O.M.; Thomsen, M. A Short-Term Cross-over Study on Oral Administration of Soluble and Insoluble Cobalt Compounds: Sex Differences in Biological Levels. Int. Arch. Occup. Environ. Health 1993, 65, 233–240. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: Burlington, MA, USA, 2007. [Google Scholar] [CrossRef]
- Harp, M.J.; Scoular, F.I. Cobalt Metabolism of Young College Women on Self-Selected Diets. J. Nutr. 1952, 47, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Edmonds, C.J.; Barnaby, C.F. Absorption and Retention of Cobalt in Man by Whole-Body Counting. Health Phys. 1972, 22, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sorbie, J.; Olatunbosun, D.; Corbett, W.E.; Valberg, L.S. Cobalt Excretion Test for the Assessment of Body Iron Stores. Can. Med. Assoc. J. 1971, 104, 777–782. [Google Scholar] [PubMed]
- Reuber, S.; Kreuzer, M.; Kirchgessner, M. Interactions of Cobalt and Iron in Absorption and Retention. J. Trace Elem. Electrolytes Health Dis. 1994, 8, 151–158. [Google Scholar] [PubMed]
- Hoffmann, P. Speciation of Iron. In Handbook of Elemental Speciation II—Species in the Environment, Food, Medicine and Occupational Health; Wiley: Hoboken, NJ, USA, 2005; pp. 200–217. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Tvermoes, B.E.; Unice, K.M.; Finley, B.L.; Kerger, B.D. A Review of the Health Hazards Posed by Cobalt. Crit. Rev. Toxicol. 2013, 43, 316–362. [Google Scholar] [CrossRef] [PubMed]
- Collecchi, P.; Esposito, M.; Brera, S.; Mora, E.; Mazzucotelli, A.; Oddone, M. The Distribution of Arsenic and Cobalt in Patients with Laryngeal Carcinoma. J. Appl. Toxicol. 