DNA Damage Response in Early Breast Cancer: A Phase III Cohort in the Phobos Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
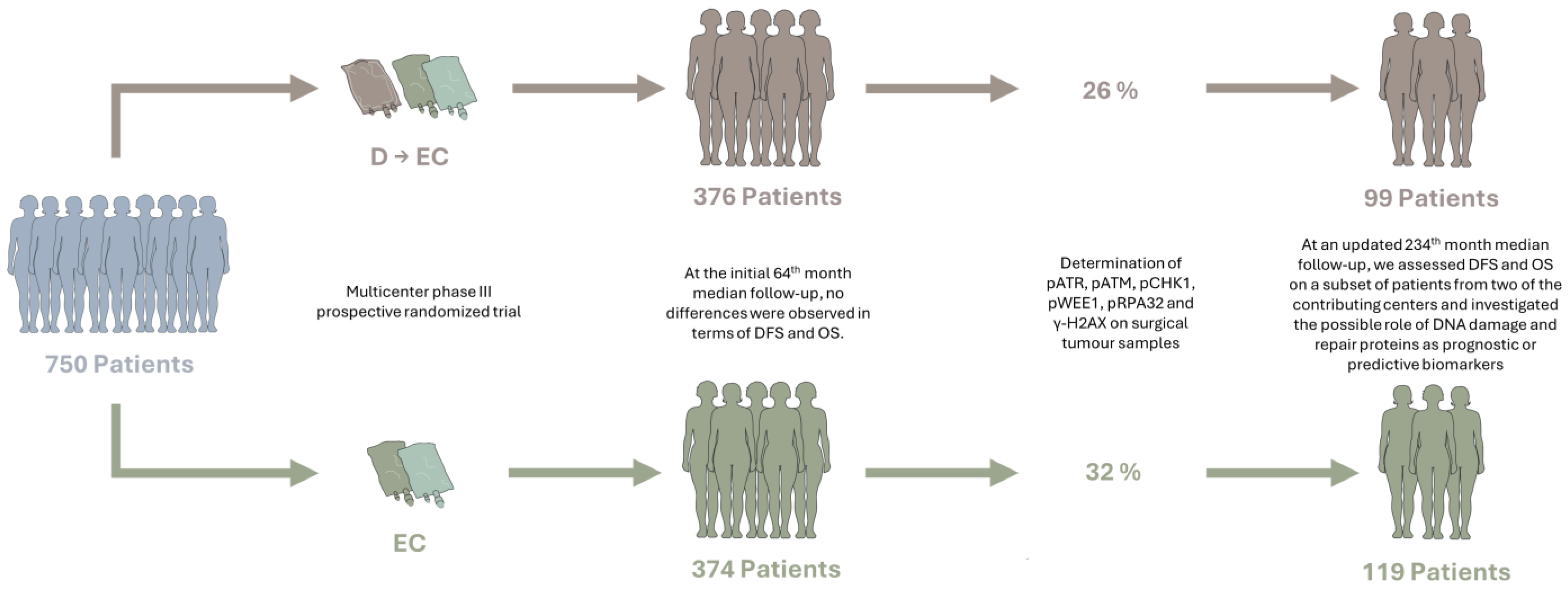
2.1. Study Design
2.2. Study Participants
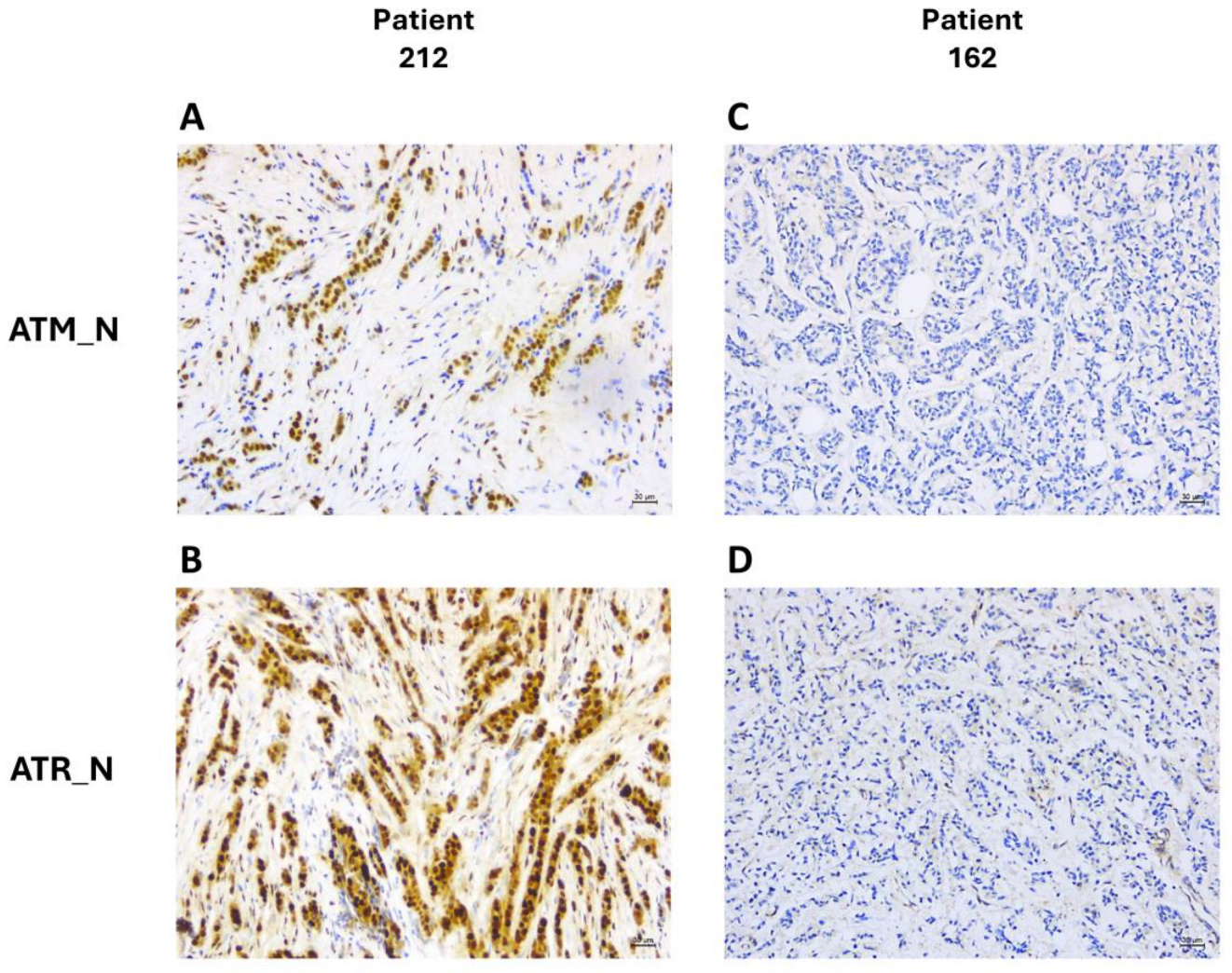
2.3. Immunohistochemistry
2.4. K-Means and Hierarchical Clustering Analysis
2.5. Statistical Analysis
2.5.1. Baseline Characteristics and Treatment Allocation
2.5.2. Survival Analysis
2.5.3. Correlation Analysis between DDR Biomarkers
2.5.4. Cox Elastic Net Analysis
2.5.5. Multivariate Analysis
2.5.6. Validation in TCGA BC Cohort
3. Results
3.1. Original Study and Baseline Characteristics of Patients
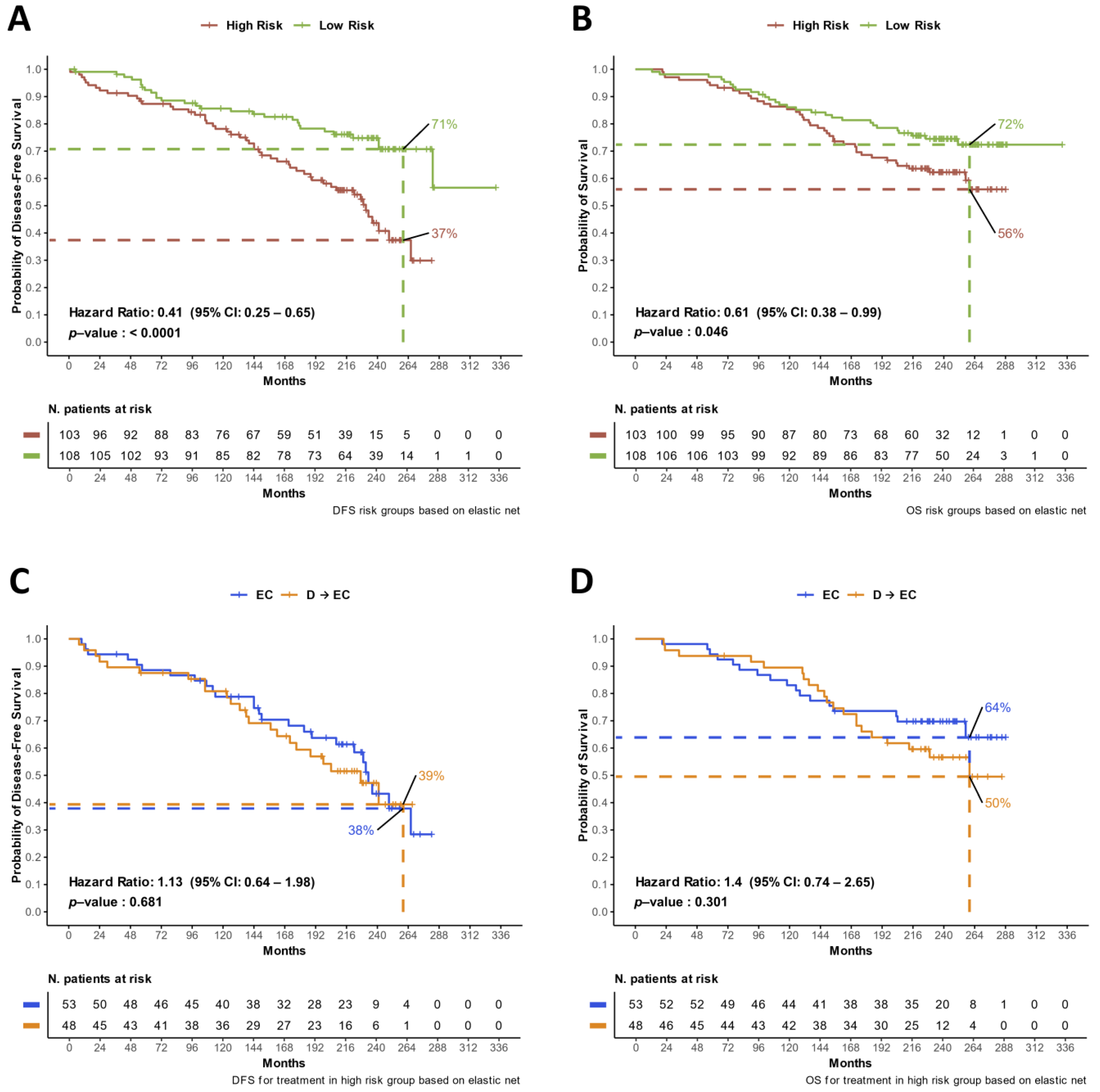
3.2. Updated DFS and OS Analyses
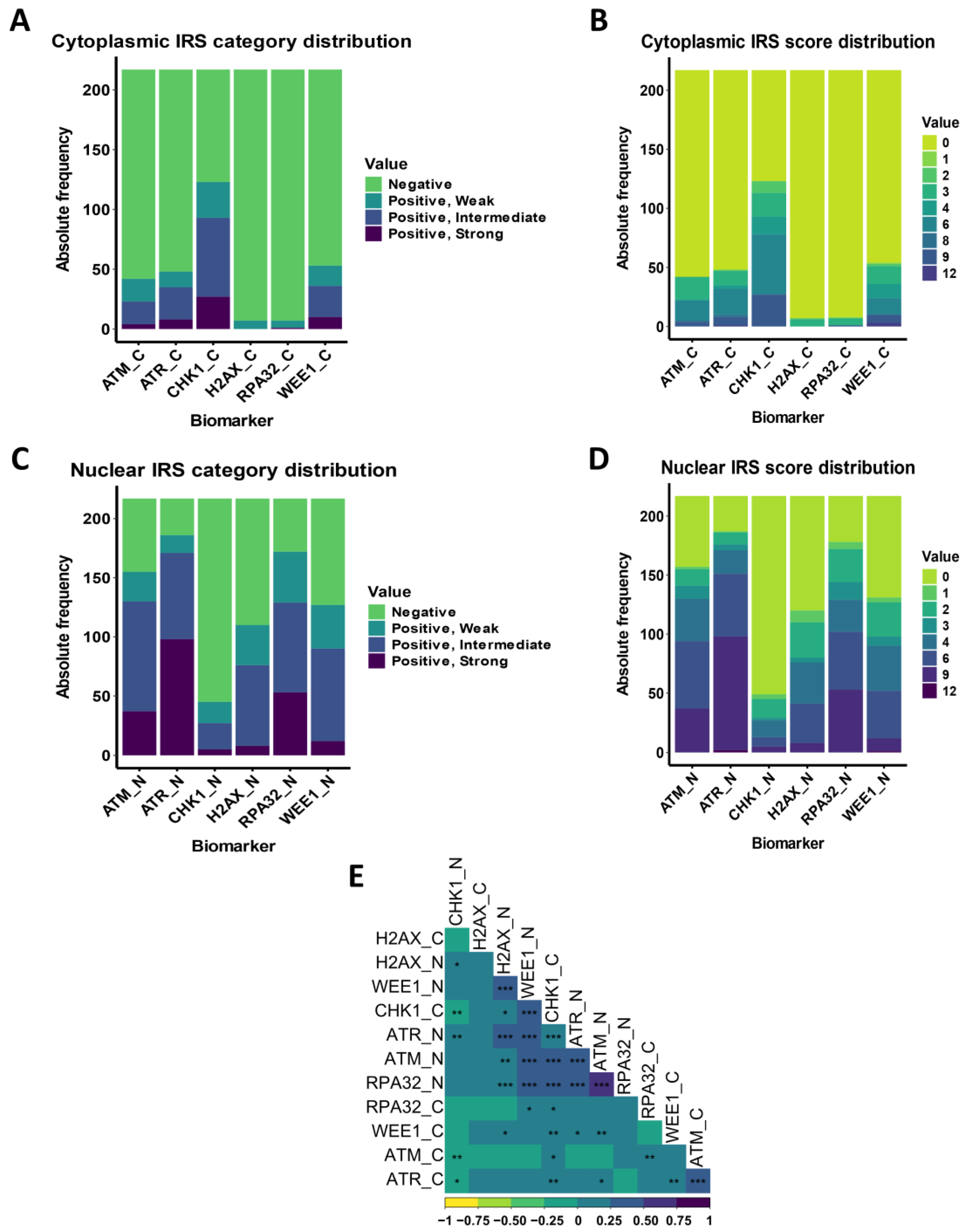
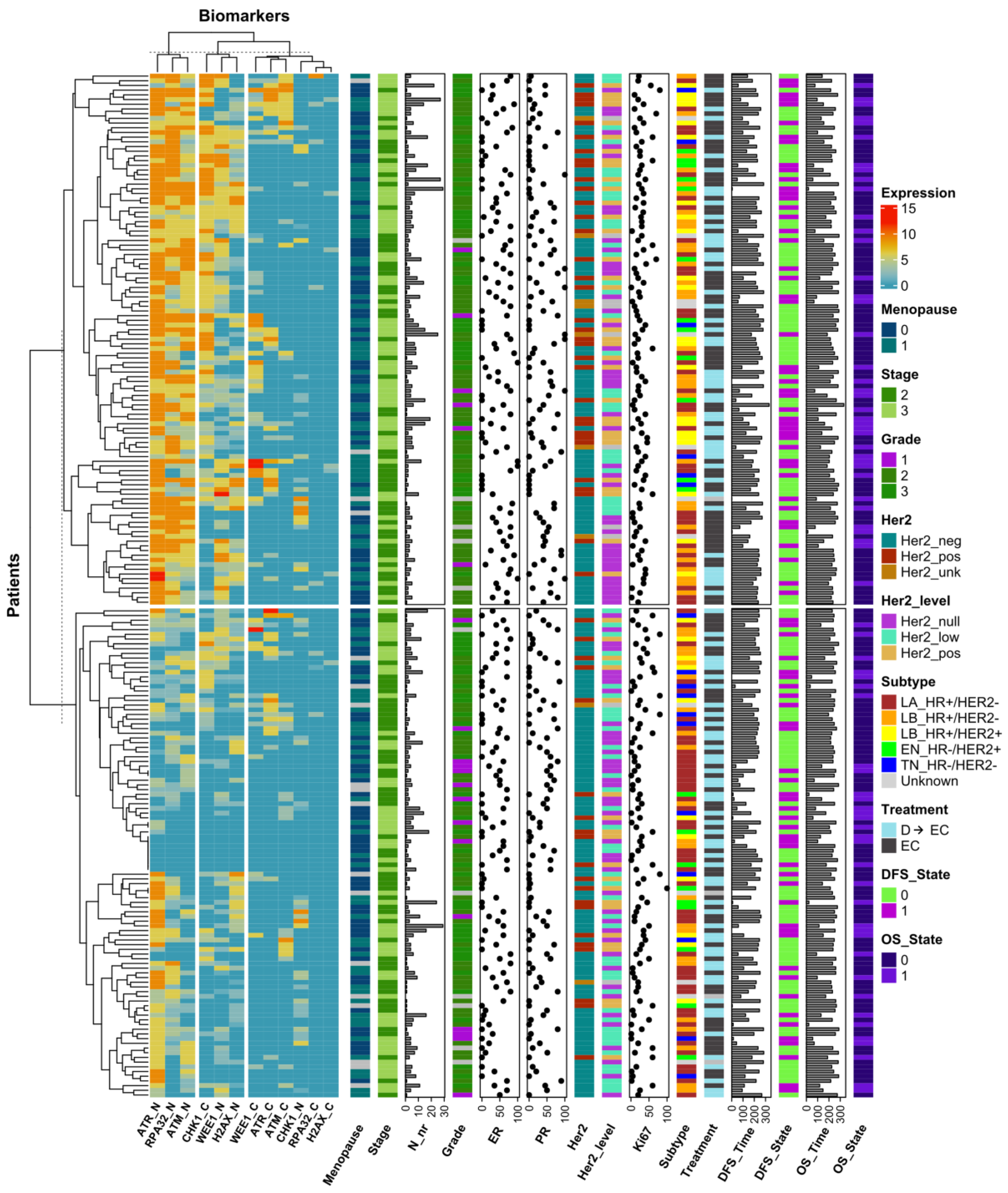
3.3. DDR Biomarker Expressions and Correlation with Clinical–Pathological Characteristics
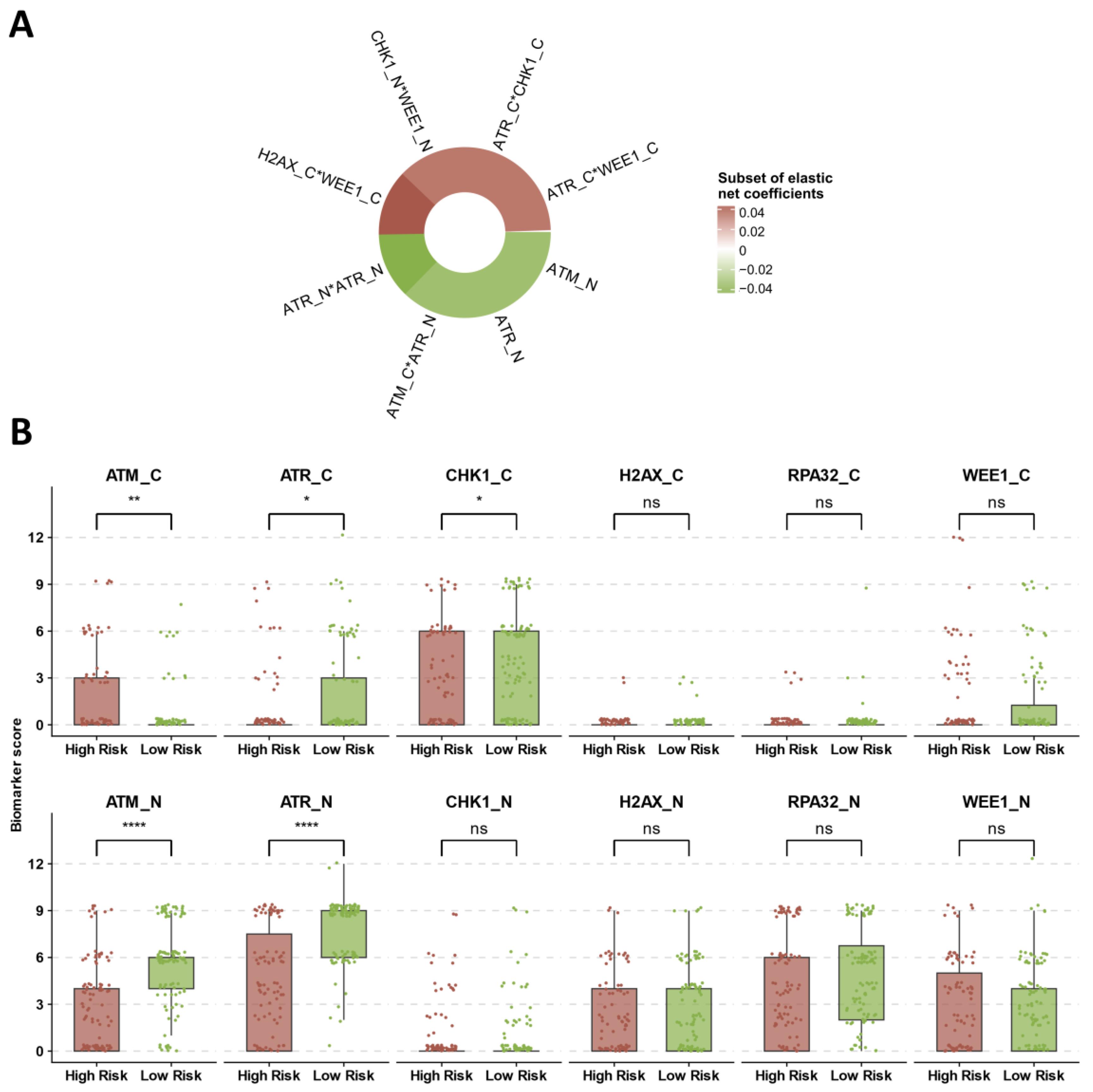
3.4. Identification of Prognostic DDR Groups through Cox Elastic Net
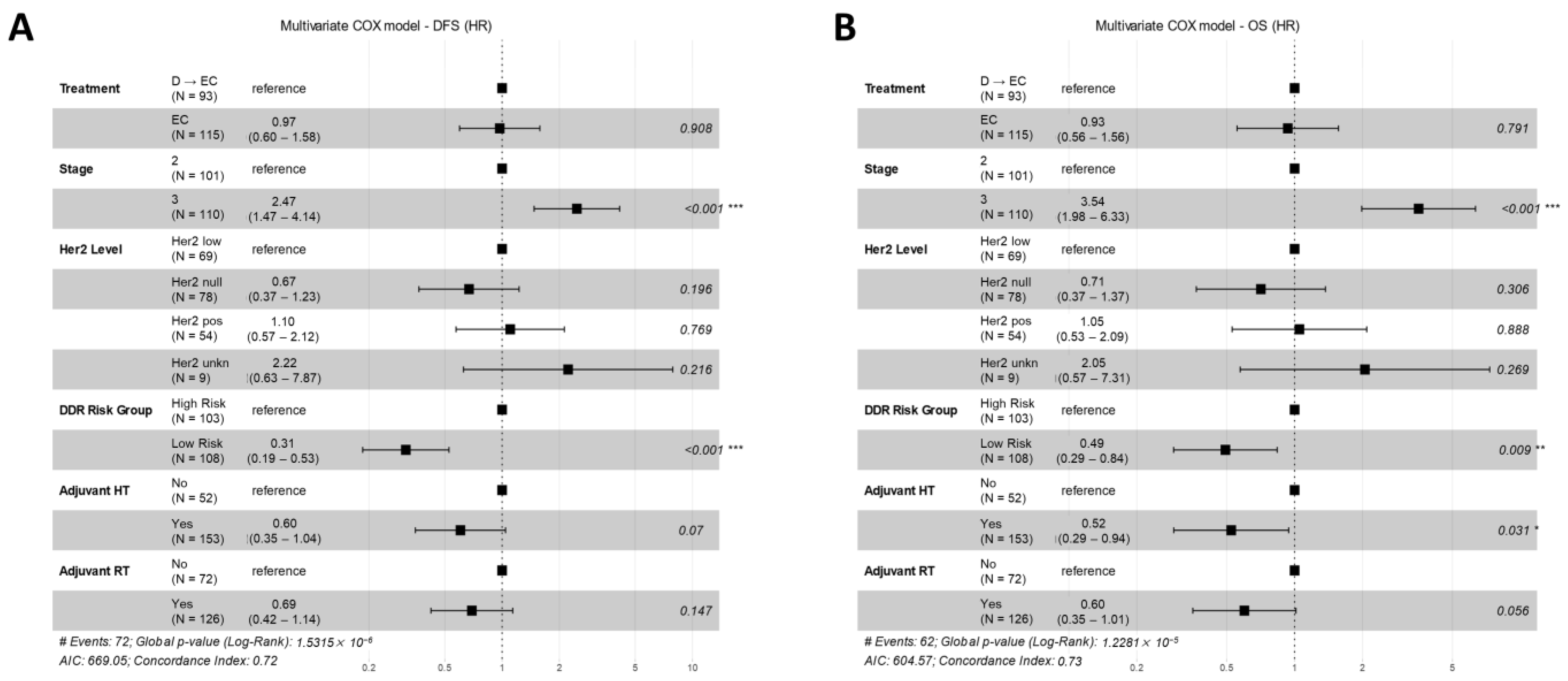
3.5. Multivariate Models for Treatment Arm and DDR Risk Group Effects on DFS and OS
3.6. Evaluating the Validation of the DDR Prognostic Effect in the TCGA BC Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. USA: NIH [updated 31 January 2024]. Cancer Stat Facts: Female Breast Cancer. Available online: http://seer.cancer.gov/statfacts/html/breast.html (accessed on 10 May 2024).
