Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Sample Collection
2.2. Proteomics Screening Analysis
2.3. Differentiated miRNA Expression Analysis in Blood Plasma Samples
2.3.1. miRNA Extraction from Plasma
2.3.2. miRNA Quantification
2.3.3. miRNA Profiling Using a Takara Platform
2.4. miRNA Validation, cDNA Synthesis from Plasma Samples, and Quantitative Real-Time PCR
2.5. Protein Targets of miRNA-1285 in the Literature
2.6. Tissue Lysis and Protein Expression Analysis with Western Blot for Protein Target Validation
2.7. Statistics and Calculations
3. Results
3.1. Patient Recruitment and Sample Collection
3.2. Proteomics Screening Analysis and Selection of Protein Targets for Validation
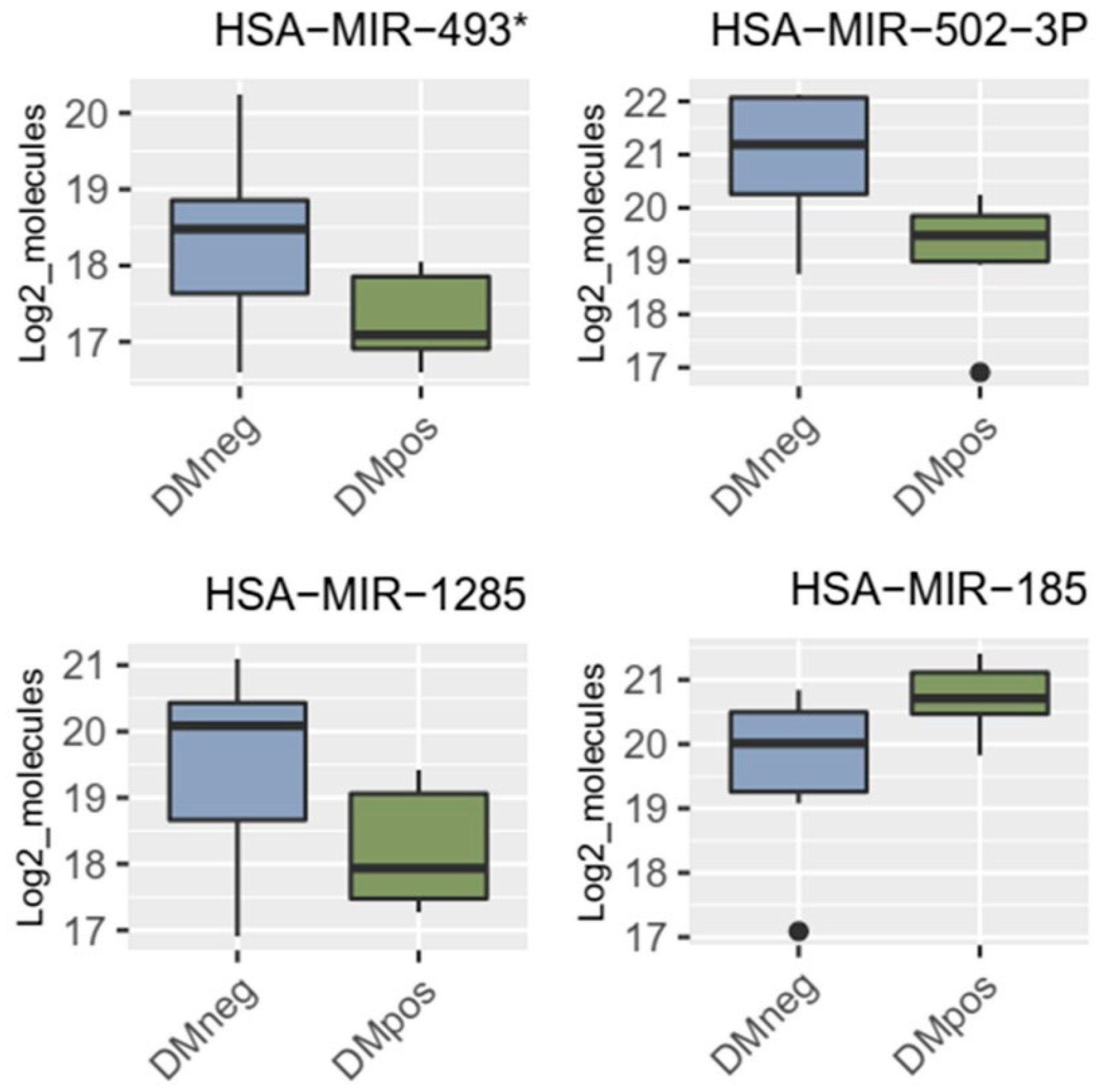
3.3. miRNA Microarray Results and Selection of miRNA Targets
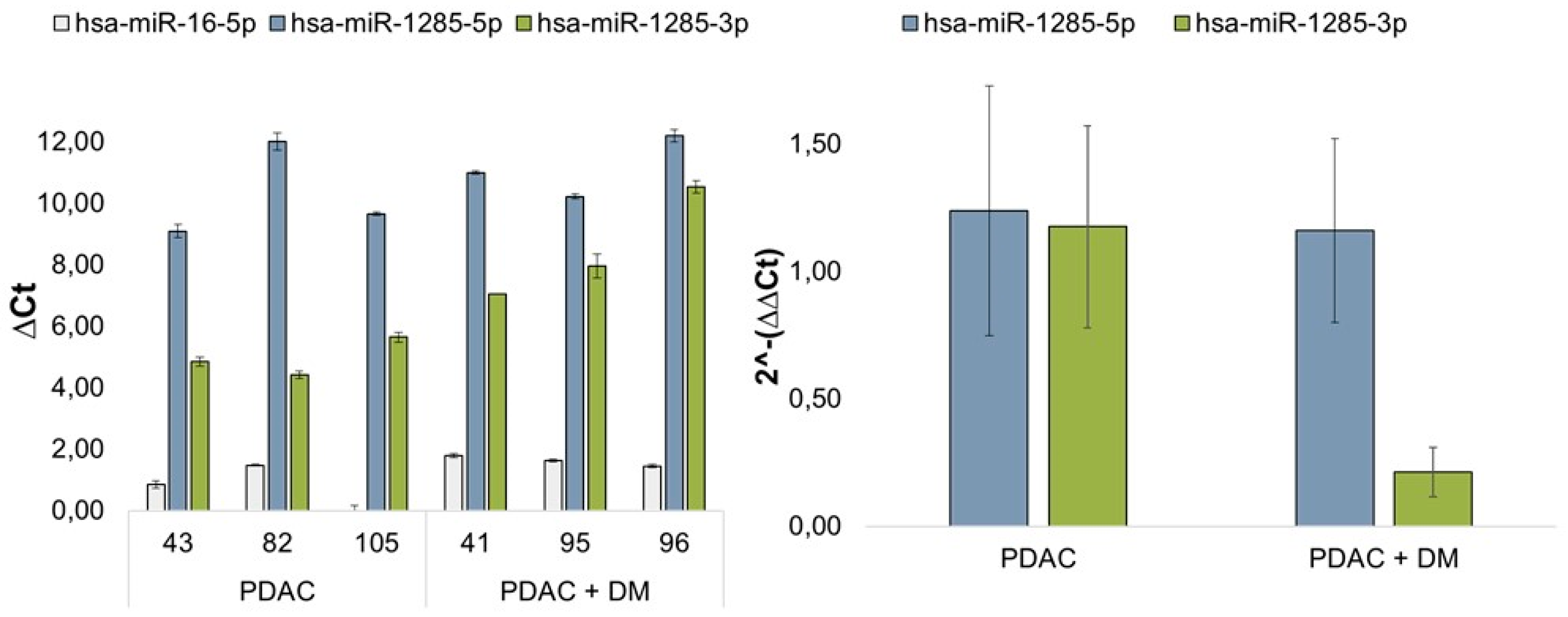
3.4. Results of miRNA Validation via qRT-PCR in Blood Plasma Samples of the Patient Collective
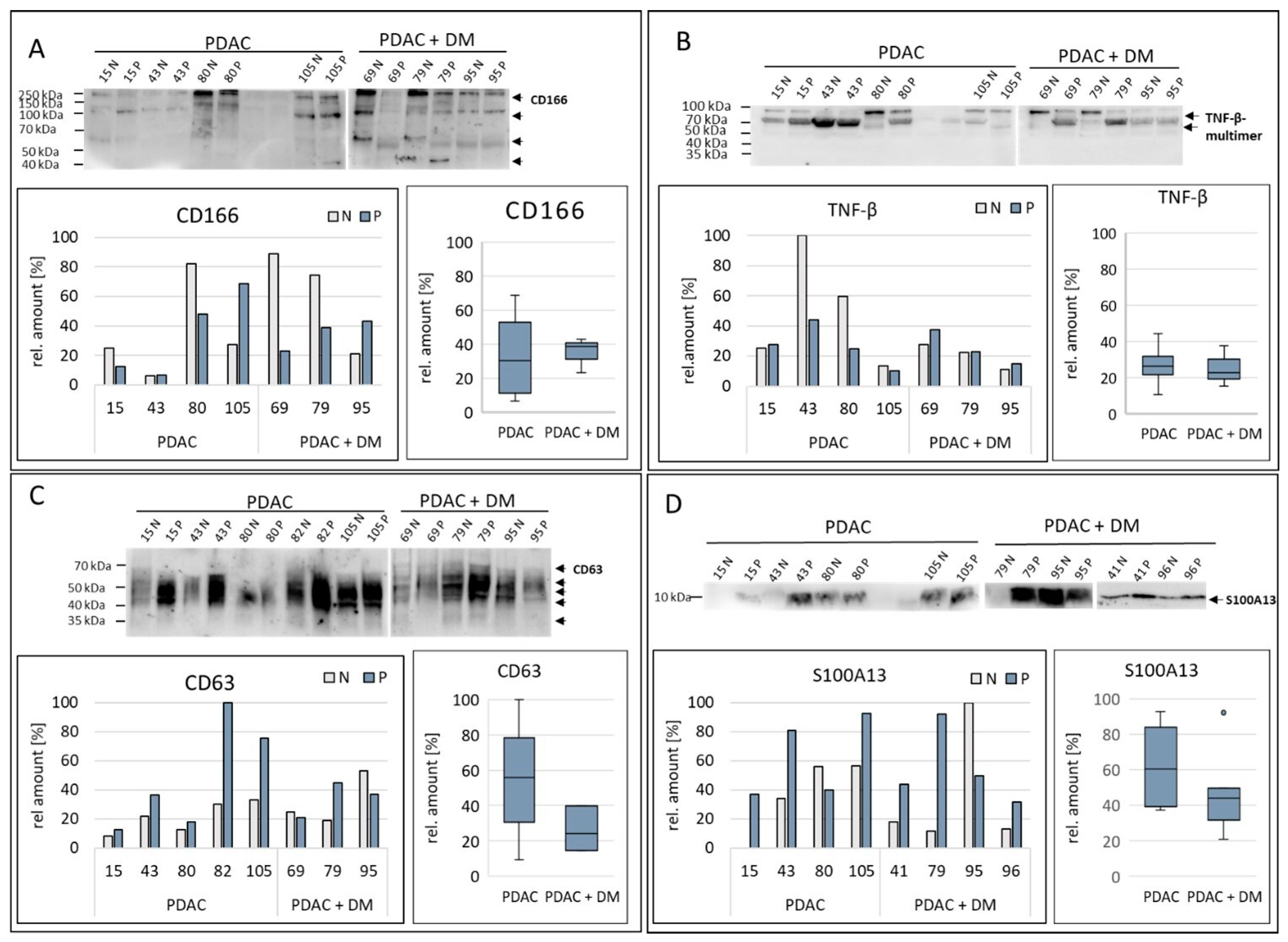
3.5. Results of Protein Validation/Target Protein Analysis in Tissue Samples via Western Blot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quante, A.S.; Ming, C.; Rottmann, M.; Engel, J.; Boeck, S.; Heinemann, V.; Westphalen, C.B.; Strauch, K. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016, 5, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Fink, U.; Russell, R.C.; Spittle, M.F.; Harris, A.L.; Spiessi, G.; Blatter, J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 1996, 73, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; van Santvoort, H.C.; van Tienhoven, G.; de Wilde, R.F.; Wilmink, J.W.; van Eijck, C.H.J.; Groot Koerkamp, B.; et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.H.; Biorserud, C.; Bjornsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Gronbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer (NORPACT-1): A multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2024, 9, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Pecoraro, C.; Panzeca, G.; Xu, G.; Roeten, M.S.F.; Cascioferro, S.; Giovannetti, E.; Diana, P.; Parrino, B. 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Nortopsentin Derivatives against Pancreatic Ductal Adenocarcinoma: Synthesis, Cytotoxic Activity, and Inhibition of CDK1. Mar. Drugs 2023, 21, 412. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; De Franco, M.; Pecoraro, C.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Sut, S.; et al. Structural Manipulations of Marine Natural Products Inspire a New Library of 3-Amino-1,2,4-Triazine PDK Inhibitors Endowed with Antitumor Activity in Pancreatic Ductal Adenocarcinoma. Mar. Drugs 2023, 21, 288. [Google Scholar] [CrossRef]
- Iriana, S.; Ahmed, S.; Gong, J.; Annamalai, A.A.; Tuli, R.; Hendifar, A.E. Targeting mTOR in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2016, 6, 99. [Google Scholar] [CrossRef]
- Jancik, S.; Drabek, J.; Radzioch, D.; Hajduch, M. Clinical relevance of KRAS in human cancers. J. Biomed. Biotechnol. 2010, 2010, 150960. [Google Scholar] [CrossRef]
- Suda, K.; Tomizawa, K.; Mitsudomi, T. Biological and clinical significance of KRAS mutations in lung cancer: An oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010, 29, 49–60. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef]