1986, 6, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Forbes, R.M.; Cooper, A.R.; Mitchell, H.H. On the occurrence of beryllium, boron, cobalt, and mercury in human tissues. J. Biol. Chem. 1954, 209, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, P.J. Accumulation of Metals in the Tissues of Occupationally Exposed Workers. Environ. Geochem. Health 1988, 10, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Koizumi, M.; Yoshida, A. Metal Concentrations in Human Pancreatic Juice. Arch. Environ. Health Int. J. 1987, 42, 356–360. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Parr, R.M. Concentrations of Some Trace Elements in Hair, Liver and Kidney from Autopsy Subjects—Relationship between Hair and Internal Organs. Sci. Total Environ. 1988, 76, 29–40. [Google Scholar] [CrossRef]
- Teraoka, H. Distribution of 24 Elements in the Internal Organs of Normal Males and the Metallic Workers in Japan. Arch. Environ. Health Int. J. 1981, 36, 155–165. [Google Scholar] [CrossRef]
- YAMAGATA, N.; MURATA, S.; TORII, T. The Cobalt Content of Human Body. J. Radiat. Res. 1962, 3, 4–8. [Google Scholar] [CrossRef][Green Version]
- Yukawa, M.; Suzuki-Yasumoto, M.; Amano, K.; Terai, M. Distribution of Trace Elements in the Human Body Determined by Neutron Activation Analysis. Arch. Environ. Health Int. J. 1980, 35, 36–44. [Google Scholar] [CrossRef]
- GARDNER, F.H. The Use of Cobaltous Chloride in the Anemia Associated with Chronic Renal Disease. J. Lab. Clin. Med. 1953, 41, 56–64. [Google Scholar]
- Licht, A.; Oliver, M.; Rachmilewitz, E.A. Optic Atrophy Following Treatment with Cobalt Chloride in a Patient with Pancytopenia and Hypercellular Marrow. Isr. J. Med. Sci. 1972, 8, 61–66. [Google Scholar]
- Rohn, R.J.; Bond, W.H.; Klotz, L.J. The Effect of Cobalt-Iron Therapy in Iron Deficiency Anemia in Infants. J. Indiana State Med. Assoc. 1953, 46, 1253–1260. [Google Scholar]
- Wolf, J. Treatment of sickle-cell anemia with cobalt chloride. Arch. Intern. Med. 1954, 93, 387–396. [Google Scholar] [CrossRef]