- Bonilla, L.; Ben-Aharon, I.; Vidal, L.; Gafter-Gvili, A.; Leibovici, L.; Stemmer, S.M. Dose-dense chemotherapy in nonmetastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. J. Natl. Cancer Inst. 2010, 102, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Gnant, M.; Loibl, S.; Cameron, D.; Regan, M.M.; Denkert, C.; Poortmans, P.; Weber, W.P.; Thürlimann, B.; et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023, 34, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Zhao, F.; Martino, S.; Ligibel, J.A.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. J. Clin. Oncol. 2015, 33, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment, and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Perez, E.A.; Olson, J.A.; et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Asakawa, H.; Koizumi, H.; Koike, A.; Takahashi, M.; Wu, W.; Iwase, H.; Fukuda, M.; Ohta, T. Prediction of breast cancer sensitivity to neoadjuvant chemotherapy based on status of DNA damage repair proteins. Breast Cancer Res. 2010, 12, R17. [Google Scholar] [CrossRef]
- Santarpia, L.; Iwamoto, T.; Di Leo, A.; Hayashi, N.; Bottai, G.; Stampfer, M.; André, F.; Turner, N.C.; Symmans, W.F.; Hortobágyi, G.N.; et al. DNA repair gene patterns as prognostic and predictive factors in molecular breast cancer subtypes. Oncologist 2013, 18, 1063–1073. [Google Scholar] [CrossRef]
- Santarpia, L.; Iwamoto, T.; Di Leo, A.; Hayashi, N.; Stampfer, M.; Guarducci, C.; Symmans, W.F.; Hortobagyi, G.N.; Pusztai, L.; Giampaolo, B. DNA repair metagene signature as a prognostic and predictive factor in molecular breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1012. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Ercolani, C.; Pizzuti, L.; Angelucci, D.; Sergi, D.; Marinelli, C.; Iezzi, L.; Sperati, F.; Terrenato, I.; Mazzotta, M.; et al. Prognostic relevance of DNA damage and repair biomarkers in elderly patients with hormone-receptor-positive breast cancer treated with neoadjuvant hormone therapy: Evidence from the real-world setting. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Paull, T.T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 2007, 26, 7741–7748. [Google Scholar] [CrossRef]
- Ma, M.; Rodriguez, A.; Sugimoto, K. Activation of ATR-related protein kinase upon DNA damage recognition. Curr. Genet. 2020, 66, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wakeman, T.; Yong, S.; Wu, X.; Kornbluth, S.; Wang, X.F. Protein phosphatase 2A-dependent dephosphorylation of replication protein A is required for the repair of DNA breaks induced by replication stress. Mol. Cell. Biol. 2009, 29, 5696–5709. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Neizer-Ashun, F.; Bhattacharya, R. Reality CHEK: Understanding the biology and clinical potential of CHK1. Cancer Lett. 2021, 497, 202–211. [Google Scholar] [CrossRef]
- Elbæk, C.R.; Petrosius, V.; Benada, J.; Erichsen, L.; Damgaard, R.B.; Sørensen, C.S. WEE1 kinase protects the stability of stalled DNA replication forks by limiting CDK2 activity. Cell Rep. 2022, 38, 110261. [Google Scholar] [CrossRef] [PubMed]
