Simple Summary
Individualized medicine means understanding how each tumor is different from normal cells and how each tumor is different from other tumors, including profiling mutations, non-mutational epigenetic changes, and differences in gene expression. This allows the discovery of key processes each tumor absolutely depends on for survival and growth, which are intrinsic weaknesses. This profiling means selecting treatments to specifically target each tumor’s survival-dependent pathways, killing them. Tip60 is a master controller of processes that maintain genome stability and signaling regulating gene expression. While disrupted in many cancers, Tip60 is essential for cell survival, and inhibiting Tip60 kills tumors. While we understand some key aspects of the molecular roles Tip60 plays, much more remains to be discovered. A more complete understanding of the diverse roles and functions of Tip60 in cancer, and how targeting Tip60 kills cancer cells, will lead to better treatments for patients and increased survival.
Abstract
Precision (individualized) medicine relies on the molecular profiling of tumors’ dysregulated characteristics (genomic, epigenetic, transcriptomic) to identify the reliance on key pathways (including genome stability and epigenetic gene regulation) for viability or growth, and then utilises targeted therapeutics to disrupt these survival-dependent pathways. Non-mutational epigenetic changes alter cells’ transcriptional profile and are a key feature found in many tumors. In contrast to genetic mutations, epigenetic changes are reversable, and restoring a normal epigenetic profile can inhibit tumor growth and progression. Lysine acetyltransferases (KATs or HATs) protect genome stability and integrity, and Tip60 is an essential acetyltransferase due to its roles as an epigenetic and transcriptional regulator, and as master regulator of the DNA double-strand break response. Tip60 is commonly downregulated and mislocalized in many cancers, and the roles that mislocalized Tip60 plays in cancer are not well understood. Here we categorize and discuss Tip60-regulated genes, evaluate Tip60-interacting proteins based on cellular localization, and explore the therapeutic potential of Tip60-targeting compounds as epigenetic inhibitors. Understanding the multiple roles Tip60 plays in tumorigenesis will improve our understanding of tumor progression and will inform therapeutic options, including informing potential combinatorial regimes with current chemotherapeutics, leading to improvements in patient outcomes.
1. Introduction
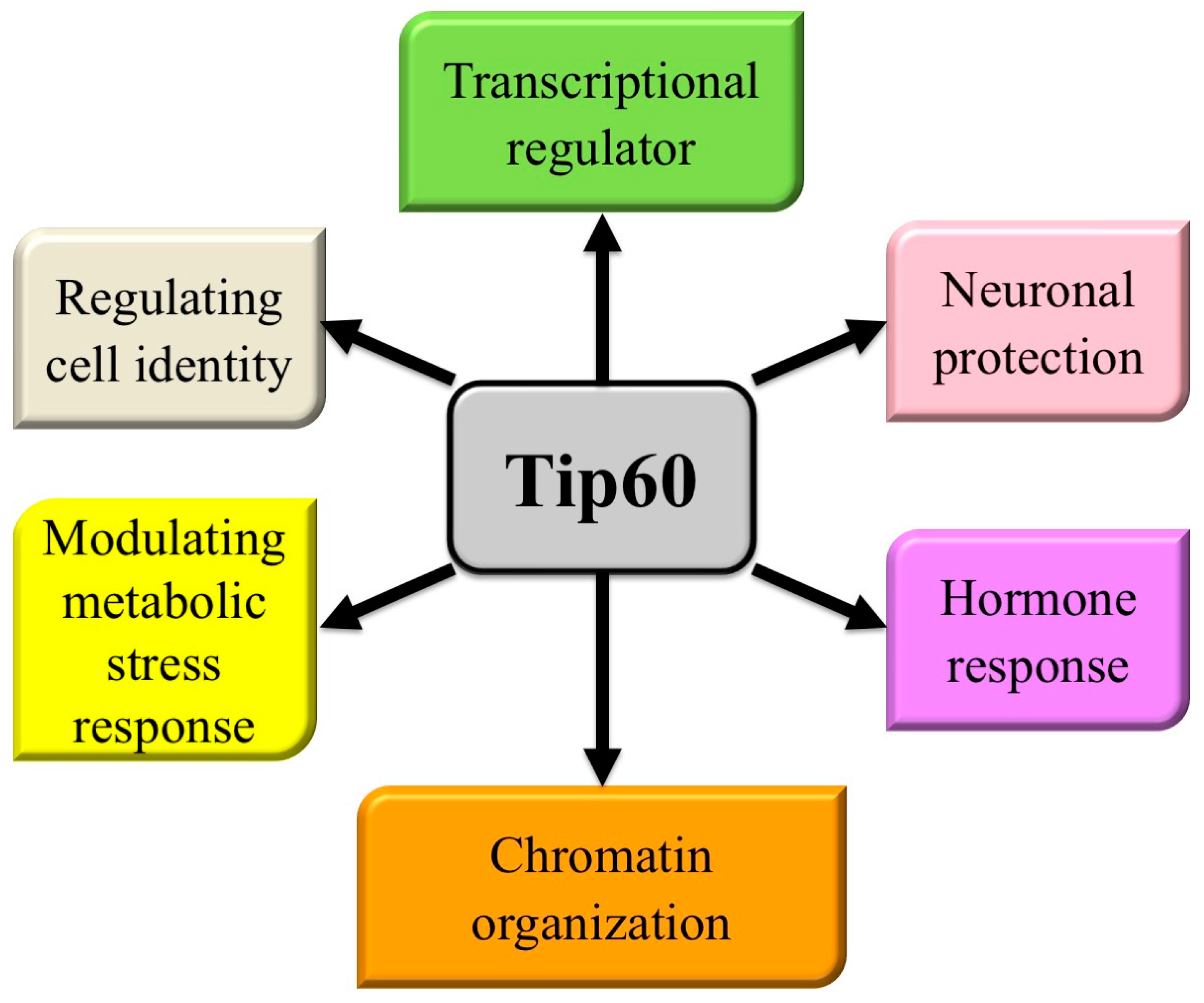
Modern molecular medicine (individualized or precision medicine) relies on profiling tumors (including using genomic, epigenetic, transcriptomic, or proteomic fingerprinting) to identify tumor cells’ reliance on key pathways for survival or growth, and then uses small molecule inhibitors (often inhibiting the activity of a single molecule) or biologics to disrupt these essential survival-dependent pathways [1,2,3]. The specificity of these treatments is enhanced by using biomarkers paired to the inhibitor to select sensitive patient cohorts (by excluding patients that are treatment insensitive), which leads to improved patient outcomes. The next generation of molecular medicines will target molecules that are key regulators of multiple processes, both improving their efficacy and enhancing their range of use to include more tumor types/profiles and other diseases [4,5,6]. One such multi-process molecule is Tip60 (Tat-interactive protein 60-kDa) (Figure 1), a member of the MYST family of acetyltransferases (one of five acetyltransferases families [7,8,9,10]).

Figure 1.
Pathways regulated by Tip60. Cellular processes in which Tip60 has a significant known role.
2. Tip60
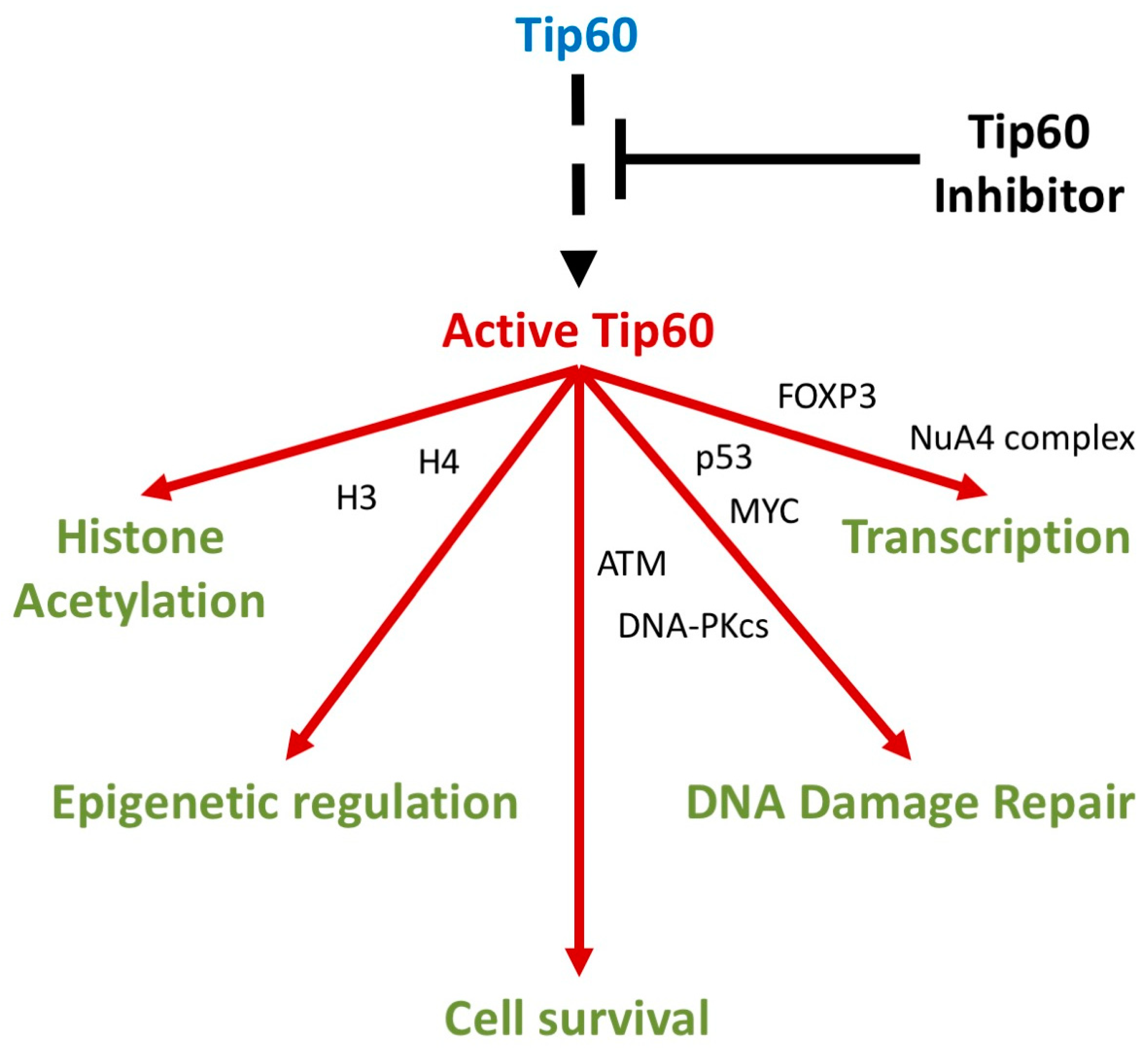
Tip60 is a versatile lysine acetyltransferase, acetylating the ε-amino groups of lysine residues in various proteins [11]. Significant Tip60-dependent processes include regulating genome stability, transcriptional and epigenetic regulation, immunoregulation, and membrane receptor expression (altering receptor pathway activity) (Table 1, Figure 2) [12,13]. Tip60 activity governs diverse cellular activities required for cell survival through modifications to key substrates including histones 2A, 3, and 4 (facilitating chromatin remodeling) and non-histone proteins (including p53, MYC, and ATM) [14,15].

Figure 2.
Key molecular processes regulated by Tip60 activity. Molecular signaling cascades (arrows indicate key pathways/cascades) where Tip60 has a significant known molecular role. Key Tip60-interacting proteins indicated (proteins between arrows indicate overlapping roles in adjacent processes).