- Populo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Pantel, K. Liquid Biopsy: Current Status and Future Perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef]
- Mellby, L.D.; Nyberg, A.P.; Johansen, J.S.; Wingren, C.; Nordestgaard, B.G.; Bojesen, S.E.; Mitchell, B.L.; Sheppard, B.C.; Sears, R.C.; Borrebaeck, C.A.K. Serum Biomarker Signature-Based Liquid Biopsy for Diagnosis of Early-Stage Pancreatic Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Kato, K.; Oono, K.; Tsuchiya, N.; Sudo, K.; Shimomura, A.; Tamura, K.; Shiino, S.; Kinoshita, T.; Daiko, H.; et al. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI Cancer Spectr. 2023, 7, pkac080. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Diabetes and pancreatic cancer. Mol. Carcinog. 2012, 51, 64–74. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Honselmann, K.C.; Elser, Y.; Boeckmann, T.; Bolm, L.; Winkel, M.T.; Deichmann, S.; Braun, R.; Wellner, U.F.; Keck, T.; Lapshyn, H. Recent onset diabetes is associated with better survival in pancreatic ductal adenocarcinoma—An analysis of preoperative symptoms within the DGAV StuDoQ|Pancreas Registry. Surgery 2023, 174, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Cheng, T.; Zhang, Z.; Raulefs, S.; Shi, K.; Steiger, K.; Maeritz, N.; Kleigrewe, K.; Hofmann, T.; Benitz, S.; et al. Glycemic Variability Promotes Both Local Invasion and Metastatic Colonization by Pancreatic Ductal Adenocarcinoma. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 429–449. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Appert, H.E.; Myles, J.; Howard, J.M. Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann. Surg. 1992, 215, 350–355. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Zheng, P.; Zhao, W.; Han, N. MicroRNA-1285-5p influences the proliferation and metastasis of non-small-cell lung carcinoma cells via downregulating CDH1 and Smad4. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317705513. [Google Scholar] [CrossRef]
- Liu, J.; Yan, J.; Zhou, C.; Ma, Q.; Jin, Q.; Yang, Z. miR-1285-3p acts as a potential tumor suppressor miRNA via downregulating JUN expression in hepatocellular carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 219–225. [Google Scholar] [CrossRef]
- Tian, S.; Huang, S.; Wu, S.; Guo, W.; Li, J.; He, X. MicroRNA-1285 inhibits the expression of p53 by directly targeting its 3′ untranslated region. Biochem. Biophys. Res. Commun. 2010, 396, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Seki, N.; Yoshino, H.; Yamasaki, T.; Yamada, Y.; Nohata, N.; Fuse, M.; Nakagawa, M.; Enokida, H. Tumor suppressive microRNA-1285 regulates novel molecular targets: Aberrant expression and functional significance in renal cell carcinoma. Oncotarget 2012, 3, 44–57. [Google Scholar] [CrossRef]
- Huang, H.; Xiong, G.; Shen, P.; Cao, Z.; Zheng, L.; Zhang, T.; Zhao, Y. MicroRNA-1285 inhibits malignant biological behaviors of human pancreatic cancer cells by negative regulation of YAP1. Neoplasma 2017, 64, 358–366. [Google Scholar] [CrossRef]