- Vici, P.; Brandi, M.; Giotta, F.; Foggi, P.; Schittulli, F.; Di Lauro, L.; Gebbia, N.; Massidda, B.; Filippelli, G.; Giannarelli, D.; et al. A multicenter phase III prospective randomized trial of high-dose epirubicin in combination with cyclophosphamide (EC) versus docetaxel followed by EC in node-positive breast cancer. GOIM (Gruppo Oncologico Italia Meridionale) 9902 study. Ann. Oncol. 2012, 23, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja, E.; Paesmans, M.; Beauduin, M.; Vindevoghel, A.; Cornez, N.; Finet, C.; Ries, F.; Closon-Dejardin, M.T.; Kerger, J.; Gobert, P.; et al. Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian multicentre study. J. Clin. Oncol. 2009, 27, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: A patient-level meta-analysis of 100 000 women from 86 randomised trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, W.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Shen, Z.; Lin, F.; Sun, Y.J.; Min, D.L.; Tang, L.N.; He, A.N.; Yao, Y. Paclitaxel-based versus docetaxel-based regimens in metastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2013, 29, 117–125. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja, E.; Durbecq, V.; Rosa, D.D.; Colozza, M.; Larsimont, D.; Piccart-Gebhart, M.; Cardoso, F. HER-2 overexpression/amplification and its interaction with taxane-based therapy in breast cancer. Ann. Oncol. 2008, 19, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52, 56–73. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, J.; Liu, D.; Ren, F.; Wu, J.; Shen, K. Distribution, dynamic evolution, and clinical outcomes of patients with advanced breast cancer according to HER2 expression. BMC Cancer 2023, 23, 173. [Google Scholar] [CrossRef]
