Definition of a Multi-Omics Signature for Esophageal Adenocarcinoma Prognosis Prediction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
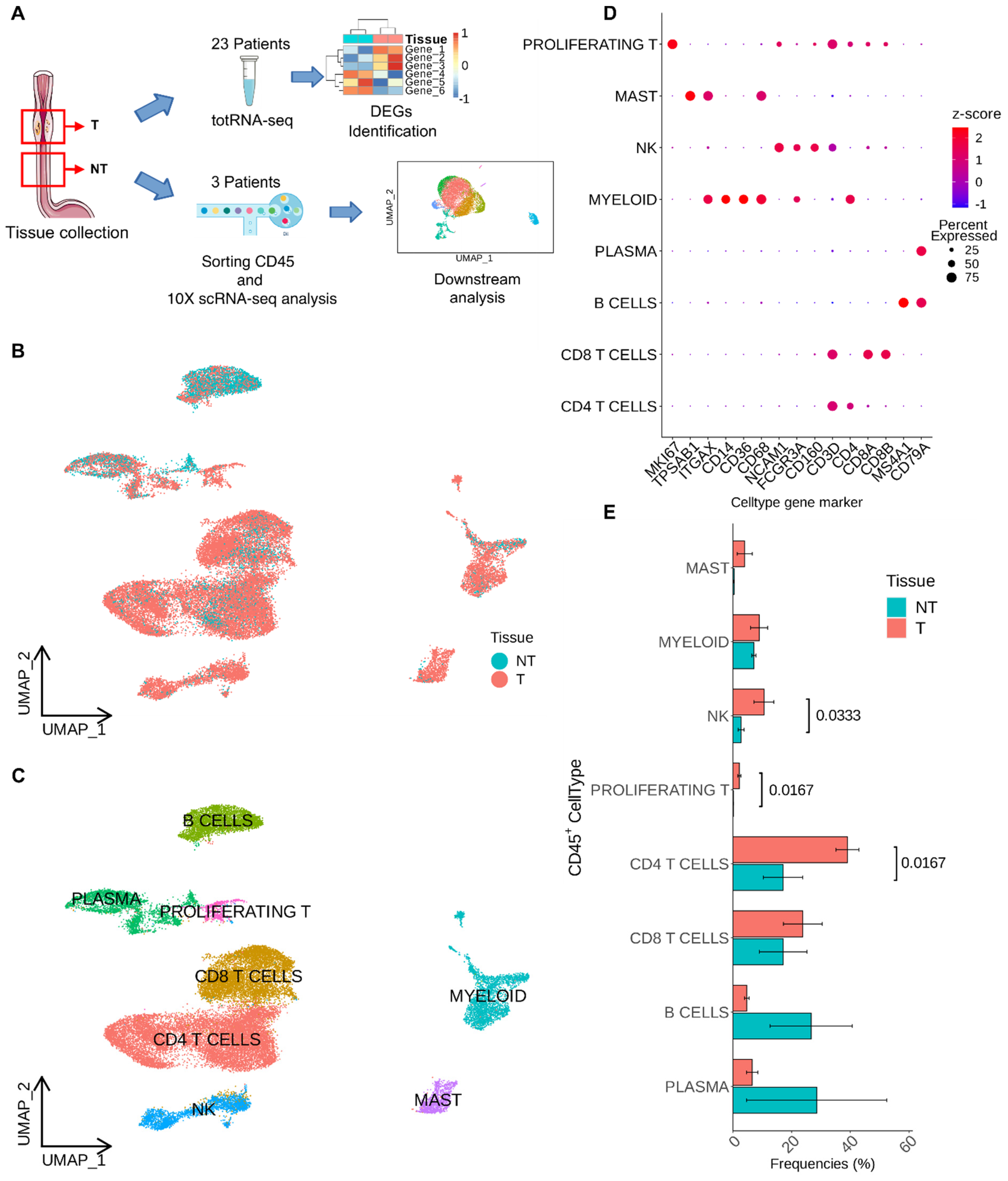
2.1. Patients’ Recruitment, Tissue Collection, and Experimental Workflow
2.2. Single-Cell Sequencing: Cells’ Preparation, Library Preparation, and Sequencing
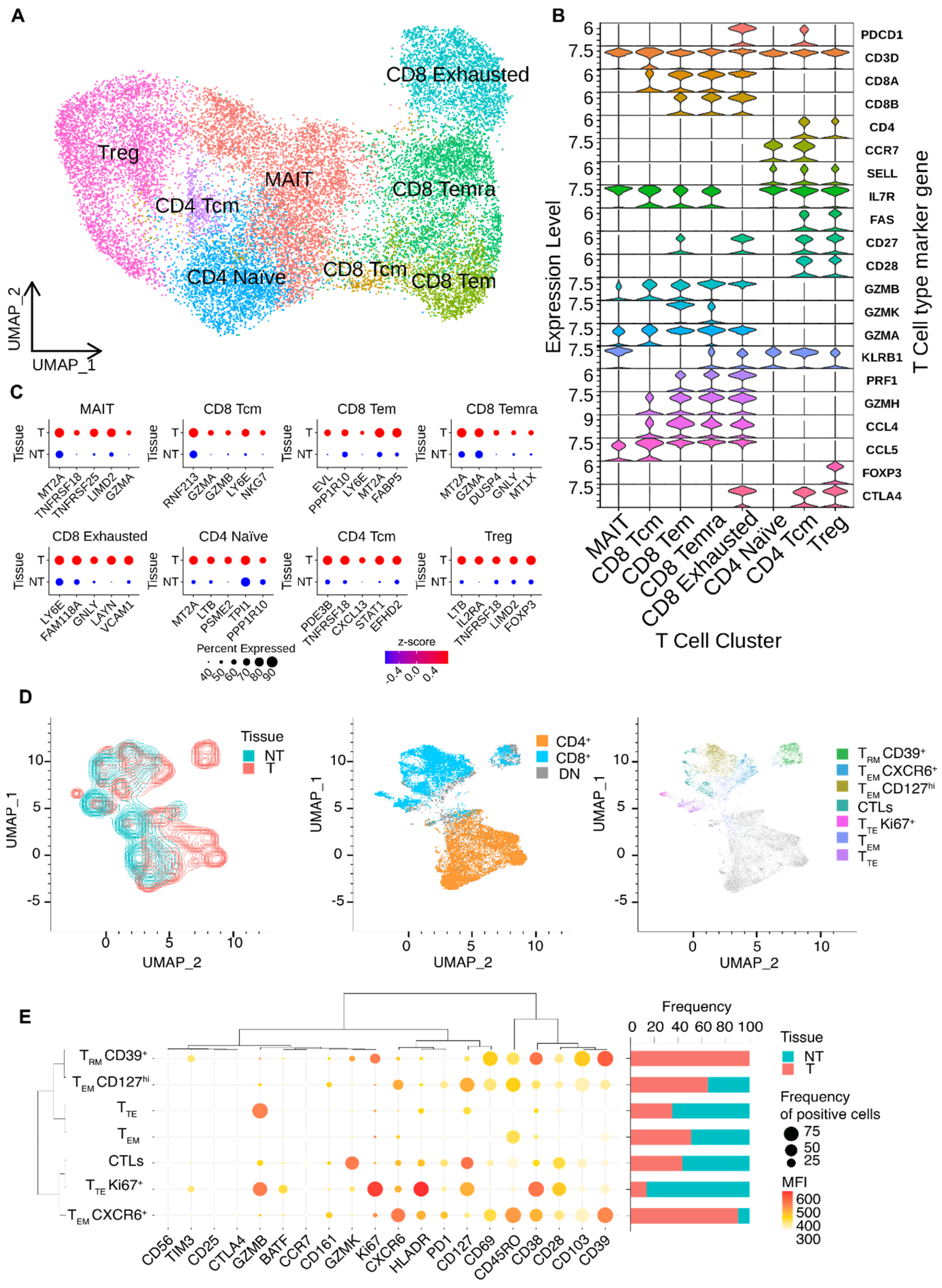
2.3. Analysis of Single-Cell RNA Sequencing Data
2.4. Identification of TF Regulons
2.5. Polychromatic Flow Cytometry
2.6. Computational Analysis of Flow Cytometry Data
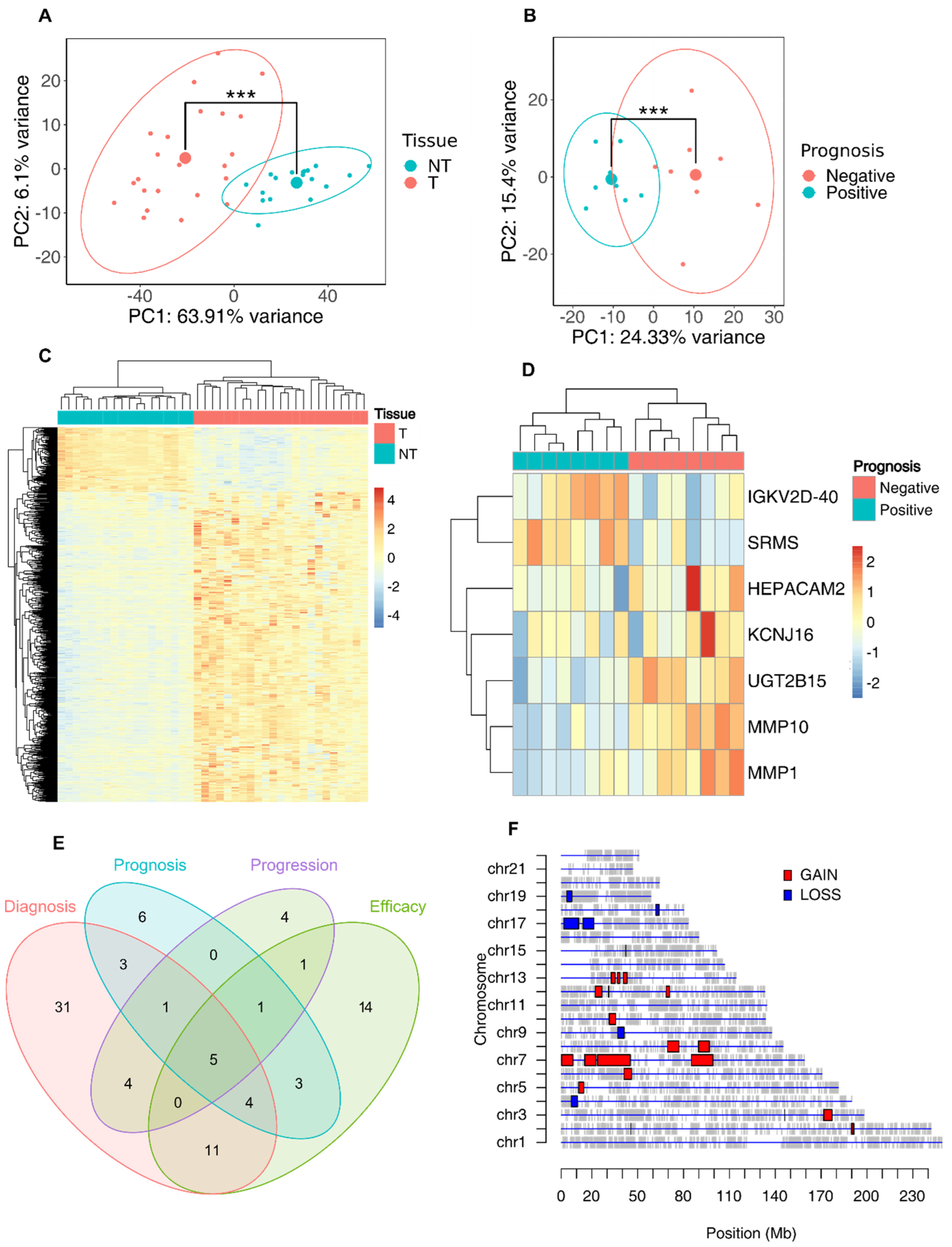
2.7. Analysis of Bulk RNA Sequencing Data
2.8. SODEGIR Analysis
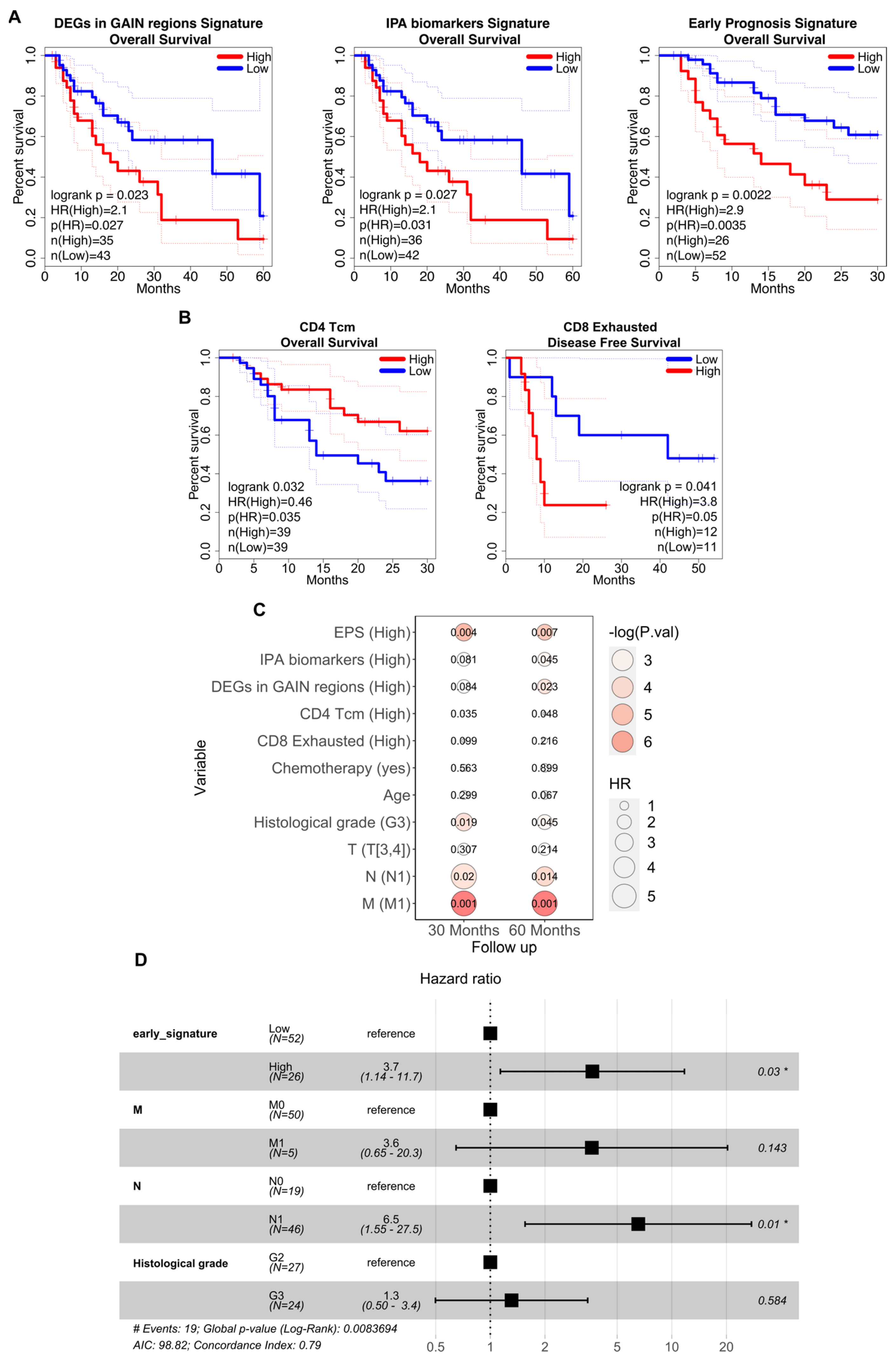
2.9. Survival Analysis
3. Results
3.1. Single-Cell Level Analysis of Esophageal Adenocarcinoma Immune Infiltrate
3.2. Dissection of T Cells’ Heterogeneity in Esophageal Adenocarcinoma
3.3. Whole-Transcriptome Profiling of Esophageal Adenocarcinoma Tissues for the Identification of a Prognostic Signature