- Hu, X.H.; Dai, J.; Shang, H.L.; Zhao, Z.X.; Hao, Y.D. miR-1285-3p is a potential prognostic marker in human osteosarcoma and functions as a tumor suppressor by targeting YAP1. Cancer Biomark. Sect. A Dis. Markers 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Asnaghi, L.; Bruno, P.; Priulla, M.; Nicolin, A. mTOR: A protein kinase switching between life and death. Pharmacol. Res. 2004, 50, 545–549. [Google Scholar] [CrossRef]
- Basso, D.; Fabris, C.; Del Favero, G.; Piccoli, A.; Angonese, C.; Pasquali, C.; Castoro, C.; Plebani, M.; Leandro, G.; Burlina, A.; et al. How does liver dysfunction influence serum CA 19-9 in pancreatic cancer? Ital. J. Gastroenterol. 1990, 22, 1–6. [Google Scholar]
- Narimatsu, H.; Iwasaki, H.; Nakayama, F.; Ikehara, Y.; Kudo, T.; Nishihara, S.; Sugano, K.; Okura, H.; Fujita, S.; Hirohashi, S. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998, 58, 512–518. [Google Scholar]
- Scara, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [CrossRef]
- Rachdaoui, N. Insulin: The Friend and the Foe in the Development of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 1770. [Google Scholar] [CrossRef]
- Wang, C.C.; Goalstone, M.L.; Draznin, B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 2004, 53, 2735–2740. [Google Scholar] [CrossRef]
- D’Oria, R.; Laviola, L.; Giorgino, F.; Unfer, V.; Bettocchi, S.; Scioscia, M. PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel potential insights in endothelial dysfunction in preeclampsia. Pregnancy Hypertens. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- van Kempen, L.C.; Nelissen, J.M.; Degen, W.G.; Torensma, R.; Weidle, U.H.; Bloemers, H.P.; Figdor, C.G.; Swart, G.W. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J. Biol. Chem. 2001, 276, 25783–25790. [Google Scholar] [CrossRef]
- Sulaj, A.; Kopf, S.; Grone, E.; Grone, H.J.; Hoffmann, S.; Schleicher, E.; Haring, H.U.; Schwenger, V.; Herzig, S.; Fleming, T.; et al. ALCAM a novel biomarker in patients with type 2 diabetes mellitus complicated with diabetic nephropathy. J. Diabetes Its Complicat. 2017, 31, 1058–1065. [Google Scholar] [CrossRef]
- Hebron, K.E.; Li, E.Y.; Arnold Egloff, S.A.; von Lersner, A.K.; Taylor, C.; Houkes, J.; Flaherty, D.K.; Eskaros, A.; Stricker, T.P.; Zijlstra, A. Alternative splicing of ALCAM enables tunable regulation of cell-cell adhesion through differential proteolysis. Sci. Rep. 2018, 8, 3208. [Google Scholar] [CrossRef]
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Piva, F.; Marinelli, O.; Maggi, F.; Bianchi, F.; Bittoni, A.; Berardi, R.; Giampieri, R.; et al. Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival. Front. Oncol. 2019, 9, 874. [Google Scholar] [CrossRef]
- Kahlert, C.; Weber, H.; Mogler, C.; Bergmann, F.; Schirmacher, P.; Kenngott, H.G.; Matterne, U.; Mollberg, N.; Rahbari, N.N.; Hinz, U.; et al. Increased expression of ALCAM/CD166 in pancreatic cancer is an independent prognostic marker for poor survival and early tumour relapse. Br. J. Cancer 2009, 101, 457–464. [Google Scholar] [CrossRef]
- Hong, X.; Michalski, C.W.; Kong, B.; Zhang, W.; Raggi, M.C.; Sauliunaite, D.; De Oliveira, T.; Friess, H.; Kleeff, J. ALCAM is associated with chemoresistance and tumor cell adhesion in pancreatic cancer. J. Surg. Oncol. 2010, 101, 564–569. [Google Scholar] [CrossRef]
