Ultrasound Combination to Improve the Efficacy of Current Boron Neutron Capture Therapy for Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. BNCT for the Treatment of HNC
3. Current BPA-Based BNCT Issues
4. Ultrasound as a Strategy to Enhance the Effectiveness of BPA-Based BNCT
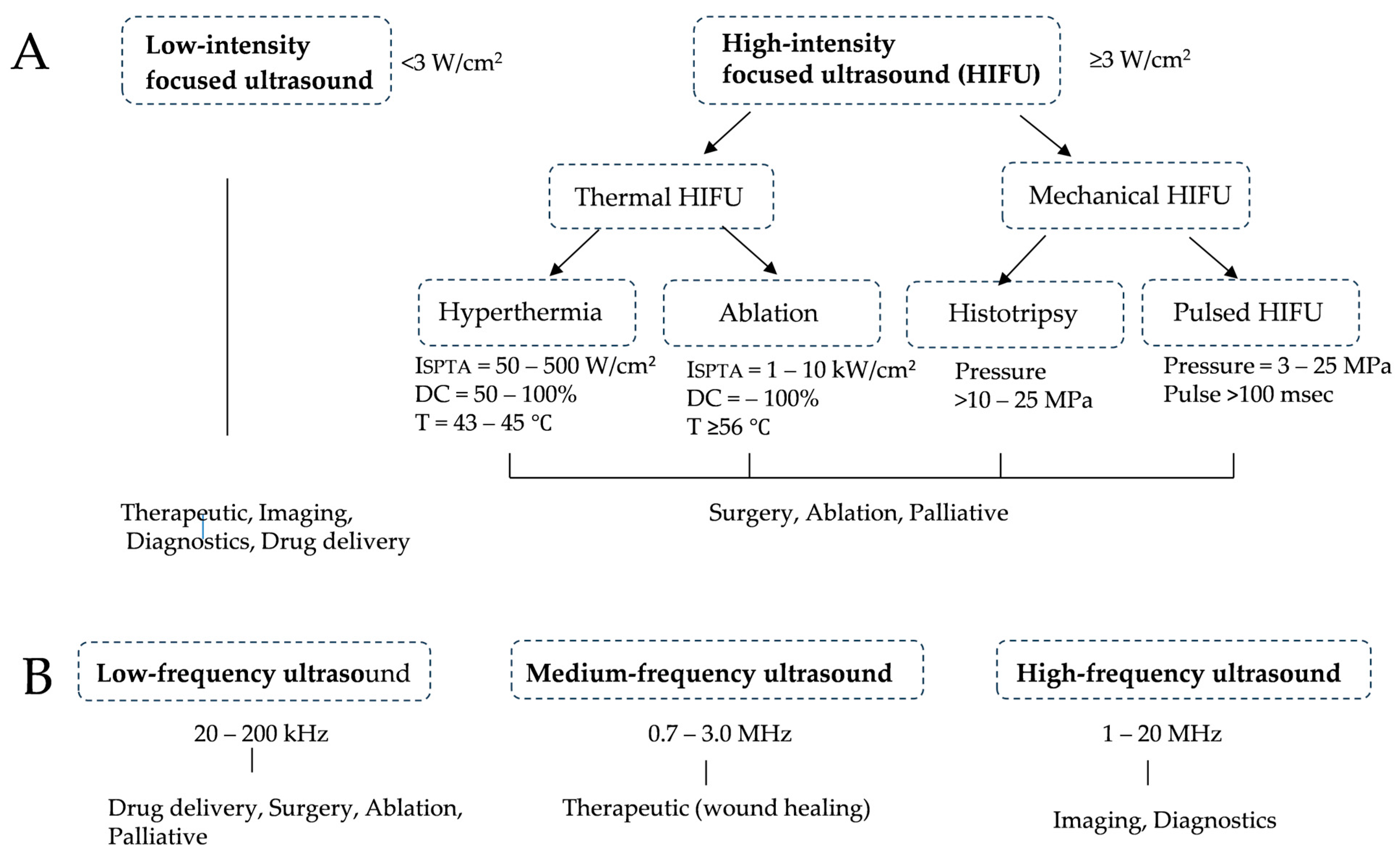
4.1. How Ultrasound Works and Its Application to Cancer Treatment
4.2. Research on Ultrasound Combined with Microbubbles (MBs) to Improve the Delivery of Drugs to Tumors
4.3. Research on Improving the Effectiveness of RT with Ultrasound
4.4. Research on the Use of FUS to Shrink Tumors
5. Previous BNCT Studies Using Ultrasound
6. Proposed Plans to Reduce the Recurrence of BPA-BNCT-Treated HNC by Ultrasound
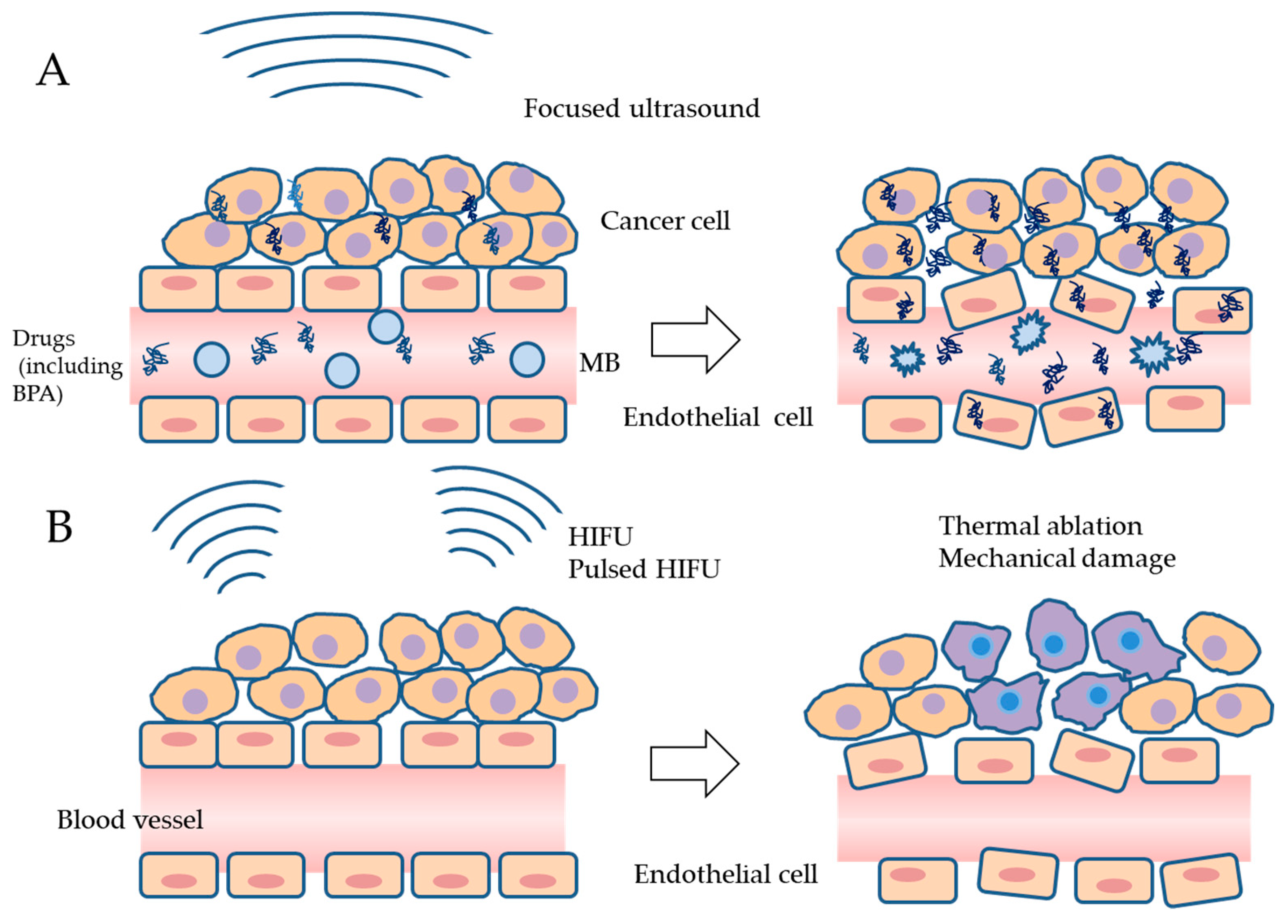
6.1. Increases in 10B Concentrations in Tumors and Endothelial Cells by USMB
6.2. Prevention of the Repopulation of Surviving Cells after BPA-BNCT by HIFU
7. Future Prospectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coderre, J.A.; Morris, G.M. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J. Neurooncol. 2009, 91, 199–206. [Google Scholar] [CrossRef]
- Kanno, H.; Nagata, H.; Ishiguro, A.; Tsuzuranuki, S.; Nakano, S.; Nonaka, T.; Kiyohara, K.; Kimura, T.; Sugawara, A.; Okazaki, Y.; et al. Designation Products: Boron Neutron Capture Therapy for Head and Neck Carcinoma. Oncologist 2021, 26, e1250–e1255. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A Critical Review of Radiation Therapy: From Particle Beam Therapy (Proton, Carbon, and BNCT) to Beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef]
- Matsumura, A.; Asano, T.; Hirose, K.; Igaki, H.; Kawabata, S.; Kumada, H. Initiatives Toward Clinical Boron Neutron Capture Therapy in Japan. Cancer Biother. Radiopharm. 2023, 38, 201–207. [Google Scholar] [CrossRef]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Coderre, J.A.; Glass, J.D.; Fairchild, R.G.; Micca, P.L.; Fand, I.; Joel, D.D. Selective delivery of boron by the melanin precursor analogue p-boronophenylalanine to tumors other than melanoma. Cancer Res. 1990, 50, 138–141. [Google Scholar]
- Hatanaka, H.; Nakagawa, Y. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1061–1066. [Google Scholar] [CrossRef]
- Detta, A.; Cruickshank, G.S. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009, 69, 2126–2132. [Google Scholar] [CrossRef]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef]
- Watanabe, T.; Sanada, Y.; Hattori, Y.; Suzuki, M. Correlation between the expression of LAT1 in cancer cells and the potential efficacy of boron neutron capture therapy. J. Radiat. Res. 2023, 64, 91–98. [Google Scholar] [CrossRef]
- Nakashima, H. The New Generation of Particle Therapy Focused on Boron Element (Boron Neutron Capture Therapy; BNCT) -The World’s First Approved BNCT Drug. Yakugaku Zasshi 2022, 142, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Igaki, H.; Ito, M.; Okamoto, H.; Nishioka, S.; Iijima, K.; Nakayama, H.; Takemori, M.; Imamichi, S.; Kashihara, T.; et al. Characterization of the relationship between neutron production and thermal load on a target material in an accelerator-based boron neutron capture therapy system employing a solid-state Li target. PLoS ONE 2019, 14, e0225587. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Mitsumoto, T.; Fujita, K.; Kashino, G.; Kinashi, Y.; Liu, Y.; Takada, M.; et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl. Radiat. Isot. 2011, 69, 1642–1645. [Google Scholar] [CrossRef]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Yura, Y.; Tada, S.; Fujita, Y.; Hamada, M. Current treatment, particle radiotherapy, and boron neutron capture therapy for advanced oral cancer in patients. Oral Sci. Int. 2019, 16, 49–68. [Google Scholar] [CrossRef]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef]