Table 2 lists many known Tip60-interacting proteins, including their described cellular functions/signaling pathways [16]. An issue worth highlighting is that much of the key work exploring the molecular roles of Tip60 has relied on using either gene knockouts (KO) or gene expression knockdown (KD) (often small interfering RNA) systems. These methods produce an asynchronous pool of Tip60 KO/KD cells (most often in cancer cell lines) which are in different stages of apoptosis, as loss of the essential Tip60 gene/protein induces cell death. This complicates the analysis of any roles of Tip60, as the Tip60-dependent signaling investigated is intrinsically entangled with the induced apoptotic signaling [17]. Additionally, many experiments have focused on very early time points in Tip60-dependent signaling cascades (often in DNA damage response pathways), which must be taken into account when reviewing our understanding of Tip60-mediated roles [8,18].

Table 1.
Cellular roles of Tip60.
Table 1.
Cellular roles of Tip60.
| Function | Molecular Process |
|---|---|
| Regulating cell identity | Stem cell identity [18,19,20,21] |
| Enhancing Treg cell induction [22,23] | |
| Transcriptional regulator | Transcription [13,24] |
| Modulating metabolic stress response | Cell survival [13,25] |
| Hormone response | AR signaling response [26,27] |
| Genome stability/chromatin remodeling | DNA damage repair [14,24] |
| Transcriptional regulation [28] | |
| Neuronal protection | Neuronal cell function [16,20] |
| Cell cycle | Regulating Mad1/2 expression [13,29] |

Table 2.
Tip60 interactive proteins.
Table 2.
Tip60 interactive proteins.
| Protein Name | Signaling Pathway |
|---|---|
| FOXP3 | Transcription |
| APBB1 (Fe65) | |
| C/EBP α | |
| Interleukin-9 receptor | |
| STAT3 | |
| HDAC7 | |
| KLF4 | |
| ATXN1 | |
| Epc1 | |
| Epc2 | |
| MBTD1 | |
| Gas41/YEATS4 | |
| MYC | |
| RELA/p65 | |
| SOX9 | |
| ATM | DNA damage response |
| P53 | |
| SIRT1 | |
| TRRAP | |
| FAM135B | |
| ATF2 | |
| p400 | |
| MOF | |
| BAF53a | |
| ANP32E | |
| RNF8 | |
| UHRF1 | |
| FLJ10914/MRGBP | |
| MORF4L1 | |
| TRIM29 | |
| ACTL6A | |
| TRCp120 | |
| P300 | Enhancing Treg cell induction |
| USP7 | |
| FOXP3 | |
| Androgen receptor | AR signaling response |
| HDAC1 | |
| RuvBL1 | Tip60 complex assembly |
| RuvBL2 | |
| ING3 | Apoptosis |
| APP | |
| YL-1 | Chromatin remodeling |
| UHRF1 | |
| P400 | |
| HDAC9 | |
| MORF4L2 | |
| JAZF1 | |
| DMAP | DNA replication |
| Mdm2 | Regulation of Tip60 |
| Cul3 | |
| ATF3 | |
| UHRF2 |
Furthermore, despite the many roles of Tip60 in different cellular processes, numerous studies have had a single-role focus, which then fails to fully profile the multi-functional activity and/or networked effects of Tip60 loss on the system; i.e., a study focused on genome stability may not explore the effects of Tip60 loss on transcriptional regulation.
3. Tip60-Modulated Transcriptional Regulation
Due to the key significance of histone modifications in regulating chromosome structure and transcription, the role of Tip60 in the regulation of transcriptional processes has been explored [12,24]. The direct role of Tip60 in regulating the expression of several genes has been described (reviewed in Table 3). As the catalytic subunit of the NuA4 (nucleosome acetyltransferase of H4) complex, Tip60 plays a vital role in transcriptional activation and is a co-activator for numerous transcription factors and demonstrates binding (along with other Tip60 complex members) to promoters regulated by E2F or c-myc [27,30]. Tip60, concurrently with other NuA4 subunits like p400, is involved in facilitating p53-mediated transcription [31]. Furthermore, Tip60, as a transcriptional co-activator of p53, increases the activation of p21 and puma, which have a role in growth arrest and apoptosis. In response to DNA damage, Tip60 acetylates p53K120 (within p53’s DNA-binding domain), regulating the selection of promoters and ultimately altering the cellular response from cell-cycle arrest to apoptosis [32]. The identification of p53K120 acetylation by Tip60 is important because it represents one post-translational modification of p53 linked to a residue which is frequently mutated in cancer (promoting tumorigenesis) [33]. While it is known that loss of Tip60 induces apoptotic cell death, which primarily appears to result from increased DNA damage induced genome instability and triggers apoptosis, it remains likely that the dysregulation of Tip60-dependent transcriptomic and/or epigenetic pathways will correspondingly contribute significantly to Tip60-dependent cell survival [18,34,35].

Table 3.
Genes activated by Tip60.
4. Tip60-Modulated Epigenetic Regulation
Tip60 has been identified as an epigenetic regulator through its role as a co-activator or corepressor of transcription factors and through its role in chromatin remodeling by histone acetylation [28,35,42]. As previously highlighted (Section 2), many approaches investigating Tip60 were conducted on an induced background of apoptotic signaling; as such, the epigenetic effects of Tip60 loss (alone and without apoptotic signaling) are poorly understood. Furthermore, it is likely that these Tip60-dependent epigenetic roles of Tip60 are tissue-specific (as is seen with the known cell-specific immunoregulatory and stem cell identity roles). Interestingly, Tip60 activity has been shown to be important for cognition-linked processes in brain tissue, where Tip60-mediated transcriptional regulation mediates cognitive function (in Drosophila) [43]. Together, the roles of Tip60 in chromatin remodeling and transcription support the need to use precise and tunable Tip60 inhibitors to better understand the epigenetic roles of Tip60 in each tissue type (without the confounding effects of apoptosis induction due to the genetic/transcript loss of Tip60) and as potential epigenetic therapeutics for the treatment of cancer and other diseases, including neurological and immune disorders.
5. Tip60-Modulated Immunoregulation
A key element regulating the immune system response to tumors is mediated through T-regulatory (Treg) cells, with Treg cells characterized by their expression of the transcription factor Foxp3 expression, which is Tip60-dependent [44,45,46]. It has been demonstrated that the activity of Tip60 significantly influences this immunoregulation through the Foxp3-driven modulation of regulatory T cells (Tregs). The Tip60–Foxp3 interaction enhances both the stability and transcriptional activity of Foxp3, where Tip60 acetylates Foxp3, preventing its polyubiquitination and degradation, ensuring increased protein levels [47], which promotes the suppressive Foxp3 Treg functions, helping drive tumor immunity [46,48,49,50,51]. This indicates that targeting Tip60 would have a significant effect on tumors’ immunological profile, mediated through Treg cells, with a strong potential for beneficial therapeutic effects in oncology and autoimmune disease treatments [52,53]. While Tip60 plays a key role in mediating the immune response through transcriptional activities, other key roles include more direct functions in protecting genome stability.
6. Tip60-Regulated Genome Stability
Cells protect genomic integrity through many mechanisms, and the DNA damage response (DDR) pathway is essential for repairing double-strand DNA breaks (DSB). The DSB response is regulated by the apical kinase ATM, and ATM activation requires acetylation by the lysine acetyltransferase Tip60, positioning Tip60 as a master regulator of the DSB response [14,54,55]. Tip60 contributes to the DDR through two key molecular pathways: DSB chromatin remodeling (involving Nu4A) and through Tip60-dependent-activation of ATM. Tip60 is recruited to damaged sites, acetylating lysine 3016 of ATM and initiating a phosphorylation cascade and DSB repair. This cascade activates the DNA repair pathway by phosphorylating H2AX (γH2AX), facilitating the recruitment of additional repair machinery. Tip60’s involvement extends to regulating cell-cycle arrest triggered by DNA damage, controlling the cell cycle through p53, and ensuring chromosomal stability during mitosis [34]. Tip60 also has an effect on the loosening of nucleosomes through interaction with the NuA4 complex members at DSB, resulting in an increase in DNA accessibility [14]. Many key proteins in the DDR, cell cycle, or chromatin remodeling pathways are regulated/acetylated by Tip60 (including ATM, H2AX, p53, Histones H4, and H2, Aurora B1, MRN, NuA4) [14,54,56,57,58,59,60,61,62,63]. Mutations that compromise cellular DDR pathways (including defects in the Tip60–ATM pathway) increase genomic instability and allow abnormal cell proliferation and tumor progression, ultimately significantly reducing patient survival [64,65]. In addition, Tip60-mediated genome instability is a feature of multiple diseases, including carcinogenesis, neurodegenerative diseases, aging, and immunodeficiency [66,67].
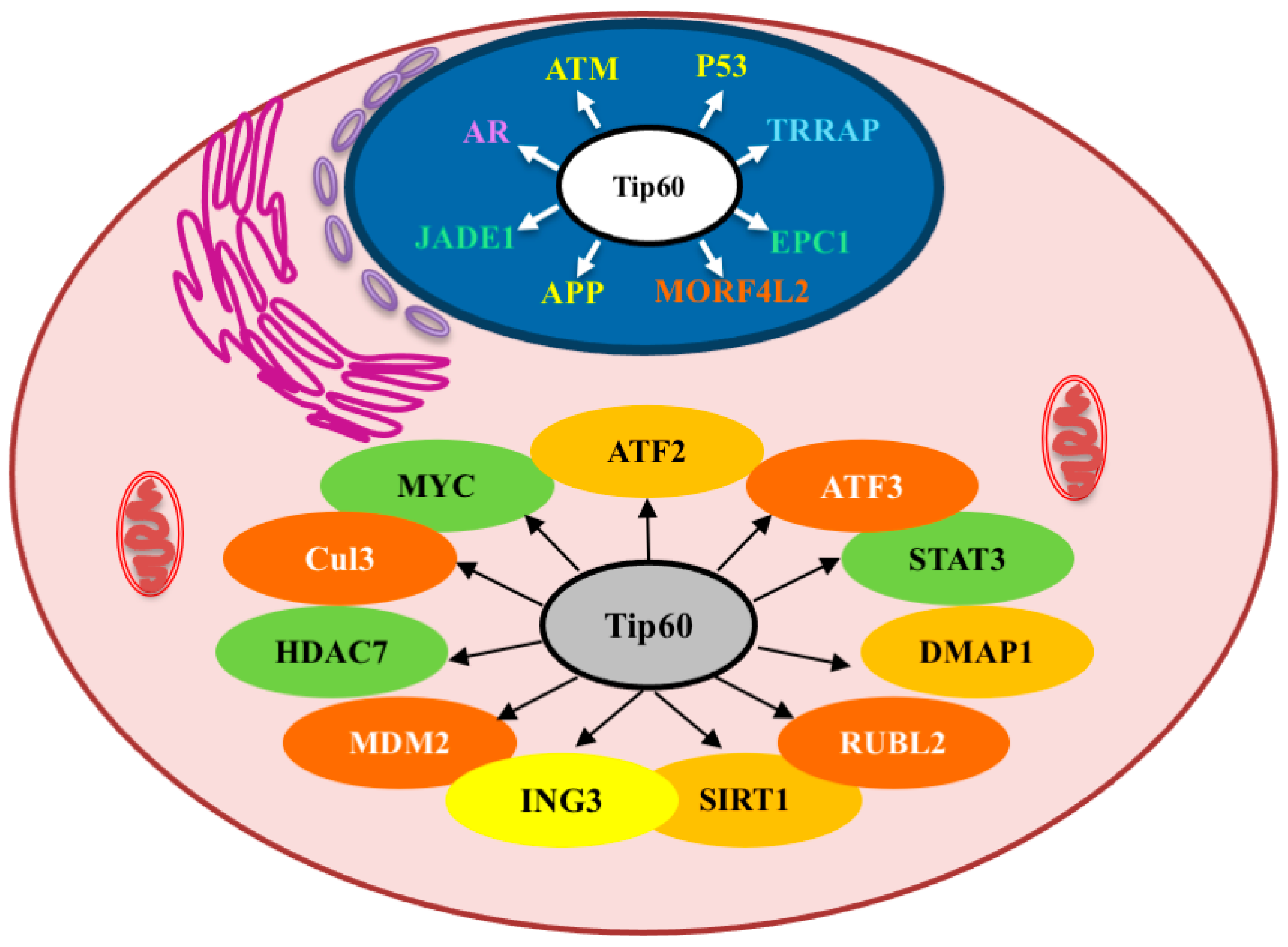
Interestingly, under “normal” non-tumorigenic conditions Tip60 is mainly found in the nucleus; however, Tip60 has been found to be strongly mislocalized to the cytoplasm in several cancers (Table 4) [68,69,70]. The effects of the mislocalization of Tip60 to the cytoplasm, and the consequences of its activity in cellular signaling while there, are poorly understood (Figure 3) but may underpin the novel pro-tumorigenic effects of Tip60 described (including simply the reduction of nuclear Tip60 levels, which inhibits its anti-tumorigenic activity).