- Miao, S.; Qiu, T.; Zhao, Y.; Wang, H.; Sun, X.; Wang, Y.; Xuan, Y.; Qin, Y.; Jiao, W. Overexpression of S100A13 protein is associated with tumor angiogenesis and poor survival in patients with early-stage non-small cell lung cancer. Thorac. Cancer 2018, 9, 1136–1144. [Google Scholar] [CrossRef]
- Massi, D.; Landriscina, M.; Piscazzi, A.; Cosci, E.; Kirov, A.; Paglierani, M.; Di Serio, C.; Mourmouras, V.; Fumagalli, S.; Biagioli, M.; et al. S100A13 is a new angiogenic marker in human melanoma. Mod. Pathol. 2010, 23, 804–813. [Google Scholar] [CrossRef]
- Landriscina, M.; Schinzari, G.; Di Leonardo, G.; Quirino, M.; Cassano, A.; D’Argento, E.; Lauriola, L.; Scerrati, M.; Prudovsky, I.; Barone, C. S100A13, a new marker of angiogenesis in human astrocytic gliomas. J. Neuro-Oncol. 2006, 80, 251–259. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Li, H.B.; Wang, J.L.; Jin, X.D.; Zhao, L.; Ye, H.L.; Kuang, Y.B.; Ma, Y.; Jiang, X.Y.; Yu, Z.Y. Comprehensive analysis of the transcriptional expressions and prognostic value of S100A family in pancreatic ductal adenocarcinoma. BMC Cancer 2021, 21, 1039. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Shi, Y.; Chen, J.; Lin, S.; Zhu, J.; Xu, X.; Lu, L.; Zou, H. Genome-wide analysis of DNA methylation identifies S100A13 as an epigenetic biomarker in individuals with chronic (>/=30 years) type 2 diabetes without diabetic retinopathy. Clin. Epigenet. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and beta1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef] [PubMed]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Sandstrom, A.; Andersson, R.; Segersvard, R.; Lohr, M.; Borrebaeck, C.A.; Wingren, C. Serum proteome profiling of pancreatitis using recombinant antibody microarrays reveals disease-associated biomarker signatures. Proteom. Clin. Appl. 2012, 6, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-cell communication: microRNAs as hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Montani, F.; Bianchi, F. Circulating Cancer Biomarkers: The Macro-revolution of the Micro-RNA. eBioMedicine 2016, 5, 4–6. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; He, W.; Zhao, P.; Ye, C. MicroRNA-433 targets AKT3 and inhibits cell proliferation and viability in breast cancer. Oncol. Lett. 2018, 15, 3998–4004. [Google Scholar] [CrossRef]
- Ding, X.M. MicroRNAs: Regulators of cancer metastasis and epithelial-mesenchymal transition (EMT). Chin. J. Cancer 2014, 33, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Shen, Y.; Chen, Z.; Li, R.; Ge, Q. No Significant Difference between Plasma miRNAs and Plasma-Derived Exosomal miRNAs from Healthy People. BioMed Res. Int. 2017, 2017, 1304816. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Ploug, T. Assessment of six commercial plasma small RNA isolation kits using qRT-PCR and electrophoretic separation: Higher recovery of microRNA following ultracentrifugation. Biol. Methods Protoc. 