- Tvermoes, B.E.; Paustenbach, D.J.; Kerger, B.D.; Finley, B.L.; Unice, K.M. Review of Cobalt Toxicokinetics Following Oral Dosing: Implications for Health Risk Assessments and Metal-on-Metal Hip Implant Patients. Crit. Rev. Toxicol. 2015, 45, 367–387. [Google Scholar] [CrossRef]
- GROSS, R.T.; KRISS, J.P.; SPAET, T.H. The Hematopoletic and Goltrogenic Effects of Cobaltous Chloride in Patients with Sickle Cell Anemia. Pediatrics 1955, 15, 284–290. [Google Scholar] [CrossRef]
- Kriss, J.P. Hypothyroidism and thyroid hyperplasia in patients treated with cobalt. J. Am. Med. Assoc. 1955, 157, 117–121. [Google Scholar] [CrossRef]
- Bowie, E.A.; Hurley, P.J. Cobalt Chloride in the Treatment of Refractory Anaemia in Patients Undergoing Long-term Haemodialysis. Aust. N. Z. J. Med. 1975, 5, 306–313. [Google Scholar] [CrossRef]
- Curtis, J.R.; Goode, G.C.; Herrington, J.; Urdaneta, L.E. Possible Cobalt Toxicity in Maintenance Hemodialysis Patients after Treatment with Cobaltous Chloride: A Study of Blood and Tissue Cobalt Concentrations in Normal Subjects and Patients with Terminal and Renal Failure. Clin. Nephrol. 1976, 5, 61–65. [Google Scholar]
- Duckham, J.M.; Lee, H.A. The Treatment of Refractory Anaemia of Chronic Renal Failure with Cobalt Chloride. Q. J. Med. 1976, 45, 277–294. [Google Scholar]
- Manifold, I.H.; Platts, M.M.; Kennedy, A. Cobalt Cardiomyopathy in a Patient on Maintenance Haemodialysis. BMJ 1978, 2, 1609. [Google Scholar] [CrossRef]
- Schirrmacher, U.O. Case of Cobalt Poisoning. BMJ 1967, 1, 544–545. [Google Scholar] [CrossRef][Green Version]
- Llobet, J.M.; Domingo, J.L.; Corbella, J. Comparative Effects of Repeated Parenteral Administration of Several Chelators on the Distribution and Excretion of Cobalt. Res. Commun. Chem. Pathol. Pharmacol. 1988, 60, 225–233. [Google Scholar]