Table 4.
Tip60-interacting proteins and their subcellular localization.

Figure 3.
Selected Tip60-interacting proteins and their cellular localizations. Pink: cytoplasm; Blue: nuclear. The colors of the selected proteins shown relate to their processes, as indicated in Figure 1 (orange/dark orange: chromatin organization; green: transcription; yellow: metabolic stress response; purple: hormone response).
Recently, it has been discovered that some KATs have additional catalytic activities, including Tip60 and p300, which display lysine isobutyrylation (Kibu) activity [128,129]. The post-translational modification Kibu has been shown on histones, where it regulates processes including metabolism (different metabolic pathways regulate the availability of acyl-CoAs required for different PTMs, such as Kibu) and transcription (through gene expression) [130,131]. As our understanding of the roles of Tip60 grows, due to improved understanding of its molecular functions, a clearer picture of its dysregulation and the consequences of this will be revealed.
7. Tip60 Regulation
Tip60 activity is regulated by multiple partners (Table 5) involving multiple mechanisms (including auto-acetylation, phosphorylation, SUMOylation), where these PTMs modulate the activity and role of Tip60 in processes like apoptosis induction. Tip60 auto-acetylation is a key regulatory mechanism regulating the DNA damage response, leading to ATM activation and the repair of double-strand breaks [132]. Additionally, it was shown that Tip60 is activated though phosphorylation by GSK3, which leads to p53-dependent apoptosis though the activation of p53 by Tip60 acetylation (of K120) [56,133]. Exploring the inhibitory mechanisms regulating Tip60 activity, it is known that the Abl kinase phosphorylates Tip60 (Y327), which indues association with FE65, inhibiting its HAT activity [134]. Furthermore, it has been shown that ATF2 (activating transcription factor-2) in conjunction with the Cul3 ubiquitin ligase, can regulate Tip60 activity (in DNA damage response signaling) by limiting the availability of Tip60, promoting its degradation [122]. To further highlight the complicated nature regulating Tip60 activity, in contrast to SIRT1-mediated deacetylation, HDAC3-mediated deacetylation extends Tip60’s half-life, mediating its availability and activity [109,135]. Interestingly, both HDAC3 and Tip60 can be localized in both the nucleus and cytoplasm, suggesting a potential stabilizing effect of HDAC3 on Tip60 [109]. Furthermore, it is likely that cell-type-specific regulation of Tip60 exists, further complicating our understanding of the effects of Tip60 regulation and the cellular effects on individual signaling pathways in each tissue.

Table 5.
Tip60 regulation.
8. Tip60 Tumor Profiling
Tip60 does not appear to act as a direct tumor suppressor or oncogene. Instead, it helps other proteins in these functions through its general acetyltransferase and transcriptional co-activator capabilities. This is demonstrated by the connection between Tip60 and p53 [56,133]. Interestingly, recent studies have shown a significant decrease in Tip60 expression in colon and lung carcinomas [33].
The involvement of Tip60 in cancer development is complex. As part of the multi-subunit NuA4 complex, Tip60 gets directed to target promoters by a variety of transcription factors. Operating within the NuA4 complex, Tip60 acetylates the nucleosomal histones H2A and H4, acting as a co-activator for the transcriptional factor. Furthermore Tip60 plays a key role in p53 activation, regulating apoptosis induction. Additionally, Tip60 is crucial for the expression of KAI1, a tumor suppressor in prostate cancer. Hence, the activity of Tip60 appears to rely on the specific context (cellular or molecular), and aberrations in lysine acetyltransferase activity can either promote or impede tumorigenesis in colon, breast, and prostate cancers [145]. Tip60 is downregulated in various cancers, such as colon, lung, breast, melanoma, prostate, gastric, lung, and pancreatic cancers. The hypothesis that eliminating the remaining Tip60 activity induces apoptosis has been confirmed, making Tip60 a promising candidate for targeted drug development as a lysine acetyltransferase inhibitor (KATi) [8,146]. A key feature of Tip60 is that its expression is essential for embryo viability [147,148], and it is vital for cell survival [149,150].
9. Tip60 Inhibitors
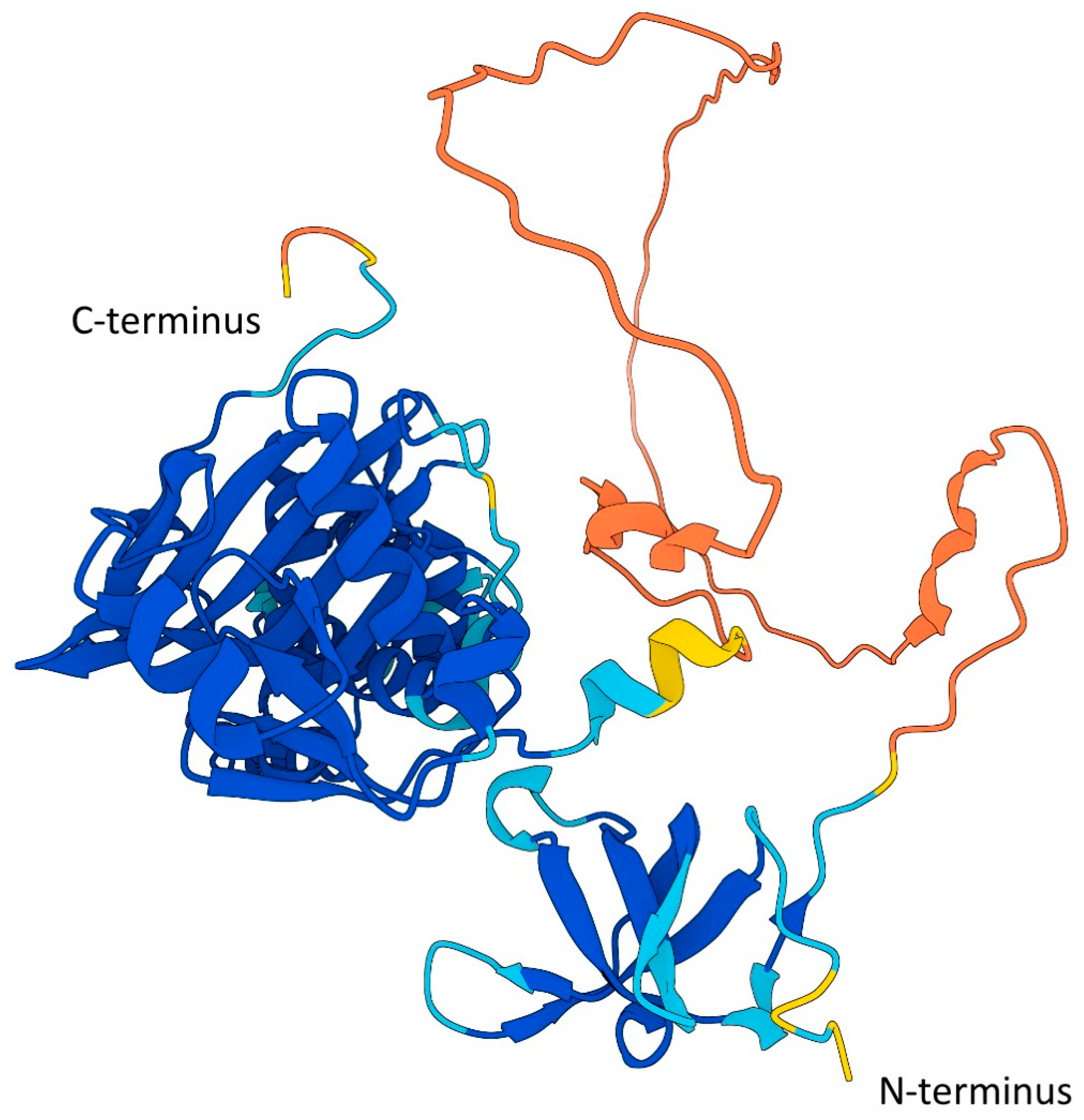
The creation of Tip60-specific inhibitors (such as TH1834) has provided new tools for precise and tailored modulation of Tip60 activity, which are now used for thorough and specific molecular investigations of Tip60 activates to be explored [15,151,152,153]. The rationally designed method used to produce the Tip60 inhibitor TH1834 was facilitated by crystallization of the catalytic domain [8,101,150,154], and it is possible that future targeted inhibitors will make use of new advances in protein structure predictions by using the full protein (Figure 4) rather than just the crystalized catalytic acetyltransferase domain [155,156].

Figure 4.
AlphaFold prediction of Tip60 structure. Based on Uniport accession A0A024R5E8 [155,156]. The region between 64aa and ~200aa has an expected position error of >30 Angstroms. Predicted template modeling (pTM) = 0.7, where a pTM score >0.5 indicates the overall prediction may be comparable to the true structure. Reside scoring—Blue: very high (pLDDT > 90); Aqua: high (90 > pLDDT > 70); Yellow: low (70 > pLDDT > 50); Orange: very low (pLDDT < 50).
Recently, it has been clear that Tip60-targeting inhibitors show significant activities against cancer and other diseases [157] (Table 6). They are categorized into three groups: bisubstrate inhibitors, synthetic compounds, and natural compounds [7]. Among Tip60 inhibitors, some are well known (including Lys-CoA, anacardic acid, pentamidine, garcinol, and curcumin), but exhibit lower specificity affecting not only Tip60 but also pCAF and CBP/p300. However, several designed small molecules, including TH1834, NU9056, and MG 149, are selective for Tip60 [8,101,150,154].

Table 6.
Specific Tip60 HAT inhibitors in pre-clinical studies.
10. Tip60 as a Biomarker
To effectively utilize Tip60 inhibitors, specific paired biomarkers to identify cells with a sensitivity (or resistance) to these inhibitors are needed (Figure 5). As a key epigenetic and genome stability regulator, Tip60 (protein levels and/or activity) is itself a potential biomarker [68]. Levels of Tip60 have been investigated in several tumor types. In breast cancer, Tip60 transcript and protein levels have been found to be downregulated [68,149], while Tip60 was overexpressed in prostate cancer [101,168], and its activity upregulated in colon cancer [79] (Table 5). While mislocalization of Tip60, from the nucleus to the cytoplasm, has been observed in several cancer types (Table 7), the exact molecular consequences of this mislocalization remain to be fully elucidated.