2016, 1, bpw003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Salcedo Allende, M.T.; Zeron-Medina, J.; Hernandez, J.; Macarulla, T.; Balsells, J.; Merino, X.; Allende, H.; Tabernero, J.; Ramon, Y.C.S. Overexpression of Yes Associated Protein 1, an Independent Prognostic Marker in Patients with Pancreatic Ductal Adenocarcinoma, Correlated with Liver Metastasis and Poor Prognosis. Pancreas 2017, 46, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Parsels, J.D.; Lohse, I.; Lawrence, T.S.; Pasca di Magliano, M.; Sun, Y.; Morgan, M.A. Fbxw7 Deletion Accelerates Kras(G12D)-Driven Pancreatic Tumorigenesis via Yap Accumulation. Neoplasia 2016, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Nemazanyy, I.; White, S.M.; Chen, H.; Nguyen, C.D.K.; Graham, G.T.; Saur, D.; Pende, M.; Yi, C. A Yap-Myc-Sox2-p53 Regulatory Network Dictates Metabolic Homeostasis and Differentiation in Kras-Driven Pancreatic Ductal Adenocarcinomas. Dev. Cell 2019, 51, 113–128.e119. [Google Scholar] [CrossRef]
- Murakami, S.; Shahbazian, D.; Surana, R.; Zhang, W.; Chen, H.; Graham, G.T.; White, S.M.; Weiner, L.M.; Yi, C. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 2017, 36, 1232–1244. [Google Scholar] [CrossRef]
- Ortillon, J.; Le Bail, J.C.; Villard, E.; Leger, B.; Poirier, B.; Girardot, C.; Beeske, S.; Ledein, L.; Blanchard, V.; Brieu, P.; et al. High Glucose Activates YAP Signaling to Promote Vascular Inflammation. Front. Physiol. 2021, 12, 665994. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, F.J., 3rd; Cohen, D.R.; Curran, T.; Bos, T.J.; Vogt, P.K.; Bohmann, D.; Tjian, R.; Franza, B.R., Jr. Fos-associated protein p39 is the product of the jun proto-oncogene. Science 1988, 240, 1010–1016. [Google Scholar] [CrossRef]
- Lamph, W.W.; Wamsley, P.; Sassone-Corsi, P.; Verma, I.M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature 1988, 334, 629–631. [Google Scholar] [CrossRef]
- Serna, R.; Ramrakhiani, A.; Hernandez, J.C.; Chen, C.L.; Nakagawa, C.; Machida, T.; Ray, R.B.; Zhan, X.; Tahara, S.M.; Machida, K. c-JUN inhibits mTORC2 and glucose uptake to promote self-renewal and obesity. iScience 2022, 25, 104325. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.Q.; Wang, Z.F.; Wu, X.L.; Zhou, C.H.; Yan, J.Y.; Hu, B.Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef]
- Maddalena, M.; Mallel, G.; Nataraj, N.B.; Shreberk-Shaked, M.; Hassin, O.; Mukherjee, S.; Arandkar, S.; Rotkopf, R.; Kapsack, A.; Lambiase, G.; et al. TP53 missense mutations in PDAC are associated with enhanced fibrosis and an immunosuppressive microenvironment. Proc. Natl. Acad. Sci. USA 2021, 118, e2025631118. [Google Scholar] [CrossRef]
- Kung, C.P.; Murphy, M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J. Endocrinol. 2016, 231, R61–R75. [Google Scholar] [CrossRef] [PubMed]
- Pertz, O.; Bozic, D.; Koch, A.W.; Fauser, C.; Brancaccio, A.; Engel, J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999, 18, 1738–1747. [Google Scholar] [CrossRef]
- Frixen, U.H.; Behrens, J.; Sachs, M.; Eberle, G.; Voss, B.; Warda, A.; Lochner, D.; Birchmeier, W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 1991, 113, 173–185. [Google Scholar] [CrossRef]