3.4. Association between the Prognostic Signatures and Patients’ Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, H.G.; Xie, S.H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Fassan, M.; Cavallin, F.; Guzzardo, V.; Kotsafti, A.; Scarpa, M.; Cagol, M.; Chiarion-Sileni, V.; Maria Saadeh, L.; Alfieri, R.; Castagliuolo, I.; et al. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer Med. 2019, 8, 6036–6048. [Google Scholar] [CrossRef]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.-T.; Huang, X.; Huang, S.; Xie, S.-J.; Xiao, Z.-D.; Zhang, H. RNA sequencing: New technologies and applications in cancer research. J. Hematol. Oncol. 2020, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef]
- Peng, J.; Sun, B.-F.; Chen, C.-Y.; Zhou, J.-Y.; Chen, Y.-S.; Chen, H.; Liu, L.; Huang, D.; Jiang, J.; Cui, G.-S.; et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019, 29, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.H.; Simonds, E.F.; Bendall, S.C.; Davis, K.L.; Amir, E.-A.D.; Tadmor, M.D.; Litvin, O.; Fienberg, H.G.; Jager, A.; Zunder, E.R.; et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell 2015, 162, 184–197. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Campbell, K.R.; Lun, A.T.L.; Wills, Q.F. Scater: Pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 2017, 33, 1179–1186. [Google Scholar] [CrossRef]
- Lun, A.T.L.; Riesenfeld, S.; Andrews, T.; Dao, T.P.; Gomes, T.; Marioni, J.C. EmptyDrops: Distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Croft, W.; Evans, R.P.T.; Pearce, H.; Elshafie, M.; Griffiths, E.A.; Moss, P. The single cell transcriptional landscape of esophageal adenocarcinoma and its modulation by neoadjuvant chemotherapy. Mol. Cancer 2022, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Lyu, Y.; Pan, H.; Zhang, J.; Stambolian, D.; Susztak, K.; Reilly, M.P.; Hu, G.; Li, M. Deep learning enables accurate clustering with batch effect removal in single-cell RNA-seq analysis. Nat. Commun. 