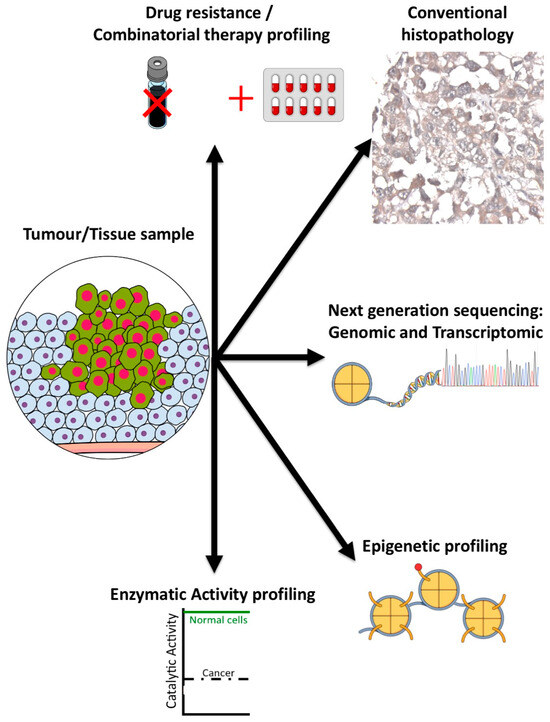
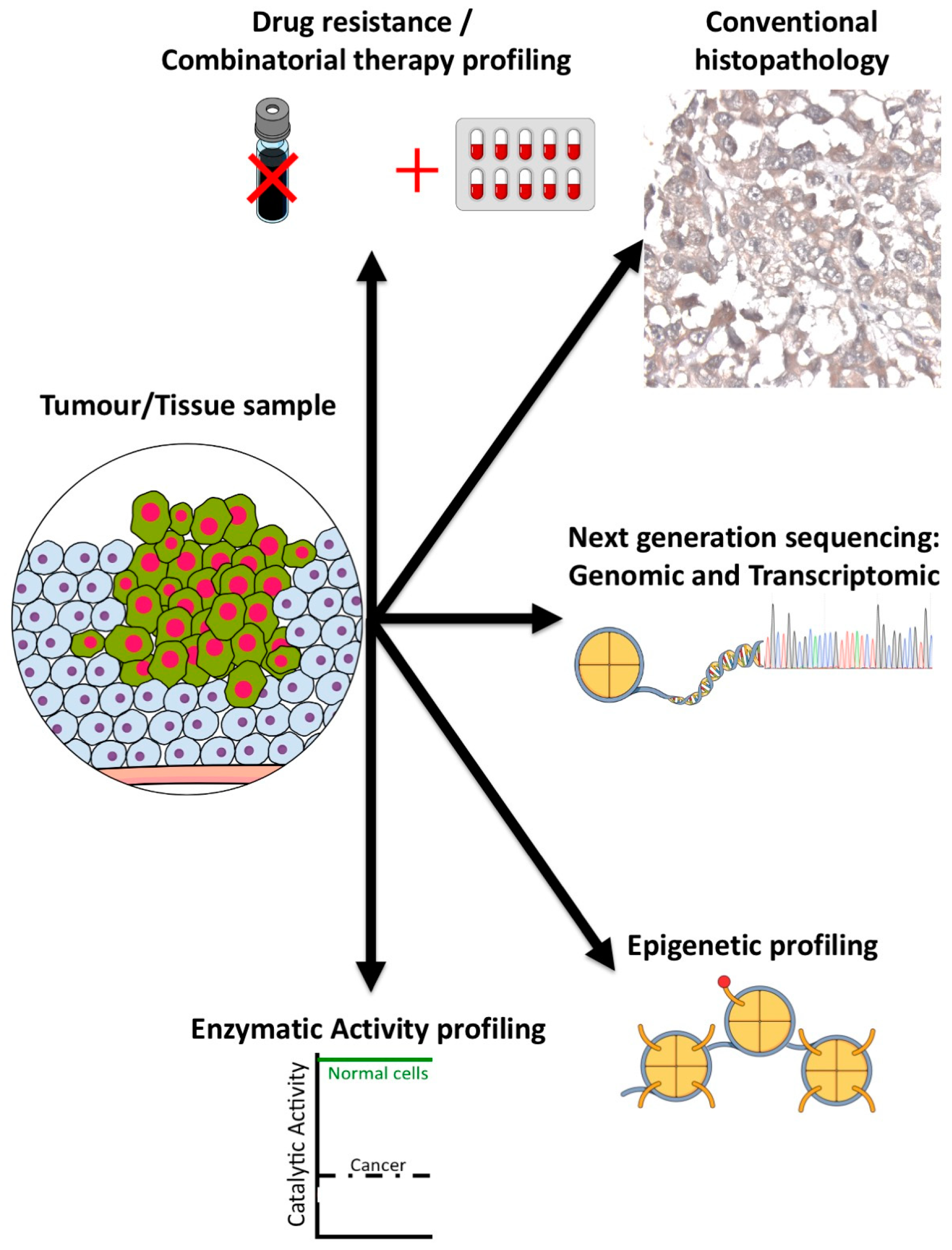
Figure 5.
Tip60 biomarker profiling. Individual biomarker methods for evaluating Tip60 in tumors (methods as indicated).

Table 7.
Tip60 or Kat5 profiling in different tumor types.
As Tip60 has also been found to be dysregulated in other diseases, including neurodegenerative disorders, this raises the potential of using Tip60 as a biomarker in these conditions [174]. It has been found that in some neurodegenerative disorders, like Alzheimer’s disease (AD), histone acetylation by Tip60 in some loci is disrupted before amyloid-β accumulation. Detecting these spots could be an early biomarker for AD diagnosis and highlights the potential use of Tip60-targeting molecules as therapeutics in these diseases [174,175]. It has also been demonstrated that drugs inhibiting Tip60 activity may be useful agents for the treatment of ischemic heart disease [158].
11. Conclusions
Since Tip60 is an essential molecule with multiple cellular roles required for cell survival, more work is needed to better understand the individual (and linked) molecular roles it plays in normal cell types and tissues. The diverse molecular functions and roles of Tip60 make it a key new molecule for therapeutic targeting that has the potential to improve treatment in multiple diseases, ranging from cancer to neurological disorders. In addition, we need to improve our current understanding of the new (or misregulated) roles that mislocalized Tip60 plays in tumors or disease. Understanding Tip60 tissue-specific roles, and how tissue or disease-specific uses of Tip60 inhibitors vary is the key to facilitate the targeted use and clinical impact of Tip60 inhibitors in disease management.
Author Contributions
Writing—original draft preparation, N.Z. and J.A.L.B.; Writing—review and editing, N.Z., J.A.L.B., E.C., M.D., A.S., and P.B.-L.; supervision, J.A.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the students and academics of the Bioscience degree (University of Limerick, Ireland) for stimulating discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Dugger, S.A.; Platt, A.; Goldstein, D.B. Drug Development in the Era of Precision Medicine. Nat. Rev. Drug Discov. 2018, 17, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Garcia-Moreno, J.; Brown, J.; Bourke, E. Clinically Applicable Inhibitors Impacting Genome Stability. Molecules 2018, 23, 1166. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-H.; Snyder, M. Omics Profiling in Precision Oncology. Mol. Cell. Proteom. 2016, 15, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Functional Precision Cancer Medicine—Moving beyond Pure Genomics. Nat. Med. 2017, 23, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering Precision Oncology to Patients with Cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef] [PubMed]
- López-Bañuelos, L.; Vega, L. Inhibition of Acetylation, Is It Enough to Fight Cancer? Crit. Rev. Oncol. Hematol. 2022, 176, 103752. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.L.; Bourke, E.; Eriksson, L.A.; Kerin, M.J. Targeting Cancer Using KAT Inhibitors to Mimic Lethal Knockouts. Biochem. Soc. Trans. 2016, 44, 979–986. [Google Scholar] [CrossRef]
- Farria, A.; Li, W.; Dent, S.Y.R. KATs in Cancer: Functions and Therapies. Oncogene 2015, 34, 4901–4913. [Google Scholar] [CrossRef]
- Sheikh, B.N.; Akhtar, A. The Many Lives of KATs—Detectors, Integrators and Modulators of the Cellular Environment. Nat. Rev. Genet. 2019, 20, 7–23. [Google Scholar] [CrossRef]
- Lee, K.K.; Workman, J.L. Histone Acetyltransferase Complexes: One Size Doesn’t Fit All. Nat. Rev. Mol. Cell. Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and Mechanisms of Non-Histone Protein Acetylation. Nat. Rev. Mol. Cell. Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, V.; Logan, I.R.; Robson, C.N. Cellular Functions of TIP60. Int. J. Biochem. Cell Biol. 2006, 38, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, X.; Price, B.D. Tip60: Connecting Chromatin to DNA Damage Signaling. Cell Cycle 2010, 9, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.L.; Hancock, W. Lysine acetylation inhibitors (KATi) as novel therapeutics. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer: Cham, Switzerland, 2024; ISBN 978-3-030-80962-1. [Google Scholar] [CrossRef]
- Li, Z.; Rasmussen, L.J. TIP60 in Aging and Neurodegeneration. Ageing Res. Rev. 2020, 64, 101195. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.K.; Thomas, T. Histone Lysine and Genomic Targets of Histone Acetyltransferases in Mammals. BioEssays 2018, 40, e1800078. [Google Scholar] [CrossRef]
- Lashgari, A.; Kougnassoukou Tchara, P.E.; Lambert, J.P.; Côté, J. New insights into the DNA repair pathway choice with NuA4/TIP60. DNA Repair 2022, 113, 103315. [Google Scholar] [CrossRef]
- Acharya, D.; Hainer, S.J.; Yoon, Y.; Wang, F.; Bach, I.; Rivera-Pérez, J.A.; Fazzio, T.G. KAT-Independent Gene Regulation by Tip60 Promotes ESC Self-Renewal but Not Pluripotency. Cell Rep. 2017, 19, 671–679. [Google Scholar] [CrossRef]
- Tominaga, K.; Sakashita, E.; Kasashima, K.; Kuroiwa, K.; Nagao, Y.; Iwamori, N.; Endo, H. Tip60/KAT5 Histone Acetyltransferase Is Required for Maintenance and Neurogenesis of Embryonic Neural Stem Cells. Int. J. Mol. Sci. 2023, 24, 2113. [Google Scholar] [CrossRef]
- Fazzio, T.G.; Huff, J.T.; Panning, B. An RNAi Screen of Chromatin Proteins Identifies Tip60-P400 as a Regulator of Embryonic Stem Cell Identity. Cell 2008, 134, 162–174. [Google Scholar] [CrossRef]
- Su, Q.; Jing, J.; Li, W.; Ma, J.; Zhang, X.; Wang, Z.; Zhou, Z.; Dai, L.; Shao, L. Impaired Tip60-Mediated Foxp3 Acetylation Attenuates Regulatory T Cell Development in Rheumatoid Arthritis. J. Autoimmun. 2019, 100, 27–39. [Google Scholar] [CrossRef]
- Fueyo-González, F.; Vilanova, G.; Ningoo, M.; Marjanovic, N.; González-Vera, J.A.; Orte, Á.; Fribourg, M. Small-Molecule TIP60 Inhibitors Enhance Regulatory T Cell Induction through TIP60-P300 Acetylation Crosstalk. iScience 2023, 26, 108491. [Google Scholar] [CrossRef]
- Ghobashi, A.H.; Kamel, M.A. Tip60: Updates. J. Appl. Genet. 2018, 59, 161–168. [Google Scholar] [CrossRef]
- Tan, K.N.; Avery, V.M.; Carrasco-Pozo, C. Metabolic Roles of Androgen Receptor and Tip60 in Androgen-Dependent Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 6622. [Google Scholar] [CrossRef]
- Xiao, H.; Chung, J.; Kao, H.-Y.; Yang, Y.-C. Tip60 Is a Co-Repressor for STAT3. J. Biol. Chem. 2003, 278, 11197–11204. [Google Scholar] [CrossRef]
- Brady, M.E.; Ozanne, D.M.; Gaughan, L.; Waite, I.; Cook, S.; Neal, D.E.; Robson, C.N. Tip60 Is a Nuclear Hormone Receptor Coactivator. J. Biol. Chem. 1999, 274, 17599–17604. [Google Scholar] [CrossRef]
- Kim, J.-W.; Song, P.I.; Jeong, M.-H.; An, J.-H.; Lee, S.-Y.; Jang, S.-M.; Song, K.-H.; Armstrong, C.A.; Choi, K.-H. TIP60 Represses Transcriptional Activity of P73β via an MDM2-Bridged Ternary Complex. J. Biol. Chem. 2008, 283, 20077–20086. [Google Scholar] [CrossRef]
- Li, H.; Cuenin, C.; Murr, R.; Wang, Z.-Q.; Herceg, Z. HAT Cofactor Trrap Regulates the Mitotic Checkpoint by Modulation of Mad1 and Mad2 Expression. EMBO J. 2004, 23, 4824–4834. [Google Scholar] [CrossRef]
- Hlubek, F.; Lohberg, C.; Meiler, J.; Jung, A.; Kirchner, T.; Brabletz, T. Tip60 Is a Cell-Type-Specific Transcriptional Regulator. J. Biochem. 2001, 129, 635–641. [Google Scholar] [CrossRef]
- Wang, P.; Bao, H.; Zhang, X.; Liu, F.; Wang, W. Regulation of Tip60-dependent P53 Acetylation in Cell Fate Decision. FEBS Lett. 2019, 593, 13–22. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Wang, J.; Wang, R.; Liu, Z.; Yu, Y.; Lu, H. ING5 Is a Tip60 Cofactor That Acetylates P53 in Response to DNA Damage. Cancer Res. 2013, 73, 3749–3760. [Google Scholar] [CrossRef]
- Avvakumov, N.; Côté, J. The MYST Family of Histone Acetyltransferases and Their Intimate Links to Cancer. Oncogene 2007, 26, 5395–5407. [Google Scholar] [CrossRef]
- Mir, U.S.; Bhat, A.; Mushtaq, A.; Pandita, S.; Altaf, M.; Pandita, T.K. Role of Histone Acetyltransferases MOF and Tip60 in Genome Stability. DNA Repair 2021, 107, 103205. [Google Scholar] [CrossRef]
- Squatrito, M.; Gorrini, C.; Amati, B. Tip60 in DNA Damage Response and Growth Control: Many Tricks in One HAT. Trends Cell Biol. 2006, 16, 433–442. [Google Scholar] [CrossRef]
- Won Jeong, K.; Chodankar, R.; Purcell, D.J.; Bittencourt, D.; Stallcup, M.R. Gene-Specific Patterns of Coregulator Requirements by Estrogen Receptor-in Breast Cancer Cells. Mol. Endocrinol. 2012, 26, 955–966. [Google Scholar] [CrossRef]