- Pazzaglia, U.E.; Apostoli, P.; Congiu, T.; Catalani, S.; Marchese, M.; Zarattini, G. Cobalt, Chromium and Molybdenum Ions Kinetics in the Human Body: Data Gained from a Total Hip Replacement with Massive Third Body Wear of the Head and Neuropathy by Cobalt Intoxication. Arch. Orthop. Trauma. Surg. 2011, 131, 1299–1308. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Ursino, N. N-Acetyl-Cysteine Reduces Blood Chromium and Cobalt Levels in Metal-on-Metal Hip Arthroplasty. Arthroplast. Today 2020, 6, 149–152. [Google Scholar] [CrossRef]
| Variable | Overall, n = 261 |
|---|---|
| Vital status | |
| Alive | 205 (78.54%) |
| Dead | 56 (21.46%) |
| Cause of death | |
| Other | 25 (44.64%) |
| Prostate cancer | 31 (55.36%) |
| Age of diagnosis | 46–86 (65.70) |
| <60 | 58 (22.22%) |
| ≥60 | 203 (77.78%) |
| Gleason | |
| <7 | 85 (32.57%) |
| 7 | 133 (50.96%) |
| >7 | 43 (16.48%) |
| PSA | 0.25–700.00 (20.94) |
| <4 | 13 (4.98%) |
| 4–10 | 143 (54.79%) |
| >10 | 105 (40.23%) |
| Prostatectomy | |
| No | 57 (22.62%) |
| Yes | 195 (77.38%) |
| Unknown | 9 |
| Radiotherapy | |
| No | 114 (48.51%) |
| Yes | 121 (51.49%) |
| Unknown | 26 |
| Chemotherapy | |
| No | 207 (92.83%) |
| Yes | 16 (7.17%) |
| Unknown | 38 |
| Hormone therapy | |
| No | 154 (64.17%) |
| Yes | 86 (35.83%) |
| Unknown | 21 |
| Cobalt level | |
| QI: 0.06–0.10 (0.09) | 59 (22.61%) |
| QII: 0.10–0.12 (0.11) | 58 (22.22%) |
| QIII: 0.12–0.15 (0.14) | 62 (23.75%) |
| QIV: 0.15–4.02 (0.27) | 82 (31.42%) |
| All Study Group (n = 261) | Non-Cancer-Related Deaths (n = 230) | Prostate Cancer-Specific Deaths (n = 236) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n (%) | Mean | IQR | Median | p 1 | n (%) | Mean | IQR | Median | p 1 | n (%) | Mean | IQR | Median | p 1 |
| Vital status | <0.01 | <0.01 | 0.02 | ||||||||||||
| Alive | 205 (79%) | 0.16 | 0.06 | 0.12 | 205 (89%) | 0.16 | 0.06 | 0.12 | 205 (87%) | 0.16 | 0.06 | 0.12 | |||
| Dead | 56 (21%) | 0.17 | 0.07 | 0.16 | 25 (11%) | 0.16 | 0.05 | 0.17 | 31 (13%) | 0.17 | 0.09 | 0.15 | |||