- Vici, P.; Di Benedetto, A.; Ercolani, C.; Pizzuti, L.; Di Lauro, L.; Sergi, D.; Sperati, F.; Terrenato, I.; Dattilo, R.; Botti, C.; et al. Predictive significance of DNA damage and repair biomarkers in triple-negative breast cancer patients treated with neoadjuvant chemotherapy: An exploratory analysis. Oncotarget 2015, 6, 42773–42780. [Google Scholar] [CrossRef]
- Abdel-Fatah, T.M.; Middleton, F.K.; Arora, A.; Agarwal, D.; Chen, T.; Moseley, P.M.; Perry, C.; Doherty, R.; Chan, S.; Green, A.R.; et al. Untangling the ATR-CHEK1 network for prognostication, prediction and therapeutic target validation in breast cancer. Mol. Oncol. 2015, 9, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Stucci, L.S.; Internò, V.; Tucci, M.; Perrone, M.; Mannavola, F.; Palmirotta, R.; Porta, C. The ATM Gene in Breast Cancer: Its Relevance in Clinical Practice. Genes 2021, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Ercolani, C.; Mottolese, M.; Sperati, F.; Pizzuti, L.; Vici, P.; Terrenato, I.; Shaaban, A.M.; Humphries, M.P.; Di Lauro, L.; et al. Analysis of the ATR-Chk1 and ATM-Chk2 pathways in male breast cancer revealed the prognostic significance of ATR expression. Sci. Rep. 2017, 7, 8078. [Google Scholar] [CrossRef]
- Bueno, R.C.; Canevari, R.A.; Villacis, R.A.; Domingues, M.A.; Caldeira, J.R.; Rocha, R.M.; Drigo, S.A.; Rogatto, S.R. ATM down-regulation is associated with poor prognosis in sporadic breast carcinomas. Ann. Oncol. 2014, 25, 69–75. [Google Scholar] [CrossRef]