- Yamagata, K.; Shino, M.; Aikawa, Y.; Fujita, S.; Kitabayashi, I. Tip60 Activates Hoxa9 and Meis1 Expression through Acetylation of H2A.Z, Promoting MLL-AF10 and MLL-ENL Acute Myeloid Leukemia. Leukemia 2021, 35, 2840–2853. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.; Cai, L.; Choi, H.J.; Ohgi, K.A.; Tran, C.; Chen, C.; Chung, C.H.; Huber, O.; Rose, D.W.; et al. Transcriptional Regulation of a Metastasis Suppressor Gene by Tip60 and B-Catenin Complexes. Nature 2005, 434, 921–926. [Google Scholar] [CrossRef]
- Legube, G.; Linares, L.K.; Tyteca, S.; Cile Caron, C.; Scheffner, M.; Chevillard-Briet, M.; Trouche, D. Role of the Histone Acetyl Transferase Tip60 in the P53 Pathway. J. Biol. Chem. 2004, 279, 44825–44833. [Google Scholar] [CrossRef]
- Tyteca, S.; Vandromme, M.; Legube, G.; Chevillard-Briet, M.; Trouche, D. Tip60 and P400 Are Both Required for UV-Induced Apoptosis but Play Antagonistic Roles in Cell Cycle Progression. EMBO J. 2006, 25, 1680–1689. [Google Scholar] [CrossRef]
- Bassi, C.; Li, Y.-T.; Khu, K.; Mateo, F.; Baniasadi, P.S.; Elia, A.; Mason, J.; Stambolic, V.; Pujana, M.A.; Mak, T.W.; et al. The Acetyltransferase Tip60 Contributes to Mammary Tumorigenesis by Modulating DNA Repair. Cell Death Differ. 2016, 23, 1198–1208. [Google Scholar] [CrossRef]
- Fazzio, T.G.; Huff, J.T.; Panning, B. Chromatin Regulation Tip(60)s the Balance in Embryonic Stem Cell Self-Renewal. Cell Cycle 2008, 7, 3302–3306. [Google Scholar] [CrossRef]
- Xu, S.; Wilf, R.; Menon, T.; Panikker, P.; Sarthi, J.; Elefant, F. Epigenetic Control of Learning and Memory in Drosophila by Tip60 HAT Action. Genetics 2014, 198, 1571–1586. [Google Scholar] [CrossRef]
- Nagai, Y.; Lam, L.; Greene, M.I.; Zhang, H. FOXP3 and Its Cofactors as Targets of Immunotherapies. Engineering 2019, 5, 115–121. [Google Scholar] [CrossRef]
- Xiao, Y.; Nagai, Y.; Deng, G.; Ohtani, T.; Zhu, Z.; Zhou, Z.; Zhang, H.; Ji, M.Q.; Lough, J.W.; Samanta, A.; et al. Dynamic Interactions between TIP60 and P300 Regulate FOXP3 Function through a Structural Switch Defined by a Single Lysine on TIP60. Cell Rep. 2014, 7, 1471–1480. [Google Scholar] [CrossRef]
- Goel, P.N.; Grover, P.; Greene, M.I. PRMT5 and Tip60 Modify FOXP3 Function in Tumor Immunity. Crit. Rev. Immunol. 2020, 40, 283–295. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T Cells: Regulation of Identity and Function. Front Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bin Dhuban, K.; D’Hennezel, E.; Nagai, Y.; Xiao, Y.; Shao, S.; Istomine, R.; Alvarez, F.; Ben-Shoshan, M.; Ochs, H.; Mazer, B.; et al. Suppression by Human FOXP3+ Regulatory T Cells Requires FOXP3-TIP60 Interactions. Sci. Immunol. 2017, 2, eaai9297. [Google Scholar] [CrossRef]
- Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-Specific Protease-7 Inhibition Impairs Tip60-Dependent Foxp3 + T-Regulatory Cell Function and Promotes Antitumor Immunity. EBioMedicine 2016, 13, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The Regulation of Immune Tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, X.; Li, B.; Greene, M.I. FOXP3 and Its Partners: Structural and Biochemical Insights into the Regulation of FOXP3 Activity. Immunol. Res. 2008, 42, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Song, X.; Greene, M.I. FoxP3 in Treg Cell Biology: A Molecular and Structural Perspective. Clin. Exp. Immunol. 2020, 199, 255–262. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Piccirillo, C.A.; Greene, M.I. FOXP3 and Tip60 Structural Interactions Relevant to IPEX Development Lead to Potential Therapeutics to Increase FOXP3 Dependent Suppressor T Cell Functions. Front. Pediatr. 2021, 9, 607292. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Chen, S.; Fernandes, N.; Price, B.D. A Role for the Tip60 Histone Acetyltransferase in the Acetylation and Activation of ATM. Proc. Natl. Acad. Sci. USA 2005, 102, 13182–13187. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, Y.; Lei, D.; Xiang, J.; Ouyang, L.; Wang, Y.; Yang, J. Recent Progress in DNA Methyltransferase Inhibitors as Anticancer Agents. Front. Pharmacol. 2022, 13, 1072651. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, J.; Zhang, W.; Gu, W. Tip60-Dependent Acetylation of P53 Modulates the Decision between Cell-Cycle Arrest and Apoptosis. Mol. Cell 2006, 24, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Searle, N.E.; Torres-Machorro, A.L.; Pillus, L. Chromatin Regulation by the NuA4 Acetyltransferase Complex Is Mediated by Essential Interactions Between Enhancer of Polycomb (Epl1) and Esa1. Genetics 2017, 205, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Ikura, T.; Tashiro, S.; Kakino, A.; Shima, H.; Jacob, N.; Amunugama, R.; Yoder, K.; Izumi, S.; Kuraoka, I.; Tanaka, K.; et al. DNA Damage-Dependent Acetylation and Ubiquitination of H2AX Enhances Chromatin Dynamics. Mol. Cell. Biol. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-L.; Xiang, J.-F.; Liu, S.-C.; Guo, T.; Yan, G.-F.; Feng, Y.; Kong, N.; Li, H.-D.; Huang, Y.; Lin, H.; et al. H2AX Facilitates Classical Non-Homologous End Joining at the Expense of Limited Nucleotide Loss at Repair Junctions. Nucleic Acids Res. 2017, 45, 10614–10633. [Google Scholar] [CrossRef][Green Version]
- Chailleux, C.; Tyteca, S.; Papin, C.; Boudsocq, F.; Puget, N.; Courilleau, C.; Grigoriev, M.; Canitrot, Y.; Trouche, D. Physical Interaction between the Histone Acetyl Transferase Tip60 and the DNA Double-Strand Breaks Sensor MRN Complex. Biochem. J. 2010, 426, 365–371. [Google Scholar] [CrossRef]
- Mo, F.; Zhuang, X.; Liu, X.; Yao, P.Y.; Qin, B.; Su, Z.; Zang, J.; Wang, Z.; Zhang, J.; Dou, Z.; et al. Acetylation of Aurora B by TIP60 Ensures Accurate Chromosomal Segregation. Nat. Chem. Biol. 2016, 12, 226–232. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Roy, K.; Price, B.D. DNA Damage-Induced Acetylation of Lysine 3016 of ATM Activates ATM Kinase Activity. Mol. Cell. Biol. 2007, 27, 8502–8509. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, K.; Fradet-Turcotte, A.; Avvakumov, N.; Lambert, J.-P.; Roques, C.; Pandita, R.K.; Paquet, E.; Herst, P.; Gingras, A.-C.; Pandita, T.K.; et al. The TIP60 Complex Regulates Bivalent Chromatin Recognition by 53BP1 through Direct H4K20me Binding and H2AK15 Acetylation. Mol. Cell 2016, 62, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Alterations of DNA Damage Repair in Cancer: From Mechanisms to Applications. Ann. Transl. Med. 2020, 8, 1685. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.L.; Lan, L.; Zou, L. DNA Repair Defects in Cancer and Therapeutic Opportunities. Genes Dev. 2022, 36, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lindahl, T. Maintenance of Genome Stability. Genom. Proteom. Bioinform. 2016, 14, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Choi, Y.-L.; Kwon, M.; Park, P.J. Mechanisms and Consequences of Cancer Genome Instability: Lessons from Genome Sequencing Studies. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 283–312. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.; Casey, M.C.; Shalaby, A.; Kalinina, O.; Curran, C.; Webber, M.; Callagy, G.; Holian, E.; Bourke, E.; Kerin, M.J.; et al. Quantifying Tip60 (Kat5) Stratifies Breast Cancer. Sci. Rep. 2019, 9, 3819. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Pereira-Smith, O.M. Identification of an Alternatively Spliced Form of the Tat Interactive Protein (Tip60), Tip60(β). Gene 2000, 258, 141–146. [Google Scholar] [CrossRef]
- Gorrini, C.; Squatrito, M.; Luise, C.; Syed, N.; Perna, D.; Wark, L.; Martinato, F.; Sardella, D.; Verrecchia, A.; Bennett, S.; et al. Tip60 Is a Haplo-Insufficient Tumour Suppressor Required for an Oncogene-Induced DNA Damage Response. Nature 2007, 448, 1063–1067. [Google Scholar] [CrossRef]