| Age of diagnosis | 0.80 | >0.90 | 0.60 | ||||||||||||
| <60 | 58 (22%) | 0.22 | 0.08 | 0.13 | 55 (24%) | 0.22 | 0.07 | 0.12 | 55 (23%) | 0.22 | 0.08 | 0.12 | |||
| ≥60 | 203 (78%) | 0.14 | 0.07 | 0.13 | 175 (76%) | 0.14 | 0.06 | 0.13 | 181 (77%) | 0.14 | 0.06 | 0.12 | |||
| Gleason | <0.01 | <0.01 | <0.01 | ||||||||||||
| <7 | 85 (33%) | 0.21 | 0.08 | 0.15 | 81 (35%) | 0.21 | 0.08 | 0.15 | 71 (30%) | 0.21 | 0.07 | 0.15 | |||
| 7 | 133 (51%) | 0.13 | 0.05 | 0.12 | 119 (52%) | 0.13 | 0.05 | 0.12 | 127 (54%) | 0.13 | 0.05 | 0.12 | |||
| >7 | 43 (16%) | 0.15 | 0.07 | 0.13 | 30 (13%) | 0.16 | 0.08 | 0.13 | 38 (16%) | 0.15 | 0.06 | 0.13 | |||
| PSA | 0.06 | 0.15 | 0.11 | ||||||||||||
| <4 | 13 (5.0%) | 0.13 | 0.04 | 0.12 | 12 (5.2%) | 0.13 | 0.04 | 0.11 | 10 (4.2%) | 0.13 | 0.03 | 0.11 | |||
| 4–10 | 143 (55%) | 0.17 | 0.06 | 0.12 | 136 (59%) | 0.17 | 0.06 | 0.12 | 131 (56%) | 0.17 | 0.05 | 0.12 | |||
| >10 | 105 (40%) | 0.16 | 0.08 | 0.14 | 82 (36%) | 0.15 | 0.08 | 0.13 | 95 (40%) | 0.15 | 0.07 | 0.13 | |||
| Prostatectomy | 0.03 | 0.03 | 0.04 | ||||||||||||
| No | 57 (23%) | 0.16 | 0.09 | 0.15 | 37 (17%) | 0.16 | 0.08 | 0.15 | 48 (21%) | 0.16 | 0.09 | 0.14 | |||
| Yes | 195 (77%) | 0.16 | 0.06 | 0.12 | 187 (83%) | 0.16 | 0.05 | 0.12 | 182 (79%) | 0.16 | 0.05 | 0.12 | |||
| Unknown | 9 | NA | NA | NA | 6 | NA | NA | NA | 6 | NA | NA | NA | |||
| Radiotherapy | >0.90 | 0.60 | 0.80 | ||||||||||||
| No | 114 (49%) | 0.18 | 0.06 | 0.12 | 107 (51%) | 0.18 | 0.05 | 0.12 | 103 (48%) | 0.18 | 0.06 | 0.12 | |||
| Yes | 121 (51%) | 0.14 | 0.06 | 0.12 | 104 (49%) | 0.14 | 0.06 | 0.12 | 112 (52%) | 0.14 | 0.05 | 0.12 | |||
| Unknown | 26 | NA | NA | NA | 19 | NA | NA | NA | 21 | NA | NA | NA | |||
| Chemotherapy | 0.30 | 0.09 | 0.60 | ||||||||||||
| No | 207 (93%) | 0.16 | 0.05 | 0.12 | 191 (95%) | 0.16 | 0.05 | 0.12 | 191 (93%) | 0.16 | 0.05 | 0.12 | |||