- Obayashi, S.; Kato, I.; Ono, K.; Masunaga, S.; Suzuki, M.; Nagata, K.; Sakurai, Y.; Yura, Y. Delivery of (10)boron to oral squamous cell carcinoma using boronophenylalanine and borocaptate sodium for boron neutron capture therapy. Oral Oncol. 2004, 40, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kamida, A.; Obayashi, S.; Kato, I.; Ono, K.; Suzuki, M.; Nagata, K.; Sakurai, Y.; Yura, Y. Effects of boron neutron capture therapy on human oral squamous cell carcinoma in a nude mouse model. Int. J. Radiat. Biol. 2006, 82, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, N.; Suzuki, M.; Sakurai, Y.; Nagata, K.; Kinashi, Y.; Masunaga, S.; Maruhashi, A.; Imahori, Y.; Kodaira, T.; Tachibana, H.; et al. Treatment results of boron neutron capture therapy using intra-arterial administration of boron compounds for recurrent head and neck cancer. Br. J. Radiol. 2008, 81, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Wittig, A.; Collette, L.; Moss, R.; Sauerwein, W.A. Early clinical trial concept for boron neutron capture therapy: A critical assessment of the EORTC trial 11001. Appl. Radiat. Isot. 2009, 67, S59–S62. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kato, I.; Aihara, T.; Hiratsuka, J.; Yoshimura, K.; Niimi, M.; Kimura, Y.; Ariyoshi, Y.; Haginomori, S.; Sakurai, Y.; et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J. Radiat. Res. 2014, 55, 146–153. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef] [PubMed]
- Koivunoro, H.; Kankaanranta, L.; Seppälä, T.; Haapaniemi, A.; Mäkitie, A.; Joensuu, H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother. Oncol. 2019, 137, 153–158. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Y.H.; Chu, P.Y.; Liu, H.M.; Peir, J.J.; Lin, K.H.; Huang, W.S.; Lo, W.L.; Lee, J.C.; Lin, T.Y.; et al. Boron Neutron Capture Therapy Followed by Image-Guided Intensity-Modulated Radiotherapy for Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial. Cancers 2023, 15, 2762. [Google Scholar] [CrossRef]
- Masunaga, S.; Ono, K.; Sakurai, Y.; Takagaki, M.; Kobayashi, T.; Kinashi, Y.; Suzuki, M. Evaluation of apoptosis and micronucleation induced by reactor neutron beams with two different cadmium ratios in total and quiescent cell populations within solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, D.S.; Saifi, O.; Mackeyev, Y.; Malouff, T.; Krishnan, S. Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT. Cells 2023, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Hiratsuka, J.; Ishikawa, H.; Kumada, H.; Ohnishi, K.; Kamitani, N.; Suzuki, M.; Sakurai, H.; Harada, T. Fatal carotid blowout syndrome after BNCT for head and neck cancers. Appl. Radiat. Isot. 2015, 106, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.L.; Chang, F.C.; Wang, C.W.; Igawa, K.; Wu, S.H.; Lo, W.L.; Chen, Y.W. Prevention and early management of carotid blowout syndrome