- Siret, C.; Dobric, A.; Martirosyan, A.; Terciolo, C.; Germain, S.; Bonier, R.; Dirami, T.; Dusetti, N.; Tomasini, R.; Rubis, M.; et al. Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma. Br. J. Cancer 2018, 118, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Maftouh, M.; Avan, A.; Sciarrillo, R.; Granchi, C.; Leon, L.G.; Rani, R.; Funel, N.; Smid, K.; Honeywell, R.; Boggi, U.; et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br. J. Cancer 2014, 110, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Facchiano, F.; Facchiano, A.; Facchiano, A.M. The role of transglutaminase-2 and its substrates in human diseases. Front. Biosci. A J. Virtual Libr. 2006, 11, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Darfler, M.M.; Alvarez, H.; Hood, B.L.; Conrads, T.P.; Habbe, N.; Krizman, D.B.; Mollenhauer, J.; Feldmann, G.; Maitra, A. Application of a global proteomic approach to archival precursor lesions: Deleted in malignant brain tumors 1 and tissue transglutaminase 2 are upregulated in pancreatic cancer precursors. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2008, 8, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Iacobuzio-Donahue, C.A.; Ashfaq, R.; Maitra, A.; Adsay, N.V.; Shen-Ong, G.L.; Berg, K.; Hollingsworth, M.A.; Cameron, J.L.; Yeo, C.J.; Kern, S.E.; et al. Highly expressed genes in pancreatic ductal adenocarcinomas: A comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003, 63, 8614–8622. [Google Scholar]
- Verma, A.; Wang, H.; Manavathi, B.; Fok, J.Y.; Mann, A.P.; Kumar, R.; Mehta, K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006, 66, 10525–10533. [Google Scholar] [CrossRef]


| Sample | Age | Gender | BMI | Histology | DM | HbA1C | CA 19-9 |
|---|---|---|---|---|---|---|---|
| 43 | 78 | m | 32.7 | PDAC (no resection) | y, insulin-dependent | 11.3 | 10,974 |
| 68 | 77 | m | 29.7 | PDAC (no resection) | y (not known pre-OP) | 6.9 | 719 |
| 35 | 69 | f | 25.2 | PDAC (no resection) | y, insulin-dependent | 9.8 | 1248 |
| 87 | 80 | f | 26.6 | PDAC (no resection) | y, insulin-dependent | 8.1 | 1803 |
| 66 | 79 | m | 24.3 | PDAC (no resection) | n | 5.6 | 726.8 |
| 40 | 59 | m | 27.7 | PDAC (no resection) | y, insulin-independent | 7.3 | 1294 |
| 42 | 73 | m | 22.4 | PDAC (no resection) | n | 5.7 | 599.6 |
| 36 | 61 | f | 25.1 | PDAC (no resection) | n | 5.8 | 601.8 |
| 8 | 76 | m | 22.6 | PDAC (resected) | n | 4.7 | 256.6 |
| 78 | 81 | m | 36.4 | PDAC (resected) | n | 5.1 | 84.3 |
| 82 | 82 | m | 22 | PDAC (resected) | n | 5.1 | 118.9 |
| 71 | 73 | m | 28.7 | PDAC (resected) | y, insulin-dependent | 6.2 | 189.4 |
| 79 | 70 | m | 32.3 | PDAC (resected) | y, insulin-dependent | 9.7 | 3340 |
| 69 | 64 | m | 31.2 | PDAC (resected) | y, insulin-dependent | 7.6 | 1147 |
| 44 | 62 | m | 27.8 | PDAC (resected) | n | 5.4 | 17.3 |
| 80 | 82 | f | 27.3 | PDAC (resected) | n | 5.9 | 247.3 |
| 95 | 69 | m | 20 | PDAC (resected) | y, insulin-dependent | 6.2 | 0.6 |
| 105 | 73 | f | 19.3 | PDAC (resected) | n | 6.1 | 368.3 |
| 99 | 80 | f | 20.3 | PDAC (resected) | n | 6 | 1289 |
| 51 | 63 | f | 20.2 | PDAC (resected) | n | 4.9 | 335.5 |
| 62 | 75 | f | 23.4 | PDAC (resected) | n | 5.2 | 24.9 |
| 80 | 82 | f | 29.4 | PDAC (resected) | n | 4.1 | 3917 |
| 15 | 78 | f | 25.6 | PDAC (resected) | n | 6.2 | 208.6 |