- Ye, C.; Cai, Q.; Dai, Q.; Shu, X.O.; Shin, A.; Gao, Y.T.; Zheng, W. Expression patterns of the ATM gene in mammary tissues and their associations with breast cancer survival. Cancer 2007, 109, 1729–1735. [Google Scholar] [CrossRef]
- Meyer, A.; John, E.; Dörk, T.; Sohn, C.; Karstens, J.H.; Bremer, M. Breast cancer in female carriers of ATM gene alterations: Outcome of adjuvant radiotherapy. Radiother. Oncol. 2004, 72, 319–323. [Google Scholar] [CrossRef]
- Stagni, V.; Manni, I.; Oropallo, V.; Mottolese, M.; Di Benedetto, A.; Piaggio, G.; Falcioni, R.; Giaccari, D.; Di Carlo, S.; Sperati, F.; et al. ATM kinase sustains HER2 tumorigenicity in breast cancer. Nat. Commun. 2015, 6, 6886. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M.; Al-Saif, M.; Arafah, M.; Eldali, A.M.; Tulbah, A.; Al-Tweigeri, T.; Semlali, A.; Khabar, K.S.; Aboussekhra, A. Clinical and functional significance of tumor/stromal ATR expression in breast cancer patients. Breast Cancer Res. 2020, 22, 49. [Google Scholar] [CrossRef]
- Feng, X.; Li, H.; Dean, M.; Wilson, H.E.; Kornaga, E.; Enwere, E.K.; Tang, P.; Paterson, A.; Lees-Miller, S.P.; Magliocco, A.M.; et al. Low ATM protein expression in malignant tumor as well as cancer-associated stroma are independent prognostic factors in a retrospective study of early-stage hormone-negative breast cancer. Breast Cancer Res. 2015, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.; De Souza, K.; Ali, R.; Rakha, E.A.; Green, A.R.; Madhusudan, S. Clinicopathological significance of ataxia telangiectasia-mutated (ATM) kinase and ataxia telangiectasia-mutated and Rad3-related (ATR) kinase in MYC overexpressed breast cancers. Breast Cancer Res. Treat. 