for patients receiving head and neck salvage boron neutron capture therapy (BNCT). J. Dent. Sci. 2021, 16, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Sato, M.; Kato, T.; Takayama, K.; Suzuki, M.; Yamaguchi, H.; Seto, I.; Kikuchi, Y.; Murakami, M.; Takai, Y. Profile analysis of adverse events after boron neutron capture therapy for head and neck cancer: A sub-analysis of the JHN002 study. J. Radiat. Res. 2022, 63, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Kinashi, Y.; Suzuki, M.; Takagaki, M.; Masunaga, S.I. The combined effect of electroporation and borocaptate in boron neutron capture therapy for murine solid tumors. Jpn. J. Cancer Res. 2000, 91, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Olaiz, N.; Monti Hughes, A.; Pozzi, E.C.C.; Thorp, S.; Curotto, P.; Trivillin, V.A.; Ramos, P.S.; Palmieri, M.A.; Marshall, G.; Schwint, A.E.; et al. Enhancement in the Therapeutic Efficacy of In Vivo BNCT Mediated by GB-10 with Electroporation in a Model of Oral Cancer. Cells 2023, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.E.; Ter Haar, G. Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 638. [Google Scholar] [CrossRef]
- ter Haar, G.R.; Daniels, S. Evidence for ultrasonically induced cavitation in vivo. Phys. Med. Biol. 1981, 26, 1145–1149. [Google Scholar] [CrossRef]
- Bader, K.B.; Vlaisavljevich, E.; Maxwell, A.D. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 2019, 45, 1056–1080. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A.; Kruse, D.E.; Caskey, C.F.; Zhao, S.; Dayton, P.A.; Ferrara, K.W. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef] [PubMed]
- McLaughlan, J.; Rivens, I.; Leighton, T.; Ter Haar, G. A study of bubble activity generated in ex vivo tissue by high intensity focused ultrasound. Ultrasound Med. Biol. 2010, 36, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Dev. Ther. 2013, 7, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Ma, Y.; Guo, Y.; Zhang, C.; Guo, X.; Tu, J.; Yu, A.C.H.; Wu, J.; Zhang, D. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason. Sonochem. 2017, 37, 279–285. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Popa, M.; Mayoral Safont, M.; Murvold, E.; Haugse, R.; Langer, A.; Dimcevski, G.; Lam, C.; Bjånes, T.; Gilja, O.H.; et al. SonoVue® vs. Sonazoid™ vs. Optison™: Which Bubble Is Best for Low-Intensity Sonoporation of Pancreatic Ductal Adenocarcinoma? Pharmaceutics 2022, 14, 98. [Google Scholar] [CrossRef]
- Sharma, D.; Leong, K.X.; Czarnota, G.J. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4393. [Google Scholar] [CrossRef]
- Rapoport, N.; Payne, A.; Dillon, C.; Shea, J.; Scaife, C.; Gupta, R. Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J. Ther. Ultrasound 2013, 1, 11. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, A.; Huynh, R.N.; Wadajkar, A.S.; Lapidus, R.G.; Kim, A.J.; Raub, C.B.; Frenkel, V. Pulsed focused ultrasound lowers interstitial fluid pressure and increases nanoparticle delivery and penetration in head and neck squamous cell carcinoma xenograft tumors. Phys. Med. Biol. 2020, 65, 125017. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; Ruiz de Garibay, G.; Langer, A.; Liu, J.B.; Dhir, T.; Leitch, C.; Wessner, C.E.; Mayoral, M.; Zhang, B.; Popa, M.; et al. Selecting the optimal parameters for sonoporation of pancreatic cancer in a pre-clinical model. Cancer Biol. Ther. 2021, 22, 204–215. [Google Scholar] [CrossRef]