| 41 | 75 | f | 27.6 | PDAC (resected) | y, insulin-independent | 7.6 | 127.4 |
| 86 | 58 | f | 27.9 | PDAC (resected) | n | 6 | 218.4 |
| 96 | 55 | f | 36.1 | PDAC (resected) | y, insulin-independent | 5.3 | 1 |
| 43 | 78 | f | 23 | PDAC (resected) | n | 5.9 | 211.3 |
| 29 | 61 | m | 24.5 | chronic pancreatitis | n | 5.6 | 9.5 |
| 1 | 57 | m | 32 | chronic pancreatitis | y, insulin-dependent | 7.6 | 11 |
| 100 | 35 | m | 29.6 | chronic pancreatitis | y, insulin-dependent | 9.9 | 9.8 |
| 89 | 61 | m | 26.8 | chronic pancreatitis | y, insulin-independent | 7.6 | 5.2 |
| 88 | 44 | f | 22.1 | chronic pancreatitis | y, insulin-independent | 6.6 | 45.5 |
| 56 | 45 | f | 13.9 | chronic pancreatitis | n | 4.7 | 17 |
| 76 | 69 | f | 27.5 | chronic pancreatitis | y, insulin-independent | 5.7 | - |
| 52 | 33 | f | 22 | chronic pancreatitis | n | 5.6 | <0.6 |
| 67 | 78 | f | 27.1 | chronic pancreatitis | n | 6.4 | 9.3 |
| 9 | 71 | f | 25.3 | chronic pancreatitis | y, insulin-dependent | 7.6 | 20.6 |
| 85 | 65 | m | 27.7 | distal CCC | y, insulin-independent | 6.9 | 32.1 |
| 58 | 74 | m | 32.7 | distal CCC | n | 5.1 | 64.3 |
| 24 | 81 | m | 23.9 | distal CCC | n | 5.4 | 85.7 |
| 73 | 78 | m | 27.7 | distal CCC | n | 5.3 | 8 |
| 53 | 68 | m | 23.2 | distal CCC | n | 5.9 | 76 |
| 93 | 77 | m | 24 | distal CCC | n | 5.6 | 159 |
| 75 | 74 | f | 27.7 | NET Pancreas | n | 5.4 | 5.8 |
| Pathway ID | Pathway Description | Protein Count | |
|---|---|---|---|
| 1 | hsa05200 | Pathways in cancer | 29 |
| 2 | hsa04060 | Cytokine-cytokine receptor interaction | 22 |
| 3 | hsa04151 | PI3K-Akt signaling pathway | 16 |
| 4 | hsa05418 | Fluid shear stress and atherosclerosis | 14 |
| 5 | hsa04514 | Cell adhesion molecules (CAMs) | 14 |
| 6 | hsa05205 | Proteoglycans in cancer | 13 |
| 7 | hsa04010 | MAPK signaling pathway | 13 |
| 8 | hsa05225 | Hepatocellular carcinoma | 12 |
| 9 | hsa04015 | Rap1 signaling pathway | 12 |
| 10 | hsa04380 | Osteoclast differentiation | 11 |
| 11 | hsa04630 | Jak-STAT signaling pathway | 11 |
| 12 | hsa05152 | Tuberculosis | 11 |
| 13 | hsa04014 | Ras signaling pathway | 11 |
| 14 | hsa04640 | Hematopoietic cell lineage | 10 |
| 15 | hsa05161 | Hepatitis B | 10 |
| 16 | hsa05226 | Gastric cancer | 10 |
| 17 | hsa05223 | non-small cell lung cancer | 9 |
| 18 | hsa05218 | Melanoma | 9 |
| 19 | hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 9 |
| 20 | hsa05162 | Measles | 9 |
| 21 | hsa05166 | HTLV-I infection | 9 |
| 22 | hsa05165 | Human papillomavirus infection | 9 |
| 23 | hsa05214 | Glioma | 8 |
| 24 | hsa05212 | Pancreatic cancer | 8 |
| Protein | Regulation PDAC + DM | Potential Validation Method |
|---|---|---|
| BDNF | ↑ | WB |
| CD166 | ↑ | WB |
| CD63 | ↑ | WB |
| CXCL13 | ↑ | WB |
| CXCL16 | ↑ | WB |
| S100A13 | ↓ | WB |
| TNF-β | ↓ | WB |
| miRNA | logFC | AveExpr | t | p. Value | adj. p. Value | B |
|---|---|---|---|---|---|---|
| HSA-MIR-502-3P | 1.793496999 | 20.34749718 | 3.313711041 | 0.003136645 | 0.366987411 | −1.957157871 |
| HSA-MIR-493* | 1.007480749 | 17.97002518 | 2.405485145 | 0.024933313 | 0.906929645 | −3.328008992 |
| HSA-MIR-1285 | 1.250109763 | 19.05366401 | 2.132340797 | 0.044305461 | 0.906929645 | −3.704638277 |