2020, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Z.; Han, Y.; Han, L.; Zou, X.; Zhou, B.; Hu, R.; Hao, J.; Bai, S.; Xiao, H.; et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat. Commun. 2020, 11, 6268. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, B.; Flerin, C.; Davie, K.; De Waegeneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saelens, W.; Cannoodt, R.; Rouchon, Q.; et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020, 15, 2247–2276. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. Erratum: The Human Transcription Factors. Cell 2018, 175, 598–599, Correction on: Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Moerman, T.; Santos, S.A.; González-Blas, C.B.; Simm, J.; Moreau, Y.; Aerts, J.; Aerts, S. GRNBoost2 and Arboreto: Efficient and scalable inference of gene regulatory networks. Bioinformatics 2019, 35, 2159–2161. [Google Scholar] [CrossRef]
- Lugli, E.; Zanon, V.; Mavilio, D.; Roberto, A. FACS analysis of memory T lymphocytes. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1514, pp. 31–47. [Google Scholar] [CrossRef]
- Brummelman, J.; Haftmann, C.; Núñez, N.G.; Alvisi, G.; Mazza, E.M.C.; Becher, B.; Lugli, E. Development, application and computational analysis of high-dimensional fluorescent antibody panels for single-cell flow cytometry. Nat. Protoc. 2019, 14, 1946–1969. [Google Scholar] [CrossRef]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic gene expression in a single cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.; Colombo, G.I.; Piacentini, L. DaMiRseq—An R/Bioconductor package for data mining of RNA-Seq data: Normalization, feature selection and classification. Bioinformatics 2018, 34, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Solari, A.; Battaglia, C.; Bicciato, S. PREDA: An R-package to identify regional variations in genomic data. Bioinformatics 2011, 27, 2446–2447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Therneau, T.M. A Package for Survival Analysis in R. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 30 June 2024).