- Zhou, B.; Lian, Q.; Jin, C.; Lu, J.; Xu, L.; Gong, X.; Zhou, P. Human clinical trial using diagnostic ultrasound and microbubbles to enhance neoadjuvant chemotherapy in HER2- negative breast cancer. Front. Oncol. 2022, 12, 992774. [Google Scholar] [CrossRef] [PubMed]
- Haram, M.; Hansen, R.; Bouget, D.; Myhre, O.F.; Davies, C.L.; Hofsli, E. Treatment of Liver Metastases With Focused Ultrasound and Microbubbles in Patients With Colorectal Cancer Receiving Chemotherapy. Ultrasound Med. Biol. 2023, 49, 2081–2088. [Google Scholar] [CrossRef]
- Lai, P.; Tarapacki, C.; Tran, W.T.; El Kaffas, A.; Lee, J.; Hupple, C.; Iradji, S.; Giles, A.; Al-Mahrouki, A.; Czarnota, G.J. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience 2016, 3, 98–108. [Google Scholar] [CrossRef]
- Daecher, A.; Stanczak, M.; Liu, J.B.; Zhang, J.; Du, S.; Forsberg, F.; Leeper, D.B.; Eisenbrey, J.R. Localized microbubble cavitation-based antivascular therapy for improving HCC treatment response to radiotherapy. Cancer Lett. 2017, 411, 100–105. [Google Scholar] [CrossRef]
- Deng, H.; Cai, Y.; Feng, Q.; Wang, X.; Tian, W.; Qiu, S.; Wang, Y.; Li, Z.; Wu, J. Ultrasound-Stimulated Microbubbles Enhance Radiosensitization of Nasopharyngeal Carcinoma. Cell. Physiol. Biochem. 2018, 48, 1530–1542. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Unger, M.; Patties, I.; Melzer, A.; Landgraf, L. Focused Ultrasound-Induced Cavitation Sensitizes Cancer Cells to Radiation Therapy and Hyperthermia. Cells 2020, 9, 2595. [Google Scholar] [CrossRef]
- Chen, X.; Cvetkovic, D.; Chen, L.; Ma, C.M. An in-vivo study of the combined therapeutic effects of pulsed non-thermal focused ultrasound and radiation for prostate cancer. Int. J. Radiat. Biol. 2023, 99, 1716–1723. [Google Scholar] [CrossRef]
- Hundt, W.; Yuh, E.L.; Steinbach, S.; Bednarski, M.D. Effect of continuous high intensity focused ultrasound in a squamous cell carcinoma tumor model compared to muscle tissue evaluated by MRI, histology, and gene expression. Technol. Cancer Res. Treat. 2009, 8, 85–98. [Google Scholar] [CrossRef]
- Korkusuz, H.; Fehre, N.; Sennert, M.; Happel, C.; Grünwald, F. Early assessment of high-intensity focused ultrasound treatment of benign thyroid nodules by scintigraphic means. J. Ther. Ultrasound 2014, 2, 18. [Google Scholar] [CrossRef]
- Cohen, G.; Chandran, P.; Lorsung, R.M.; Aydin, O.; Tomlinson, L.E.; Rosenblatt, R.B.; Burks, S.R.; Frank, J.A. Pulsed-Focused Ultrasound Slows B16 Melanoma and 4T1 Breast Tumor Growth through Differential Tumor Microenvironmental Changes. Cancers 2021, 13, 1546. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.E.; Costa, M.; Rivens, I.; Repasky, E.E.; Ter Haar, G. Pulsed focused ultrasound can improve the anti-cancer effects of immune checkpoint inhibitors in murine pancreatic cancer. J. R. Soc. Interface 2021, 18, 20210266. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Kishore, D.; Ashar, H.; Neel, T.; Singh, A.; More, S. Focused ultrasound ablation of a large canine oral tumor achieves efficient tumor remission: A case report. Int. J. Hyperth. 2021, 38, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Sit, W.H.; Wan, J.M.; Yu, A.C. Sonoporation induces apoptosis and cell cycle arrest in human promyelocytic leukemia cells. Ultrasound Med. Biol. 2011, 37, 2149–2159. [Google Scholar] [CrossRef]