| Yes | 16 (7.2%) | 0.15 | 0.09 | 0.12 | 10 (5.0%) | 0.16 | 0.08 | 0.16 | 14 (6.8%) | 0.14 | 0.07 | 0.12 | |||
| Unknown | 38 | NA | NA | NA | 29 | NA | NA | NA | 31 | NA | NA | NA | |||
| Hormonotherapy | 0.03 | 0.04 | 0.04 | ||||||||||||
| No | 154 (64%) | 0.16 | 0.05 | 0.12 | 151 (71%) | 0.16 | 0.05 | 0.12 | 142 (65%) | 0.16 | 0.05 | 0.12 | |||
| Yes | 86 (36%) | 0.15 | 0.08 | 0.13 | 62 (29%) | 0.15 | 0.08 | 0.13 | 77 (35%) | 0.15 | 0.07 | 0.13 | |||
| Unknown | 21 | NA | NA | NA | 17 | NA | NA | NA | 17 | NA | NA | NA | |||
| Death Frequency | Univariable COX Regression | Multivariable COX Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 261 | Alive, n = 205 | Dead, n = 56 | HR | 95% CI | p | HR | 95% CI | p |
| Cobalt level | |||||||||
| QI (reference): 0.06–0.10 (0.09) | 59 (23%) | 51 (25%) | 8 (14%) | — | — | — | — | ||
| QII: 0.10–0.12 (0.11) | 58 (22%) | 51 (25%) | 7 (13%) | 0.89 | 0.32–2.45 | 0.80 | 0.93 | 0.33–2.58 | 0.90 |
| QIII: 0.12–0.15 (0.14) | 62 (24%) | 51 (25%) | 11 (20%) | 1.31 | 0.53–3.26 | 0.60 | 1.15 | 0.46–2.88 | 0.80 |
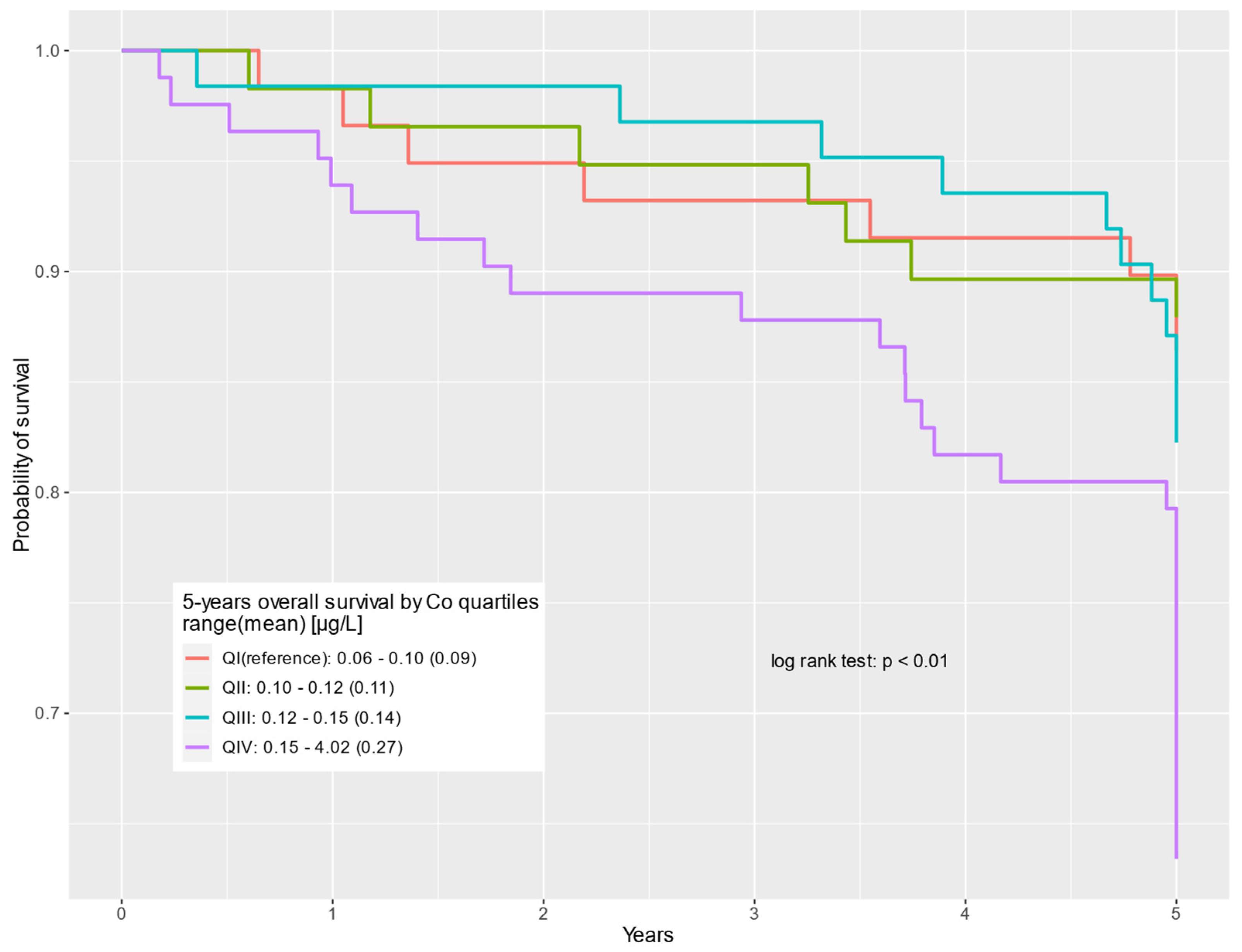
| QIV: 0.15–4.02 (0.27) | 82 (31%) | 52 (25%) | 30 (54%) | 3.02 | 1.38–6.59 | <0.01 | 2.60 | 1.17–5.82 | 0.02 |
| Death Frequency | Univariable COX Regression | Multivariable COX Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 230 | Alive, n = 205 | Dead, n = 25 | HR | 95% CI | p | HR | 95% CI | p |
| Cobalt level | |||||||||
| QI (reference): 0.06–0.10 (0.09) | 54 (23%) | 51 (25%) | 3 (12%) | — | — | — | — | ||
| QII: 0.10–0.12 (0.11) | 53 (23%) | 51 (25%) | 2 (8.0%) | 0.68 | 0.11–4.08 | 0.70 | 0.64 | 0.11–3.86 | 0.60 |
| QIII: 0.12–0.15 (0.14) | 56 (24%) | 51 (25%) | 5 (20%) | 1.63 | 0.39–6.84 | 0.50 | 1.55 | 0.37–6.52 | 0.50 |
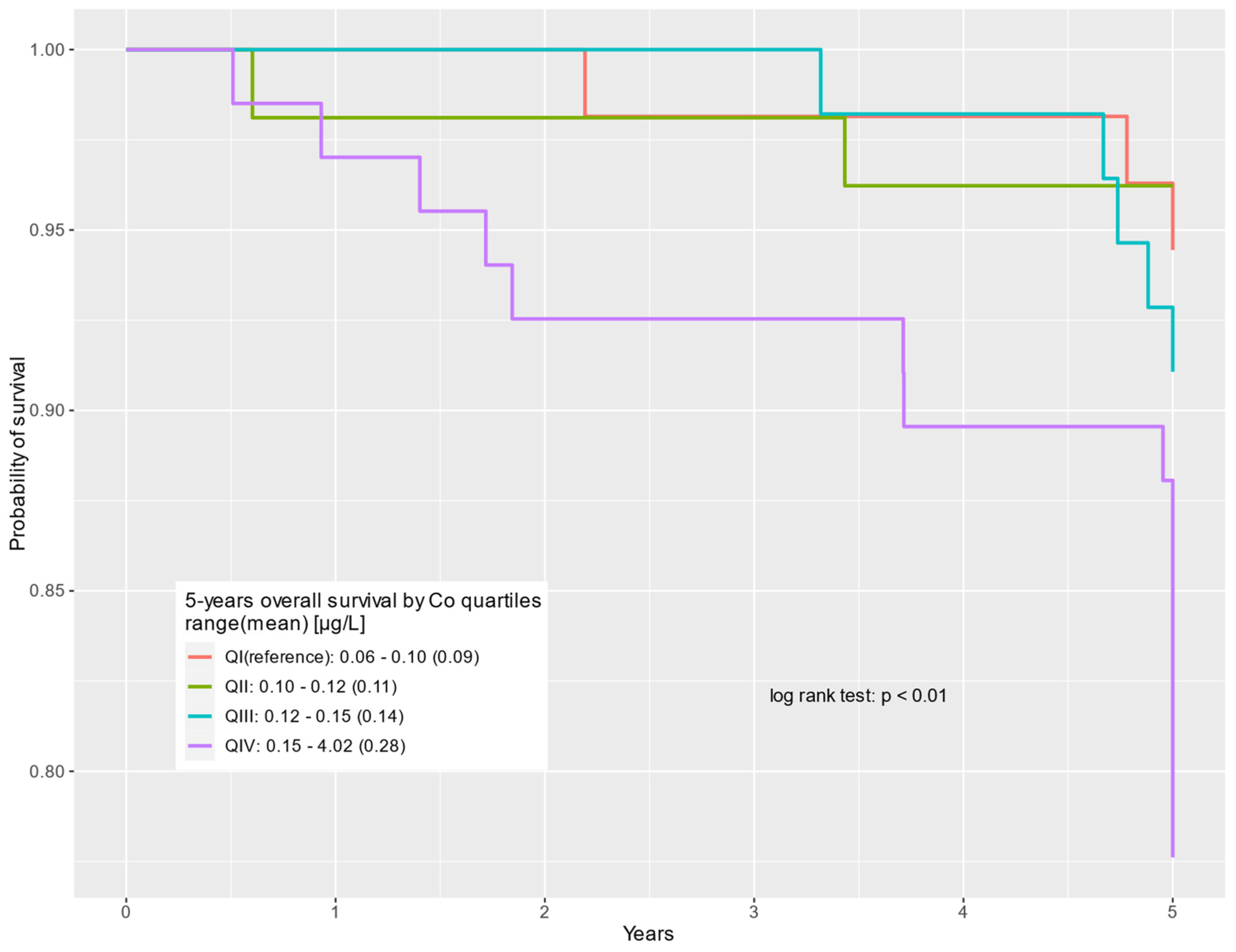
| QIV: 0.15–4.02 (0.28) | 67 (29%) | 52 (25%) | 15 (60%) | 4.39 | 1.27–15.20 | 0.02 | 3.67 | 1.03–13.00 | 0.04 |