- Kwan, S.; Sheel, A.; Song, C.; Zhang, X.; Jiang, T.; Dang, H.; Cao, Y.; Ozata, D.M.; Mou, H.; Yin, H.; et al. Depletion of TRRAP Induces P53-Independent Senescence in Liver Cancer by Down-Regulating Mitotic Genes. Hepatology 2020, 71, 275–290. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jang, S.-M.; Kim, C.-H.; An, J.-H.; Kang, E.-J.; Choi, K.-H. New Molecular Bridge between RelA/P65 and NF-ΚB Target Genes via Histone Acetyltransferase TIP60 Cofactor. J. Biol. Chem. 2012, 287, 7780–7791. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, Y.; Zhang, T.; Bai, L.; Wang, Y.; Duan, C. E3 Ligase UHRF2 Stabilizes the Acetyltransferase TIP60 and Regulates H3K9ac and H3K14ac via RING Finger Domain. Protein Cell 2017, 8, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gou, D.; Xiang, J.; Pan, X.; Gao, Q.; Zhou, P.; Liu, Y.; Hu, J.; Wang, K.; Tang, N. O-GlcNAc Modified-TIP60/KAT5 Is Required for PCK1 Deficiency-Induced HCC Metastasis. Oncogene 2021, 40, 6707–6719. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Sakai, M.; Shimokawa, T.; Yamada, Y.; Nakamura, Y.; Furukawa, Y. C20orf20 (MRG-Binding Protein) as a Potential Therapeutic Target for Colorectal Cancer. Br. J. Cancer 2010, 102, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Nakagawa, S.; Saku, A.; Isobe, Y.; Yamaguchi, R.; Sheridan, P.; Takane, K.; Ikenoue, T.; Zhu, C.; Miura, M.; et al. Bromodomain Protein BRD8 Regulates Cell Cycle Progression in Colorectal Cancer Cells through a TIP60-Independent Regulation of the Pre-RC Complex. iScience 2023, 26, 106563. [Google Scholar] [CrossRef] [PubMed]
- Mattera, L.; Escaffit, F.; Pillaire, M.-J.; Selves, J.; Tyteca, S.; Hoffmann, J.-S.; Gourraud, P.-A.; Chevillard-Briet, M.; Cazaux, C.; Trouche, D. The P400/Tip60 Ratio Is Critical for Colorectal Cancer Cell Proliferation through DNA Damage Response Pathways. Oncogene 2009, 28, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, J.; Zhang, S.; Liu, X.; Long, X.; Lan, J.; Zhou, M.; Zheng, L.; Zhou, J. LINC00839 Promotes Colorectal Cancer progression by Recruiting RUVBL1/Tip60 Complexes to Activate. EMBO Rep. 2022, 23, e54128. [Google Scholar] [CrossRef]
- Hong, Y.J.; Park, J.; Hahm, J.Y.; Kim, S.H.; Lee, D.H.; Park, K.-S.; Seo, S.-B. Regulation of UHRF1 Acetylation by TIP60 Is Important for Colon Cancer Cell Proliferation. Genes Genom. 2022, 44, 1353–1361. [Google Scholar] [CrossRef]
- Cui, H.; Guo, M.; Xu, D.; Ding, Z.-C.; Zhou, G.; Ding, H.-F.; Zhang, J.; Tang, Y.; Yan, C. The Stress-Responsive Gene ATF3 Regulates the Histone Acetyltransferase Tip60. Nat. Commun. 2015, 6, 6752. [Google Scholar] [CrossRef]
- Schleicher, E.M.; Dhoonmoon, A.; Jackson, L.M.; Khatib, J.B.; Nicolae, C.M.; Moldovan, G.-L. The TIP60-ATM Axis Regulates Replication Fork Stability in BRCA-Deficient Cells. Oncogenesis 2022, 11, 33. [Google Scholar] [CrossRef]
- Fujimoto, M.; Takii, R.; Matsumoto, M.; Okada, M.; Nakayama, K.I.; Nakato, R.; Fujiki, K.; Shirahige, K.; Nakai, A. HSF1 Phosphorylation Establishes an Active Chromatin State via the TRRAP–TIP60 Complex and Promotes Tumorigenesis. Nat. Commun. 2022, 13, 4355. [Google Scholar] [CrossRef] [PubMed]
- Obri, A.; Ouararhni, K.; Papin, C.; Diebold, M.-L.; Padmanabhan, K.; Marek, M.; Stoll, I.; Roy, L.; Reilly, P.T.; Mak, T.W.; et al. ANP32E Is a Histone Chaperone That Removes H2A.Z from Chromatin. Nature 2014, 505, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Takahashi, H.; Sato, S.; Tomomori-Sato, C.; Saraf, A.; Washburn, M.P.; Florens, L.; Conaway, R.C.; Conaway, J.W.; Hatakeyama, S. TRIM29 Regulates the Assembly of DNA Repair Proteins into Damaged Chromatin. Nat. Commun. 2015, 6, 7299. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Guan, H.; Yan, S.; Hu, S.; Song, M.; Guo, Z.-P.; Xie, D.-F.; Liu, Y.; Liu, X.; Zhang, S.; et al. TIP60 K430 SUMOylation Attenuates Its Interaction with DNA-PKcs in S-Phase Cells: Facilitating Homologous Recombination and Emerging Target for Cancer Therapy. Sci. Adv. 2020, 6, eaba7822. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Jin, J.; Tomomori-Sato, C.; Sato, S.; Sorokina, I.; Parmely, T.J.; Conaway, R.C.; Conaway, J.W. Identification of New Subunits of the Multiprotein Mammalian TRRAP/TIP60-Containing Histone Acetyltransferase Complex. J. Biol. Chem. 2003, 278, 42733–42736. [Google Scholar] [CrossRef] [PubMed]
- Procida, T.; Friedrich, T.; Jack, A.P.M.; Peritore, M.; Bönisch, C.; Eberl, H.C.; Daus, N.; Kletenkov, K.; Nist, A.; Stiewe, T.; et al. JAZF1, A Novel P400/TIP60/NuA4 Complex Member, Regulates H2A.Z Acetylation at Regulatory Regions. Int. J. Mol. Sci. 2021, 22, 678. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Bronner, C.; Zaayter, L.; Ahmad, T.; Richert, L.; Alhosin, M.; Ibrahim, A.; Hamiche, A.; Mely, Y.; Mousli, M. Interaction of the Epigenetic Integrator UHRF1 with the MYST Domain of TIP60 inside the Cell. J. Exp. Clin. Cancer Res. 2017, 36, 188. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ashraf, W.; Ibrahim, A.; Zaayter, L.; Muller, C.; Hamiche, A.; Mély, Y.; Bronner, C.; Mousli, M. TIP60 Governs the Auto-ubiquitination of UHRF1 through USP7 Dissociation from the UHRF1/USP7 Complex. Int. J. Oncol. 2021, 59, 89. [Google Scholar] [CrossRef]
- Idrissou, M.; Boisnier, T.; Sanchez, A.; Khoufaf, F.Z.H.; Penault-Llorca, F.; Bignon, Y.-J.; Bernard-Gallon, D. TIP60/P400/H4K12ac Plays a Role as a Heterochromatin Back-up Skeleton in Breast Cancer. Cancer Genom. Proteom. 2020, 17, 687–694. [Google Scholar] [CrossRef]
- Peng, L.; Ling, H.; Yuan, Z.; Fang, B.; Bloom, G.; Fukasawa, K.; Koomen, J.; Chen, J.; Lane, W.S.; Seto, E. SIRT1 Negatively Regulates the Activities, Functions, and Protein Levels of HMOF and TIP60. Mol. Cell. Biol. 2012, 32, 2823–2836. [Google Scholar] [CrossRef]
- Qin, B.; Yu, J.; Nowsheen, S.; Wang, M.; Tu, X.; Liu, T.; Li, H.; Wang, L.; Lou, Z. UFL1 Promotes Histone H4 Ufmylation and ATM Activation. Nat. Commun. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; He, Z.; Zhang, A.; Yan, Z.; Zhang, X.; Hu, S.; Wang, N.; He, H. Tip60 and P300 Function Antagonistically in the Epigenetic Regulation of HPV18 E6/E7 Genes in Cervical Cancer HeLa Cells. Genes Genom. 2020, 42, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Akbar, H.; Cao, J.; Wang, D.; Yuan, X.; Zhang, M.; Muthusamy, S.; Song, X.; Liu, X.; Aikhionbare, F.; Yao, X.; et al. Acetylation of Nup62 by TIP60 Ensures Accurate Chromosome Segregation in Mitosis. J. Mol. Cell Biol. 2022, 14, 56. [Google Scholar] [CrossRef]
- Cheng, Z.; Ke, Y.; Ding, X.; Wang, F.; Wang, H.; Ahmed, K.; Liu, Z.; Xu, Y.; Aikhionbare, F.; Yan, H.; et al. Functional Characterization of TIP60 Sumoylation in UV-Irradiated DNA Damage Response. Oncogene 2008, 27, 931–941. [Google Scholar] [CrossRef]
- Sliva, D.; Zhu, Y.X.; Tsai, S.; Kamine, J.; Yang, Y.-C. Tip60 Interacts with Human Interleukin-9 Receptor α-Chain. Biochem. Biophys. Res. Commun. 1999, 263, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-J.; Loewenstein, P.M.; Green, M. Identification of a Panel of MYC and Tip60 Co-Regulated Genes Functioning Primarily in Cell Cycle and DNA Replication. Genes Cancer 2018, 9, 101–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, J.; Wen, M.; Gao, P.; Feng, M.; Wei, G. RUVBL1 Ubiquitination by DTL Promotes RUVBL1/2-β-Catenin-Mediated Transcriptional Regulation of NHEJ Pathway and Enhances Radiation Resistance in Breast Cancer. Cell Death Dis. 2024, 15, 259. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Lungchukiet, P.; Quarni, W.; Yang, S.; Zhang, X.; Bai, W. Fe65 Suppresses Breast Cancer Cell Migration and Invasion through Tip60 Mediated Cortactin Acetylation. Sci. Rep. 2015, 5, 11529. [Google Scholar] [CrossRef]
- Chen, W.; Salto-Tellez, M.; Palanisamy, N.; Ganesan, K.; Hou, Q.; Tan, L.K.; Sii, L.H.; Ito, K.; Tan, B.; Wu, J.; et al. Targets of Genome Copy Number Reduction in Primary Breast Cancers Identified by Integrative Genomics. Genes Chromosomes Cancer 2007, 46, 288–301. [Google Scholar] [CrossRef]
- Coffey, K.; Blackburn, T.J.; Cook, S.; Golding, B.T.; Griffin, R.J.; Hardcastle, I.R.; Hewitt, L.; Huberman, K.; McNeill, H.V.; Newell, D.R.; et al. Characterisation of a Tip60 Specific Inhibitor, NU9056, in Prostate Cancer. PLoS ONE 2012, 7, e45539. [Google Scholar] [CrossRef]
- Miyajima, N.; Maruyama, S.; Bohgaki, M.; Kano, S.; Shigemura, M.; Shinohara, N.; Nonomura, K.; Hatakeyama, S. TRIM68 Regulates Ligand-Dependent Transcription of Androgen Receptor in Prostate Cancer Cells. Cancer Res. 2008, 68, 3486–3494. [Google Scholar] [CrossRef]
- Ito, S.; Kayukawa, N.; Ueda, T.; Taniguchi, H.; Morioka, Y.; Hongo, F.; Ukimura, O. MRGBP Promotes AR-Mediated Transactivation of KLK3 and TMPRSS2 via Acetylation of Histone H2A.Z in Prostate Cancer Cells. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 794–802. [Google Scholar] [CrossRef]
- Gaughan, L.; Brady, M.E.; Cook, S.; Neal, D.E.; Robson, C.N. Tip60 Is a Co-Activator Specific for Class I Nuclear Hormone Receptors. J. Biol. Chem. 2001, 276, 46841–46848. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, D.L.; Martinka, M.; Li, G. Prognostic Significance of Nuclear ING3 Expression in Human Cutaneous Melanoma. Clin. Cancer Res. 2007, 13, 4111–4116. [Google Scholar] [CrossRef]