- Nofiele, J.T.; Karshafian, R.; Furukawa, M.; Al Mahrouki, A.; Giles, A.; Wong, S.; Czarnota, G.J. Ultrasound-activated microbubble cancer therapy: Ceramide production leading to enhanced radiation effect in vitro. Technol. Cancer Res. Treat. 2013, 12, 53–60. [Google Scholar] [CrossRef]
- Bergs, J.W.; Krawczyk, P.M.; Borovski, T.; ten Cate, R.; Rodermond, H.M.; Stap, J.; Medema, J.P.; Haveman, J.; Essers, J.; van Bree, C.; et al. Inhibition of homologous recombination by hyperthermia shunts early double strand break repair to non-homologous end-joining. DNA Repair 2013, 12, 38–45. [Google Scholar] [CrossRef]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.E.; Rivens, I.; Civale, J.; Symonds-Tayler, R.; Ter Haar, G. Relationship between thermal dose and cell death for “rapid” ablative and “slow” hyperthermic heating. Int. J. Hyperth. 2019, 36, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.W.; Hall, T.L.; Ives, K.; Wolf, J.S., Jr.; Fowlkes, J.B.; Cain, C.A. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J. Urol. 2006, 175, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, V.A.; Fowlkes, J.B.; Roberts, W.W.; Schade, G.R.; Xu, Z.; Khokhlova, T.D.; Hall, T.L.; Maxwell, A.D.; Wang, Y.N.; Cain, C.A. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. Int. J. Hyperth. 2015, 31, 145–162. [Google Scholar] [CrossRef]
- Alkins, R.D.; Brodersen, P.M.; Sodhi, R.N.; Hynynen, K. Enhancing drug delivery for boron neutron capture therapy of brain tumors with focused ultrasound. Neuro Oncol. 2013, 15, 1225–1235. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chan, P.C.; Chou, L.S.; Chang, C.W.; Yang, F.Y.; Liu, R.S.; Chiou, S.H.; Chen, Y.W.; Yen, S.H.; Wang, H.E. Pulsed-focused ultrasound enhances boron drug accumulation in a human head and neck cancer xenograft-bearing mouse model. Mol. Imaging Biol. 2014, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Chang, W.Y.; Li, J.J.; Wang, H.E.; Chen, J.C.; Chang, C.W. Pharmacokinetic analysis and uptake of 18F-FBPA-Fr after ultrasound-induced blood-brain barrier disruption for potential enhancement of boron delivery for neutron capture therapy. J. Nucl. Med. 2014, 55, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Masunaga, S.-i.; Kato, I.; Iwai, S.; Nakazawa, M.; Ono, K.; Yura, Y. Enhancing effect of ultrasound on boron concentrations in an oral squamous cell carcinoma cell line SAS for boron neutron capture therapy. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 487–492. [Google Scholar] [CrossRef]
- Fan, C.H.; Wang, T.W.; Hsieh, Y.K.; Wang, C.F.; Gao, Z.; Kim, A.; Nagasaki, Y.; Yeh, C.K. Enhancing Boron Uptake in Brain Glioma by a Boron-Polymer/Microbubble Complex with Focused Ultrasound. ACS Appl. Mater. Interfaces 2019, 11, 11144–11156. [Google Scholar] [CrossRef]
- Monti Hughes, A.; Hu, N. Optimizing Boron Neutron Capture Therapy (BNCT) to Treat Cancer: An Updated Review on the Latest Developments on Boron Compounds and Strategies. Cancers 2023, 15, 4091. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Panadero, R.; Lucantoni, F.; Gamero-Sandemetrio, E.; Cruz-Merino, L.; Álvaro, T.; Noguera, R. The tumour microenvironment as an integrated framework to understand cancer biology. Cancer Lett. 2019, 461, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Li, J.; Hosmane, N.S.; Zhu, Y. Next generation of boron neutron capture therapy (BNCT) agents for cancer treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef] [PubMed]