2019, 175, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Cabañas Morafraile, E.; Pérez-Peña, J.; Fuentes-Antrás, J.; Manzano, A.; Pérez-Segura, P.; Pandiella, A.; Galán-Moya, E.M.; Ocaña, A. Genomic Correlates of DNA Damage in Breast Cancer Subtypes. Cancers 2021, 13, 2117. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; El-Deiry, W.S. Anti-cancer immune responses to DNA damage response inhibitors: Molecular mechanisms and progress toward clinical translation. Front. Oncol. 2022, 12, 998388. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, M.B.; Sterle, H.A.; Massari, N.A.; Táquez Delgado, M.A.; Formoso, K.; Herrero Ducloux, M.V.; Martinel Lamas, D.; Cremaschi, G.A.; Medina, V.A. Study of the antitumour effects and the modulation of immune response by histamine in breast cancer. Br. J. Cancer 2020, 122, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Sirek, T.; Sirek, A.; Borawski, P.; Ryguła, I.; Król-Jatręga, K.; Opławski, M.; Boroń, D.; Chalcarz, M.; Ossowski, P.; Dziobek, K.; et al. Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 6546. [Google Scholar] [CrossRef]

| Feature | Nr. (%) |
|---|---|
| Age | Median, 51 |
| Range, 28–70 | |
| Centre | |
| IRE | 88 (40) |
| BARI | 134 (60) |
| Menopause | |
| Pre-Menopause | 100 (45) |
| Post-Menopause | 104 (47) |
| Unknown | 18 (8) |
| Stage | |
| 2 | 106 (48) |
| 3 | 116 (52) |