- Lau, E.; Ronai, Z.A. ATF2—At the Crossroad of Nuclear and Cytosolic Functions. J. Cell Sci. 2012, 125, 2815–2824. [Google Scholar] [CrossRef]
- Dai, C.; Shi, D.; Gu, W. Negative Regulation of the Acetyltransferase TIP60-P53 Interplay by UHRF1 (Ubiquitin-like with PHD and RING Finger Domains 1). J. Biol. Chem. 2013, 288, 19581–19592. [Google Scholar] [CrossRef]
- Niu, X.; Wu, T.; Yin, Q.; Gu, X.; Li, G.; Zhou, C.; Ma, M.; Su, L.; Tang, S.; Tian, Y.; et al. Combination of Paclitaxel and PXR Antagonist SPA70 Reverses Paclitaxel-Resistant Non-Small Cell Lung Cancer. Cells 2022, 11, 3094. [Google Scholar] [CrossRef]
- Yi, J.; Huang, X.; Yang, Y.; Zhu, W.-G.; Gu, W.; Luo, J. Regulation of Histone Acetyltransferase TIP60 Function by Histone Deacetylase 3. J. Biol. Chem. 2014, 289, 33878–33886. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Wang, Z.-T.; Hu, Y.-J.; Bao, F.-C.; Yuan, P.; Zhang, C.; Cao, J.-L.; Lv, W.; Hu, J. Downregulation of RUVBL1 Inhibits Proliferation of Lung Adenocarcinoma Cells by G1/S Phase Cell Cycle Arrest via Multiple Mechanisms. Tumor Biol. 2016, 37, 16015–16027. [Google Scholar] [CrossRef]
- Dohmesen, C.; Koeppel, M.; Dobbelstein, M. Specific Inhibition of Mdm2-Mediated Neddylation by Tip60. Cell Cycle 2008, 7, 222–231. [Google Scholar] [CrossRef]
- Pikor, L.A.; Lockwood, W.W.; Thu, K.L.; Vucic, E.A.; Chari, R.; Gazdar, A.F.; Lam, S.; Lam, W.L. YEATS4 Is a Novel Oncogene Amplified in Non–Small Cell Lung Cancer That Regulates the P53 Pathway. Cancer Res. 2013, 73, 7301–7312. [Google Scholar] [CrossRef]
- Eymin, B.; Claverie, P.; Salon, C.; Leduc, C.; Col, E.; Brambilla, E.; Khochbin, S.; Gazzeri, S. P14 ARF Activates a Tip60-Dependent and P53-Independent ATM/ATR/CHK Pathway in Response to Genotoxic Stress. Mol. Cell. Biol. 2006, 26, 4339–4350. [Google Scholar] [CrossRef]
- Rivero, S.; Rodríguez-Real, G.; Marín, I.; Huertas, P. MRGBP, a Member of the NuA4 Complex, Inhibits DNA Double-strand Break Repair. FEBS Open Bio. 2021, 11, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shan, J.; Liu, J.; Feng, Y.; Ke, Y.; Qi, W.; Liu, W.; Zeng, X. RNF8 Promotes Efficient DSB Repair by Inhibiting the Pro-apoptotic Activity of P53 through Regulating the Function of Tip60. Cell Prolif. 2020, 53, e12780. [Google Scholar] [CrossRef]
- Hattori, T.; Coustry, F.; Stephens, S.; Eberspaecher, H.; Takigawa, M.; Yasuda, H.; de Crombrugghe, B. Transcriptional Regulation of Chondrogenesis by Coactivator Tip60 via Chromatin Association with Sox9 and Sox5. Nucleic Acids Res. 2008, 36, 3011–3024. [Google Scholar] [CrossRef]
- Li, T.Y.; Song, L.; Sun, Y.; Li, J.; Yi, C.; Lam, S.M.; Xu, D.; Zhou, L.; Li, X.; Yang, Y.; et al. Tip60-mediated lipin 1 acetylation and ER translocation determine triacylglycerol synthesis rate. Nat Commun. 2018, 9, 1916. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shibata, E.; Dutta, A. Deubiquitination of Tip60 by USP7 Determines the Activity of the P53-Dependent Apoptotic Pathway. Mol. Cell. Biol. 2013, 33, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, S.; Gewirtz, A.M. The Histone Acetyltransferase TIP60 Interacts with C-Myb and Inactivates Its Transcriptional Activity in Human Leukemia. J. Biol. Chem. 2012, 287, 925–934. [Google Scholar] [CrossRef]
- Bararia, D.; Trivedi, A.K.; Zada, A.A.P.; Greif, P.A.; Mulaw, M.A.; Christopeit, M.; Hiddemann, W.; Bohlander, S.K.; Behre, G. Proteomic Identification of the MYST Domain Histone Acetyltransferase TIP60 (HTATIP) as a Co-Activator of the Myeloid Transcription Factor C/EBPα. Leukemia 2008, 22, 800–807. [Google Scholar] [CrossRef]
- Huang, X.; Spencer, G.J.; Lynch, J.T.; Ciceri, F.; Somerville, T.D.D.; Somervaille, T.C.P. Enhancers of Polycomb EPC1 and EPC2 Sustain the Oncogenic Potential of MLL Leukemia Stem Cells. Leukemia 2014, 28, 1081–1091. [Google Scholar] [CrossRef]
- Bhoumik, A.; Singha, N.; O’Connell, M.J.; Ronai, Z.A. Regulation of TIP60 by ATF2 Modulates ATM Activation. J. Biol. Chem. 2008, 283, 17605–17614. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Micci, F.; Thorsen, J.; Gorunova, L.; Eibak, A.M.; Bjerkehagen, B.; Davidson, B.; Heim, S. Novel Fusion of MYST/Esa1-Associated Factor 6 and PHF1 in Endometrial Stromal Sarcoma. PLoS ONE 2012, 7, e39354. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, M.V.; Zhou, M.I.; Cohen, H.T. Von Hippel-Lindau Partner Jade-1 Is a Transcriptional Co-Activator Associated with Histone Acetyltransferase Activity. J. Biol. Chem. 2004, 279, 56032–56041. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Whelan, C.M.; Berezovska, O.; Hyman, B.T. The γ Secretase-Generated Carboxyl-Terminal Domain of the Amyloid Precursor Protein Induces Apoptosis via Tip60 in H4 Cells. J. Biol. Chem. 2002, 277, 28530–28536. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Zheng, H.; Yang, X.; Liu, Y.; Wang, T.C. Tip60 Functions as a Potential Corepressor of KLF4 in Regulation of HDC Promoter Activity. Nucleic Acids Res. 2007, 35, 6137–6149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, Q.; Liu, W.; Wang, Y.; Zhao, L.; Chen, J.; Liu, H.; Liu, S.; Li, J.; Zhang, W.; et al. FAM135B Sustains the Reservoir of Tip60-ATM Assembly to Promote DNA Damage Response. Clin. Transl. Med. 2022, 12, e945. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jiang, Y.; Peng, P.; Liu, G.; Qi, S.; Liu, K.; Mei, Q.; Li, J. Quantitative Proteomics Reveals the Role of Lysine 2-Hydroxyisobutyrylation Pathway Mediated by Tip60. Oxidative Med. Cell. Longev. 2022, 2022, 4571319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Han, Z.; Halabelian, L.; Yang, X.; Ding, J.; Zhang, N.; Ngo, L.; Song, J.; Zeng, H.; He, M.; et al. Identification of Lysine Isobutyrylation as a New Histone Modification Mark. Nucleic Acids Res 2020, 49, 177–189. [Google Scholar] [CrossRef]
- Dutta, A.; Abmayr, S.M.; Workman, J.L. Diverse Activities of Histone Acylations Connect Metabolism to Chromatin Function. Mol. Cell 2016, 63, 547–552. [Google Scholar] [CrossRef]
- Chen, Y.; Sprung, R.; Tang, Y.; Ball, H.; Sangras, B.; Kim, S.C.; Falck, J.R.; Peng, J.; Gu, W.; Zhao, Y. Lysine Propionylation and Butyrylation Are Novel Post-Translational Modifications in Histones. Mol. Cell. Proteom. 2007, 6, 812–819. [Google Scholar] [CrossRef]
- Fang, X.; Lu, G.; Ha, K.; Lin, H.; Du, Y.; Zuo, Q.; Fu, Y.; Zou, C.; Zhang, P. Acetylation of TIP60 at K104 Is Essential for Metabolic Stress-Induced Apoptosis in Cells of Hepatocellular Cancer. Exp. Cell Res. 2018, 362, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Charvet, C.; Wissler, M.; Brauns-Schubert, P.; Wang, S.-J.; Tang, Y.; Sigloch, F.C.; Mellert, H.; Brandenburg, M.; Lindner, S.E.; Breit, B.; et al. Phosphorylation of Tip60 by GSK-3 Determines the Induction of PUMA and Apoptosis by P53. Mol. Cell 2011, 42, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Hwa Shin, S.; Sun Kang, S. Phosphorylation of Tip60 Tyrosine 327 by Abl Kinase Inhibits HAT Activity through Association with FE65. Open Biochem. J. 2013, 7, 66–72. [Google Scholar] [CrossRef]
- Yamagata, K.; Kitabayashi, I. Sirt1 Physically Interacts with Tip60 and Negatively Regulates Tip60-Mediated Acetylation of H2AX. Biochem. Biophys. Res. Commun. 2009, 390, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Li, T.Y.; Liu, Q.; Zhang, C.; Li, X.; Chen, Y.; Zhang, S.-M.; Lian, G.; Liu, Q.; Ruan, K.; et al. GSK3-TIP60-ULK1 Signaling Pathway Links Growth Factor Deprivation to Autophagy. Science 2012, 336, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Jiang, Q.; Bhanu, N.V.; Wu, J.; Li, W.; Garcia, B.A.; Greenberg, R.A. Phosphorylation of TIP60 Suppresses 53BP1 Localization at DNA Damage Sites. Mol. Cell. Biol. 2019, 39, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Seit-Nebi, A.; Han, X.; Aslanian, A.; Tat, J.; Liao, R.; Yates, J.R.; Sun, P. A Posttranslational Modification Cascade Involving P38, Tip60, and PRAK Mediates Oncogene-Induced Senescence. Mol. Cell 2013, 50, 699–710. [Google Scholar] [CrossRef] [PubMed]
- García-González, R.; Monte-Serrano, E.; Morejón-García, P.; Navarro-Carrasco, E.; Lazo, P.A. The VRK1 Chromatin Kinase Regulates the Acetyltransferase Activity of Tip60/KAT5 by Sequential Phosphorylations in Response to DNA Damage. Biochim. Biophys. Acta Gene Regul. Mech. 2022, 1865, 194887. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, V.; Logan, I.R.; Nelson, G.; Cook, S.; Robson, C.N. Phosphorylation of Tat-Interactive Protein 60kDa by Protein Kinase Cε Is Important for Its Subcellular Localisation. Int. J. Biochem. Cell Biol. 2008, 40, 236–244. [Google Scholar] [CrossRef]