- Fukuo, Y.; Hattori, Y.; Kawabata, S.; Kashiwagi, H.; Kanemitsu, T.; Takeuchi, K.; Futamura, G.; Hiramatsu, R.; Watanabe, T.; Hu, N.; et al. The Therapeutic Effects of Dodecaborate Containing Boronophenylalanine for Boron Neutron Capture Therapy in a Rat Brain Tumor Model. Biology 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-Based Receptor Targeting Using an Fc-Binding Peptide-Dodecaborate Conjugate and Macropinocytosis Induction for Boron Neutron Capture Therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, A.; Hayashi, Y.; Kato, K.; Kogure, Y.; Kameyama, M.; Shimamoto, H.; Daitoku, H.; Fukamizu, A.; Hirota, T.; Kimura, K. Identification of a novel nucleolar protein complex required for mitotic chromosome segregation through centromeric accumulation of Aurora B. Nucleic Acids Res. 2020, 48, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nishimura, K.; Okada, S.; Sato, S.; Suzuki, M.; Takata, T.; Nakamura, H. Cyclic RGD-Functionalized closo-Dodecaborate Albumin Conjugates as Integrin Targeting Boron Carriers for Neutron Capture Therapy. Mol. Pharm. 2020, 17, 3740–3747. [Google Scholar] [CrossRef]
- Kuthala, N.; Shanmugam, M.; Yao, C.L.; Chiang, C.S.; Hwang, K.C. One step synthesis of (10)B-enriched (10)BPO(4) nanoparticles for effective boron neutron capture therapeutic treatment of recurrent head-and-neck tumor. Biomaterials 2022, 290, 121861. [Google Scholar] [CrossRef]
- Iwagami, T.; Ishikawa, Y.; Koshizaki, N.; Yamamoto, N.; Tanaka, H.; Masunaga, S.; Sakurai, Y.; Kato, I.; Iwai, S.; Suzuki, M.; et al. Boron carbide particle as a boron compound for born neutron capture therapy. J. Nucl. Med. Radiat. Ther. 2014, 5, 2. [Google Scholar] [CrossRef]
- Kozień, D.; Żeliszewska, P.; Szermer-Olearnik, B.; Adamczyk, Z.; Wróblewska, A.; Szczygieł, A.; Węgierek-Ciura, K.; Mierzejewska, J.; Pajtasz-Piasecka, E.; Tokarski, T.; et al. Synthesis and Characterization of Boron Carbide Nanoparticles as Potential Boron-Rich Therapeutic Carriers. Materials 2023, 16, 6534. [Google Scholar] [CrossRef]
- Sforzi, J.; Lanfranco, A.; Stefania, R.; Alberti, D.; Bitonto, V.; Parisotto, S.; Renzi, P.; Protti, N.; Altieri, S.; Deagostino, A.; et al. A novel pH sensitive theranostic PLGA nanoparticle for boron neutron capture therapy in mesothelioma treatment. Sci. Rep. 2023, 13, 620. [Google Scholar] [CrossRef]
- Novopashina, D.S.; Vorobyeva, M.A.; Venyaminova, A. Recent Advances in the Synthesis of High Boron-Loaded Nucleic Acids for BNCT. Front. Chem. 2021, 9, 619052. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Lembo, D. Micro- and nanobubbles: A versatile non-viral platform for gene delivery. Int. J. Pharm. 2013, 456, 437–445. [Google Scholar] [CrossRef]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef]
- Harada, T.; Hirose, K.; Wada, Y.; Sato, M.; Ichise, K.; Aoki, M.; Kato, T.; Takeda, K.; Takai, Y. YC-1 sensitizes the antitumor effects of boron neutron capture therapy in hypoxic tumor cells. J. Radiat. Res. 2020, 61, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J. Cell. Biochem. 2016, 117, 2682–2692. [Google Scholar] [CrossRef]