| Subtype | |
| Luminal A HR+/HER2− | 83 (37) |
| Luminal B HR+/HER2− | 53 (24) |
| Luminal B HR+/HER2+ | 33 (15) |
| HER2 Enriched (HR−/HER2+) | 22 (10) |
| Triple Negative (HR−/HER2−) | 18 (8) |
| Unknown | 13 (6) |
| Her2 Level | |
| HER2 positive | 55 (25) |
| HER2 low | 70 (32) |
| HER2 null | 87 (39) |
| Uknown | 10 (4) |
| Treatment Arm | |
| EC | 119 (54) |
| D → EC | 99 (44) |
| Uknown | 4 (2) |
| Adjuvant RT | |
| No | 75 (34) |
| Yes | 133 (61) |
| Unknown | 10 (5) |
| Adjuvant HT | |
| No | 55 (25) |
| Yes | 160 (74) |
| Unknown | 3 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasniqi, E.; Ercolani, C.; Di Benedetto, A.; Di Lisa, F.S.; Filomeno, L.; Arcuri, T.; Botti, C.; Pelle, F.; Cavicchi, F.; Cappelli, S.; et al. DNA Damage Response in Early Breast Cancer: A Phase III Cohort in the Phobos Study. Cancers 2024, 16, 2628. https://doi.org/10.3390/cancers16152628
Krasniqi E, Ercolani C, Di Benedetto A, Di Lisa FS, Filomeno L, Arcuri T, Botti C, Pelle F, Cavicchi F, Cappelli S, et al. DNA Damage Response in Early Breast Cancer: A Phase III Cohort in the Phobos Study. Cancers. 2024; 16(15):2628. https://doi.org/10.3390/cancers16152628
Chicago/Turabian StyleKrasniqi, Eriseld, Cristiana Ercolani, Anna Di Benedetto, Francesca Sofia Di Lisa, Lorena Filomeno, Teresa Arcuri, Claudio Botti, Fabio Pelle, Flavia Cavicchi, Sonia Cappelli, and et al. 2024. "DNA Damage Response in Early Breast Cancer: A Phase III Cohort in the Phobos Study" Cancers 16, no. 15: 2628. https://doi.org/10.3390/cancers16152628