- Salem, M.E.; Puccini, A.; Xiu, J.; Raghavan, D.; Lenz, H.-J.; Korn, W.M.; Shields, A.F.; Philip, P.A.; Marshall, J.L.; Goldberg, R.M. Comparative Molecular Analyses of Esophageal Squamous Cell Carcinoma, Esophageal Adenocarcinoma, and Gastric Adenocarcinoma. Oncologist 2018, 23, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Killcoyne, S.; Gregson, E.; Wedge, D.C.; Woodcock, D.J.; Eldridge, M.D.; de la Rue, R.; Miremadi, A.; Abbas, S.; Blasko, A.; Kosmidou, C.; et al. Genomic copy number predicts esophageal cancer years before transformation. Nat. Med. 2020, 26, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, K.; Lehman, H.L.; Stairs, D.B.; Sinha, R.; Arga, K.Y. Proteomic and Metabolic Signatures of Esophageal Squamous Cell Carcinoma. Curr. Cancer Drug Targets 2016, 16, 721–736. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Wang, N.; Yang, M.; Liao, A.; Wang, S.; Hu, G.; Zeng, B.; Yao, Y.; Liu, D.; et al. Bioinformatic analysis suggests that UGT2B15 activates the Hippo YAP signaling pathway leading to the pathogenesis of gastric cancer. Oncol. Rep. 2018, 40, 1855–1862. [Google Scholar] [CrossRef]
- Li, D.; Yin, Y.; He, M.; Wang, J. Identification of Potential Biomarkers Associated with Prognosis in Gastric Cancer via Bioinformatics Analysis. Med. Sci. Monit. 2021, 27, e929104-1–e929104-11. [Google Scholar] [CrossRef] [PubMed]
- Quilty, F.; Byrne, A.-M.; Aird, J.; El Mashad, S.; Parra-Blanco, A.; Long, A.; Gilmer, J.F.; Medina, C. Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases. Int. J. Mol. Sci. 2020, 21, 8042. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-H.; Zhang, X.; Cao, P.-G. MMP-1/PAR-1 signal transduction axis and its prognostic impact in esophageal squamous cell carcinoma. Braz. J. Med. Biol. Res. 2012, 45, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hao, Y.; Lowe, A.W. The Adenocarcinoma-Associated Antigen, AGR2, Promotes Tumor Growth, Cell Migration, and Cellular Transformation. Cancer Res. 2008, 68, 492–497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambroia, L.; Conca Dioguardi, C.M.; Puccio, S.; Pansa, A.; Alvisi, G.; Basso, G.; Cibella, J.; Colombo, F.S.; Marano, S.; Basato, S.; et al. Definition of a Multi-Omics Signature for Esophageal Adenocarcinoma Prognosis Prediction. Cancers 2024, 16, 2748. https://doi.org/10.3390/cancers16152748
Lambroia L, Conca Dioguardi CM, Puccio S, Pansa A, Alvisi G, Basso G, Cibella J, Colombo FS, Marano S, Basato S, et al. Definition of a Multi-Omics Signature for Esophageal Adenocarcinoma Prognosis Prediction. Cancers. 2024; 16(15):2748. https://doi.org/10.3390/cancers16152748
Chicago/Turabian StyleLambroia, Luca, Carola Maria Conca Dioguardi, Simone Puccio, Andrea Pansa, Giorgia Alvisi, Gianluca Basso, Javier Cibella, Federico Simone Colombo, Salvatore Marano, Silvia Basato, and et al. 2024. "Definition of a Multi-Omics Signature for Esophageal Adenocarcinoma Prognosis Prediction" Cancers 16, no. 15: 2748. https://doi.org/10.3390/cancers16152748
APA StyleLambroia, L., Conca Dioguardi, C. M., Puccio, S., Pansa, A., Alvisi, G., Basso, G., Cibella, J., Colombo, F. S., Marano, S., Basato, S., Alfieri, R., Giudici, S., Castoro, C., & Peano, C. (2024). Definition of a Multi-Omics Signature for Esophageal Adenocarcinoma Prognosis Prediction. Cancers, 16(15), 2748. https://doi.org/10.3390/cancers16152748