- Fujita, Y.; Kato, I.; Iwai, S.; Ono, K.; Suzuki, M.; Sakurai, Y.; Ohnishi, K.; Ohnishi, T.; Yura, Y. Role of p53 mutation in the effect of boron neutron capture therapy on oral squamous cell carcinoma. Radiat. Oncol. 2009, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, T.; Journe, F.; Descamps, G.; Saussez, S.; Dragan, T.; Ghanem, G.; Krayem, M.; Van Gestel, D. Restoring p53 Function in Head and Neck Squamous Cell Carcinoma to Improve Treatments. Front. Oncol. 2021, 11, 799993. [Google Scholar] [CrossRef]
- Lindemann, A.; Patel, A.A.; Silver, N.L.; Tang, L.; Liu, Z.; Wang, L.; Tanaka, N.; Rao, X.; Takahashi, H.; Maduka, N.K.; et al. COTI-2, A Novel Thiosemicarbazone Derivative, Exhibits Antitumor Activity in HNSCC through p53-dependent and -independent Mechanisms. Clin. Cancer Res. 2019, 25, 5650–5662. [Google Scholar] [CrossRef] [PubMed]

| References Year | Number of Patients, Histology | Boron Delivery Agents and Administration | Neutron Source and Dose | Clinical Outcome |
|---|---|---|---|---|
| [28] 2012 | 30 patients with recurrent malignancies, 29 evaluable SCC = 24 Sarcoma = 1 | BPA Once = 4 Twice = 26 | Reactor First average weight GTV dose = 23 (14–37) Gy (W) Second average weight GTV dose = 22 (15–30) Gy (W) | ORR = 76% MST = 13.0 months 2-year PFS = 20%, OS = 30% |
| [27] 2014 | 62 patients with advanced or recurrent cancers SCC = 33 Mucoepidermoid Ca = 5 Adenoid cystic Ca = 4 | BSH + BPA or BPA (BPA = 72, BSH + BPA = 13) Once = 42 Twice = 17 Three times = 2 Five times = 1 | Reactor Minimum tumor dose = 17.9 (4.0–44.5) Gy-Eq | ORR = 58% MST = 10.1 months 1-year OS = 43.1% 2-year OS = 24.2% |
| [29] 2019 | 79 patients with recurrent SCC, 69 evaluable | BPA Once = 40 Twice = 39 | Reactor First median minimum GTV dose = 15 (12–18) Gy (W) Second median minimum GTV dose = 14 (10–16) Gy (W) | ORR = 68% OS = 21% |
| [26] 2021 | 21 patients with recurrent or locally advanced malignancies SCC = 8, Non-SCC = 13 (5 adenoid cystic Ca, 3 mucoepidermoid Ca, 2 acinic cell Ca, 2 salivary ductal Ca, 1 melanoma) | BPA | Cyclotron-based epithermal neutrons Median tumor mean dose =44.7 (42.9–50.6) Gy-Eq Median tumor minimum dose = 31.1 (26.1–34.3) Gy-Eq | ORR = 71% SCC: CR = 50%, PR = 25% 2-year OS = 58%, Re-reated with RT, Chem, ICIs Non-SCC: CR = 8%, PR = 62%, 2-year OS =100% Re-treated with surgery, RT, CRT |
| [30] 2023 | 14 patients with locally recurrent malignancies SCC=10 Mucoepidermoid Ca = 1 Non-keratinizing Ca = 1 Sarcoma = 1 | BPA | Reactor Average GTV dose = 21.6 (10.7–32.3) Gy-Eq Fractionated IG-IMRT Total prescription dose = 46.8 (41.4–53) Gy | ORR = 64% 1-year PFS = 25%, OS = 56% |
| Ultrasound with MB to Improve Drug Delivery | |||||
| References Year | Cancer | Purpose | Type of Ultrasound | Parameters | Effects |
| [51] 2013 | Human pancreatic cancer, nude mouse tumor | Delivery of paclitaxel-nanoparticles with MB | FUS | Frequency = 3 MHz, Acoustic pressure = 3.1 MPa 16-element annular transducer | Decreased tumor growth |
| [52] 2016 | 10 patients with inoperable pancreatic cancer | Delivery of gemcitabine with MB | FUS | Frequency = 1.9 MHz Intensity = 0.25 mW/cm2, MI = 0.2 (peak negative pressure = 0.27 MPa), DC = 0.3% | May improve the clinical effects of gemcitabine |
| [53] 2021 | Human hypopharyngeal cancer, nude mouse tumor | Delivery of nanoparticles with MB | Pulsed FUS | Frequency = 500 kHz ISPTA = 1.55 kW/cm2 Acoustic pressure = 5 MPa | Improved nanoparticle delivery |