- Clarke, T.L.; Sanchez-Bailon, M.P.; Chiang, K.; Reynolds, J.J.; Herrero-Ruiz, J.; Bandeiras, T.M.; Matias, P.M.; Maslen, S.L.; Skehel, J.M.; Stewart, G.S.; et al. PRMT5-Dependent Methylation of the TIP60 Coactivator RUVBL1 Is a Key Regulator of Homologous Recombination. Mol. Cell 2017, 65, 900–916.e7. [Google Scholar] [CrossRef]
- Col, E.; Caron, C.; Chable-Bessia, C.; Legube, G.; Gazzeri, S.; Komatsu, Y.; Yoshida, M.; Benkirane, M.; Trouche, D.; Khochbin, S. HIV-1 Tat Targets Tip60 to Impair the Apoptotic Cell Response to Genotoxic Stresses. EMBO J. 2005, 24, 2634–2645. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.R.; Lakhter, A.J.; Androphy, E.J. PIASy-Mediated Tip60 Sumoylation Regulates P53-Induced Autophagy. Cell Cycle 2012, 11, 2717–2728. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J. SIRT1 Regulates Autoacetylation and Histone Acetyltransferase Activity of TIP60. J. Biol. Chem. 2010, 285, 11458–11464. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, D.; Akanuma, N.; Kobayashi, I.S.; Heo, E.; Ando, M.; Fujii, M.; Jiang, F.; Prin, P.N.; Pan, G.; Wong, K.; et al. TIP60 Is Required for Tumorigenesis in Non-small Cell Lung Cancer. Cancer Sci. 2023, 114, 2400–2413. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.L. Patent spotlight: Small-molecule lysine acetyltransferase inhibitors (KATi). Pharmaceutical Patent Analyst. 2020, 9, 17–28. [Google Scholar] [CrossRef]
- Hu, Y.; Fisher, J.B.; Koprowski, S.; McAllister, D.; Kim, M.; Lough, J. Homozygous Disruption of the Tip60 Gene Causes Early Embryonic Lethality. Dev. Dyn. 2009, 238, 2912–2921. [Google Scholar] [CrossRef]
- Numata, A.; Kwok, H.S.; Zhou, Q.-L.; Li, J.; Tirado-Magallanes, R.; Angarica, V.E.; Hannah, R.; Park, J.; Wang, C.Q.; Krishnan, V.; et al. Lysine Acetyltransferase Tip60 Is Required for Hematopoietic Stem Cell Maintenance. Blood 2020, 136, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Idrissou, M.; Judes, G.; Daures, M.; Sanchez, A.; El Ouardi, D.; Besse, S.; Degoul, F.; Penault-Llorca, F.; Bignon, Y.-J.; Bernard-Gallon, D. TIP60 Inhibitor TH1834 Reduces Breast Cancer Progression in Xenografts in Mice. OMICS 2019, 23, 457–459. [Google Scholar] [CrossRef]
- Gao, C.; Bourke, E.; Scobie, M.; Famme, M.A.; Koolmeister, T.; Helleday, T.; Eriksson, L.A.; Lowndes, N.F.; Brown, J.A.L. Rational Design and Validation of a Tip60 Histone Acetyltransferase Inhibitor. Sci. Rep. 2014, 4, 5372. [Google Scholar] [CrossRef]
- Huang, M.; Huang, J.; Zheng, Y.; Sun, Q. Histone Acetyltransferase Inhibitors: An Overview in Synthesis, Structure-Activity Relationship and Molecular Mechanism. Eur. J. Med. Chem. 2019, 178, 259–286. [Google Scholar] [CrossRef]
- Whedon, S.D.; Cole, P.A. KATs off: Biomedical Insights from Lysine Acetyltransferase Inhibitors. Curr. Opin. Chem. Biol. 2023, 72, 102255. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Tan, H.; Liao, M.; Zhu, S.; Zheng, L.-L.; Huang, H.; Liu, B. Targeting Cancer Epigenetic Pathways with Small-Molecule Compounds: Therapeutic Efficacy and Combination Therapies. Pharmacol. Res. 2021, 173, 105702. [Google Scholar] [CrossRef]
- Ghizzoni, M.; Wu, J.; Gao, T.; Haisma, H.J.; Dekker, F.J.; George Zheng, Y. 6-Alkylsalicylates Are Selective Tip60 Inhibitors and Target the Acetyl-CoA Binding Site. Eur. J. Med. Chem. 2012, 47, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Zohourian, N.; Brown, J.A.L. Current Trends in Clinical Trials and the Development of Small Molecule Epigenetic Inhibitors as Cancer Therapeutics. Epigenomics 2024, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, T.C.; Kulik, K.R.; Lauth, A.; Smith, B.C.; Lough, J.W.; Auchampach, J.A. Pharmacological Inhibition of the Acetyltransferase Tip60 Mitigates Myocardial Infarction Injury. Dis. Model Mech. 2023, 16, dmm049786. [Google Scholar] [CrossRef]
- Nagaya, M.; Yamaoka, R.; Kanada, F.; Sawa, T.; Takashima, M.; Takamura, Y.; Inatani, M.; Oki, M. Histone Acetyltransferase Inhibition Reverses Opacity in Rat Galactose-Induced Cataract. PLoS ONE 2022, 17, e0273868. [Google Scholar] [CrossRef]
- Chen, L.; Qing, W.; Yi, Z.; Lin, G.; Peng, Q.; Zhou, F. NU9056, a KAT 5 Inhibitor, Treatment Alleviates Brain Dysfunction by Inhibiting NLRP3 Inflammasome Activation, Affecting Gut Microbiota, and Derived Metabolites in LPS-Treated Mice. Front. Nutr. 2021, 8, 701760. [Google Scholar] [CrossRef]
- Xu, L.; Qin, Y.; Liu, M.; Jiao, J.; Tu, D.; Zhang, M.; Yan, D.; Song, X.; Sun, C.; Zhu, F.; et al. The Acetyltransferase KAT5 Inhibitor NU 9056 Promotes Apoptosis and Inhibits JAK2/STAT3 Pathway in Extranodal NK/T Cell Lymphoma. Anticancer Agents Med. Chem. 2022, 22, 1530–1540. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Yang, Y.; Xu, J.; Cai, S.; Tian, Y. KAT5 Inhibitor NU9056 Suppresses Anaplastic Thyroid Carcinoma Progression through C-Myc/MiR-202 Pathway. Int. J. Endocrinol. 2022, 2022, 2014568. [Google Scholar] [CrossRef] [PubMed]
- Zong-ying, L.; Jing-tao, H. NU9056 Targets KAT5 to Regulate the Proliferation, Migration and Invasion of Esophageal Cancer Cells via ABCE1 Acetylation. SDRP J. Cell. Mol. Physiol. 2022, 4, 220–227. [Google Scholar] [CrossRef]
- Luo, F.; Tao, Y.; Wang, M.; Yang, L.; Su, R.; Pan, Z.; Tan, X. The Protective Effects of KAT5 Inhibition on Ocular Inflammation by Mediating the PI3K/AKT Pathway in a Murine Model of Allergic Conjunctivitis. Investig. Opthalmology Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef]
- Sen, U.; Nayak, A.; Khurana, J.; Sharma, D.; Gupta, A. Inhibition of PfMYST Histone Acetyltransferase Activity Blocks Plasmodium Falciparum Growth and Survival. Antimicrob. Agents Chemother. 2020, 65, e00953-20. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Fiches, G.; Jean, M.J.; Dieringer, M.; McGuinness, J.; John, S.P.; Shamay, M.; Desai, P.; Zhu, J.; Santoso, N.G. Inhibition of Tip60 Reduces Lytic and Latent Gene Expression of Kaposi’s Sarcoma-Associated Herpes Virus (KSHV) and Proliferation of KSHV-Infected Tumor Cells. Front. Microbiol. 2018, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Cregan, S.; McDonagh, L.; Gao, Y.; Barr, M.P.; O’Byrne, K.J.; Finn, S.P.; Cuffe, S.; Gray, S.G. KAT5 (Tip60) Is a Potential Therapeutic Target in Malignant Pleural Mesothelioma. Int. J. Oncol. 2016, 48, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Rao, M.; Wang, C.; Sakamaki, T.; Wang, J.; Di Vizio, D.; Zhang, X.; Albanese, C.; Balk, S.; Chang, C.; et al. Acetylation of Androgen Receptor Enhances Coactivator Binding and Promotes Prostate Cancer Cell Growth. Mol. Cell. Biol. 2003, 23, 8563–8575. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, P.; Davis, S.A.; Vashishtha, H.; Gucwa, A.L.; Ginsburg, D.S. Nuclear Localization Is Not Required for Tip60 Tumor Suppressor Activity in Breast and Lung Cancer Cells. DNA Cell Biol. 2020, 39, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Lee, D.H. KAT5 Negatively Regulates the Proliferation of Prostate Cancer LNCaP Cells via the Caspase 3-Dependent Apoptosis Pathway. Anim. Cells Syst. 2019, 23, 253–259. [Google Scholar] [CrossRef]
- Chen, G.; Cheng, Y.; Tang, Y.; Martinka, M.; Li, G. Role of Tip60 in Human Melanoma Cell Migration, Metastasis, and Patient Survival. J. Investig. Dermatol. 2012, 132, 2632–2641. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Han, S.; Shao, Z.; Lu, Z.; Huo, L.; Zhang, J.; Yang, R.; Feng, Q.; Shen, H.; et al. Tip60 Suppresses Cholangiocarcinoma Proliferation and Metastasis via PI3k-AKT. Cell Physiol. Biochem. 2018, 50, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Bai, R.; Zhao, Z.; Zhou, J.; Weng, Y. Abstract 2851: Reduced Tip60 Expression in Human Gastric Cancer. Cancer Res. 2014, 74, 2851. [Google Scholar] [CrossRef]
- Beaver, M.; Karisetty, B.C.; Zhang, H.; Bhatnagar, A.; Armour, E.; Parmar, V.; Brown, R.; Xiang, M.; Elefant, F. Chromatin and Transcriptomic Profiling Uncover Dysregulation of the Tip60 HAT/HDAC2 Epigenomic Landscape in the Neurodegenerative Brain. Epigenetics 2022, 17, 786–807. [Google Scholar] [CrossRef]
- Zhang, H.; Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Roach, T.V.; Mortazavi, S.; Mandloi, S.; Elefant, F. Tip60 Protects against Amyloid-β-Induced Transcriptomic Alterations via Different Modes of Action in Early versus Late Stages of Neurodegeneration. Mol. Cell. Neurosci. 2020, 109, 103570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).