| HSA-MIR-185 | −0.935114451 | 20.09011602 | −2.089490862 | 0.048357219 | 0.906929645 | −3.761480577 |
| miRNA Symbol | logFC | AveExpr | t | p. Value | adj. p. Value | B |
|---|---|---|---|---|---|---|
| HSA-MIR-1273 | −2.43083379 | 19.1112214 | −2.9404612 | 0.0132589 | 0.64242329 | −4.55313887 |
| HSA-MIR-1285 | −2.57078247 | 18.9186008 | −2.87550303 | 0.01490307 | 0.64242329 | −4.55444384 |
| HSA-MIR-371-5P | −1.71306615 | 18.0949013 | −2.79817459 | 0.01712805 | 0.64242329 | −4.55602414 |
| HSA-MIR-888 | −1.94922757 | 18.4567094 | −2.78193133 | 0.01763595 | 0.64242329 | −4.55635971 |
| HSA-MIR-1274B | −1.71617505 | 23.1619678 | −2.71333725 | 0.01995103 | 0.64242329 | −4.5577903 |
| HSA-MIR-122 | −2.18577019 | 21.34131 | −2.55501492 | 0.02650499 | 0.68321569 | −4.56117141 |
| HSA-MIR-195 | 2.31466878 | 21.5462484 | 2.18958784 | 0.05068444 | 0.68321569 | −4.56932364 |
| HSA-MIR-192 | −1.7910867 | 18.5855867 | −2.16196007 | 0.05319605 | 0.68321569 | −4.5699547 |
| HSA-MIR-424 | 1.94472688 | 20.6816518 | 2.14824269 | 0.05448657 | 0.68321569 | −4.57026855 |
| RNU2-1 | −1.55939277 | 20.9494415 | −2.12343529 | 0.05689613 | 0.68321569 | −4.57083695 |
| miR-Variant | Effect | Tumor Entity | Protein Target | Reference |
|---|---|---|---|---|
| miR1285-3p | Tumor suppressor | HCC | Jun ↓ | (Liu et al., 2015 [27]) |
| Tumor promoter | Neuroblastoma Hepatoblastoma Mamma carcinoma | p53 ↓ | (Tian et al., 2010 [28]) | |
| Tumor suppressor | RCC | TGM2 ↓ | (Hidaka et al., 2012 [29]) | |
| Tumor suppressor | PDAC OS | YAP ↓ | (Huang et al., 2017 [30]) (Hu et al., 2019 [31]) | |
| miR-1285-5p | Tumor promoter | NSCLC | CDH-1 ↓ Smad4 ↓ | (Zhou et al., 2017 [26]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolokotronis, T.; Majchrzak-Stiller, B.; Buchholz, M.; Mense, V.; Strotmann, J.; Peters, I.; Skrzypczyk, L.; Liffers, S.-T.; Menkene, L.M.; Wagner, M.; et al. Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma. Cancers 2024, 16, 2726. https://doi.org/10.3390/cancers16152726
Kolokotronis T, Majchrzak-Stiller B, Buchholz M, Mense V, Strotmann J, Peters I, Skrzypczyk L, Liffers S-T, Menkene LM, Wagner M, et al. Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma. Cancers. 2024; 16(15):2726. https://doi.org/10.3390/cancers16152726
Chicago/Turabian StyleKolokotronis, Theodoros, Britta Majchrzak-Stiller, Marie Buchholz, Vanessa Mense, Johanna Strotmann, Ilka Peters, Lea Skrzypczyk, Sven-Thorsten Liffers, Louise Massia Menkene, Mathias Wagner, and et al. 2024. "Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma" Cancers 16, no. 15: 2726. https://doi.org/10.3390/cancers16152726
APA StyleKolokotronis, T., Majchrzak-Stiller, B., Buchholz, M., Mense, V., Strotmann, J., Peters, I., Skrzypczyk, L., Liffers, S.-T., Menkene, L. M., Wagner, M., Glanemann, M., Betsou, F., Ammerlaan, W., Schmidt, R., Schröder, C., Uhl, W., Braumann, C., & Höhn, P. (2024). Differential miRNA and Protein Expression Reveals miR-1285, Its Targets TGM2 and CDH-1, as Well as CD166 and S100A13 as Potential New Biomarkers in Patients with Diabetes Mellitus and Pancreatic Adenocarcinoma. Cancers, 16(15), 2726. https://doi.org/10.3390/cancers16152726