| Death Frequency | Univariable COX Regression | Multivariable COX Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 236 | Alive, n = 205 | Dead, n = 31 | HR | 95% CI | p | HR | 95% CI | p |
| Cobalt level | |||||||||
| QI (reference): 0.06–0.10 (0.09) | 56 (24%) | 51 (25%) | 5 (16%) | — | — | — | — | ||
| QII: 0.10–0.12 (0.11) | 56 (24%) | 51 (25%) | 5 (16%) | 0.99 | 0.29–3.43 | >0.90 | 1.13 | 0.32–3.92 | 0.90 |
| QIII: 0.12–0.15 (0.14) | 57 (24%) | 51 (25%) | 6 (19%) | 1.17 | 0.36–3.82 | 0.80 | 0.87 | 0.26–2.93 | 0.80 |
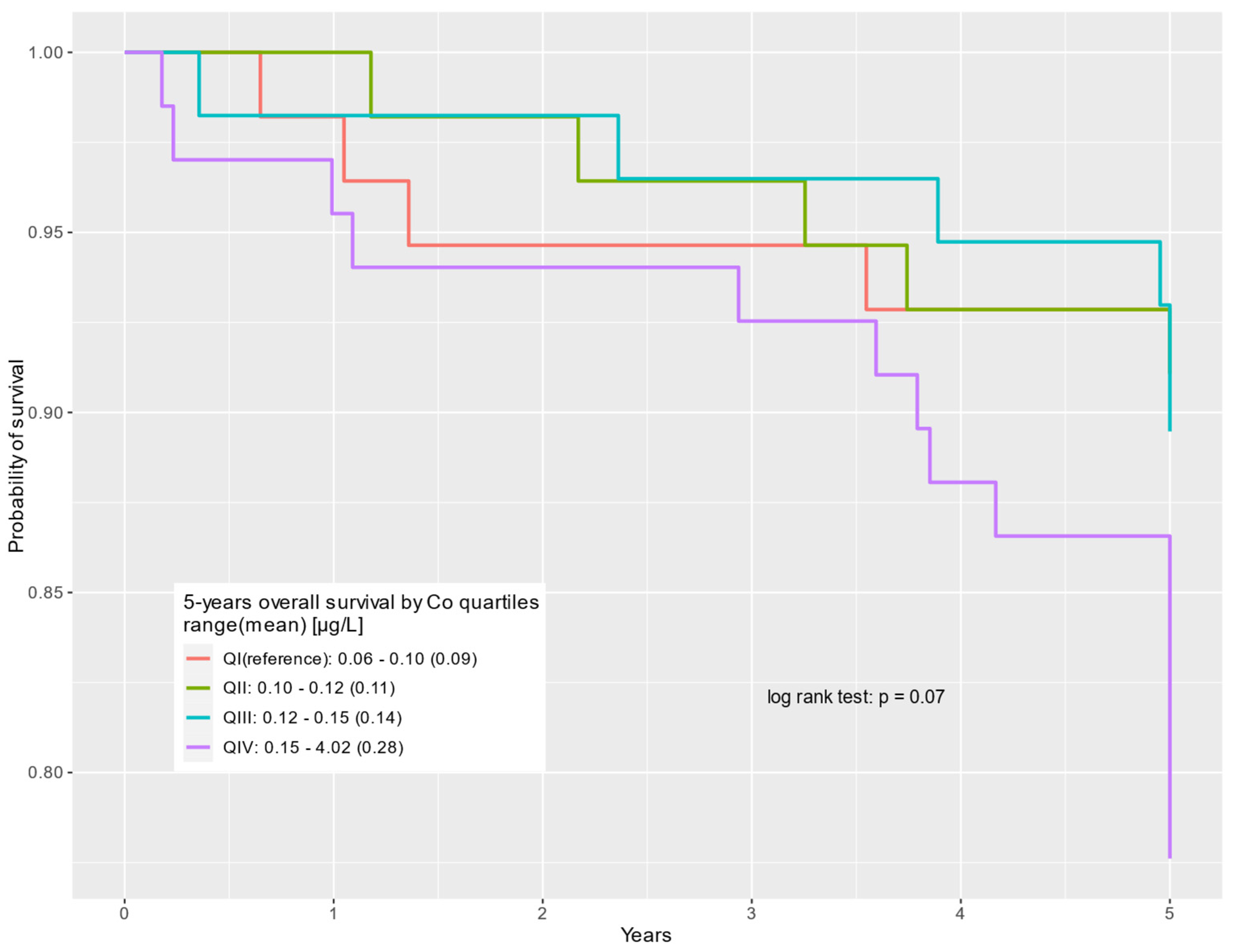
| QIV: 0.15–4.02 (0.28) | 67 (28%) | 52 (25%) | 15 (48%) | 2.64 | 0.96–7.26 | 0.06 | 2.17 | 0.76–6.18 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients. Cancers 2024, 16, 2618. https://doi.org/10.3390/cancers16152618
Pietrzak S, Marciniak W, Derkacz R, Matuszczak M, Kiljańczyk A, Baszuk P, Bryśkiewicz M, Sikorski A, Gronwald J, Słojewski M, et al. Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients. Cancers. 2024; 16(15):2618. https://doi.org/10.3390/cancers16152618
Chicago/Turabian StylePietrzak, Sandra, Wojciech Marciniak, Róża Derkacz, Milena Matuszczak, Adam Kiljańczyk, Piotr Baszuk, Marta Bryśkiewicz, Andrzej Sikorski, Jacek Gronwald, Marcin Słojewski, and et al. 2024. "Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients" Cancers 16, no. 15: 2618. https://doi.org/10.3390/cancers16152618
APA StylePietrzak, S., Marciniak, W., Derkacz, R., Matuszczak, M., Kiljańczyk, A., Baszuk, P., Bryśkiewicz, M., Sikorski, A., Gronwald, J., Słojewski, M., Cybulski, C., Gołąb, A., Huzarski, T., Dębniak, T., Lener, M. R., Jakubowska, A., Kluz, T., Soroka, M., Scott, R. J., & Lubiński, J. (2024). Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients. Cancers, 16(15), 2618. https://doi.org/10.3390/cancers16152618