| [54] 2021 | Human pancreatic cancer, nude mouse tumor | Delivery of abraxane IV and gemcitabine with MB | US | Frequency = 2.0 MHz ISPTA = 200 W/cm2 Peak negative pressure = 550~650 kPa | Decreased tumor volume Increased vascularity |
| [55] 2022 | 10 patients with breast cancer | Delivery of taxane, anthracycline, and cyclophosphamide with MB | US | Frequency = 4 MHz V-flash mode c, MI = 0.3~0.4 | Improved the effects of NAC |
| [56] 2023 | 17 patients with colorectal cancer liver metastasis | Delivery of irinotecan, calcium folinate, and fluorouracil with MB | FUS | Frequency = 1.7 MHz Acoustic pressure = 0.65 MPa, DC = 0.2~0.4% | A tendency toward tumor volume reduction |
| Ultrasound to Improve the Effectiveness of RT | |||||
| References Year | Cancer | Purpose | Type of Ultrasound | Parameters | Effects |
| [57] 2016 | Mouse breast cancer | Combination of USMB and radiation (0.2 or 8 Gy) | US | Frequency = 500 kHz Peak negative pressure = 570 kPa | Additive anti-tumor and anti-vascular effects |
| [58] 2017 | Human hepato- cellular carcinoma, nude mouse tumor | Combination of USMB and radiation (5 Gy) | US | Frequency = 4.2 MHz Peak-negative pressure = 2.5 MPa | Increased reduction in tumor growth by USMB |
| [59] 2018 | Human nasopharyngeal cancer, nude mouse tumor | Combination of USMB and radiation (0.2 or 8 Gy | US | Frequency = 238 kHz Acoustic pressure = 570 kPa MI = 0.8 | Enhanced the effects of radiation |
| [60] 2020 | Cancer cells (HNSCC, glioblastoma, prostate cancer) | Combination of ultrasound and radiation (10 Gy) or HT | FUS- cavitation | Frequency = 1.467 MHz Intensity = 1176 W/cm2 | Short FUS-cavitation sensitized cancer cells to radiation and HT |
| [61] 2023 | Human prostate cancer, nude mouse tumor | Combination of pHIFU with radiation (2 Gy) | Pulsed HIFU | Frequency = 1 MHz Intensity = 25 W (1 Hz pulse rate, 10% DC for 60 s) | Pulsd HIFU enhanced the effects of radiation |
| HIFU to Shrink Tumors | |||||
| References Year | Cancer | Purpose | Type of Ultrasound | Parameters | Effects |
| [62] 2009 | Mouse HNSCC tumor | Thermal damage on SCC by continuous HIFU | HIFU | Frequency = 1 MHz Intensity = 6830.7 W/cm2 Acoustic pressure = 142.1 kPa | Histologically revealed necrotic area |
| [63] 2020 | Benign thyroid nodule in patients | Treatment of tumors by thermal ablation | HIFU | Frequency = 3 MHz Maximum acoustic power = 125 W | Safe and effective to induce nodule shrinkage |
| [64] 2021 | Mouse melanoma or breast tumor | Mechanotransductive effects by pulsed HIFU | Pulsed HIFU | Frequency = 1.15 MHz Peak negative acoustic pressure = 6 MPa, ISPTA = 2683 W/cm2, DC = 10%, MI = 5.6 | Decreased tumor growth rates |
| [65] 2021 | Mouse pancreatic cancer | Non-ablative pulsed HIFU in combination with ICIs | Pulsed HIFU | Frequency = 1.5 MHz Peak negative power = 17 MPa | Pulsed HIFU increased the infiltration of CD8+ T cells in tumors |
| [66] 2021 | Canine oral neurilemmoma | Thermal ablation of tumor | FUS | Frequency = 1 MHz Acoustic power = 90 W DC = 50% | Complete tumor remission |
| References Year | Cancer | Purpose | BPA | Ultrasound | Effects |
|---|---|---|---|---|---|
| [76] 2013 | Rat gliosarcoma, brain tumor model | Delivery of BPA-Fr with MRI-guided FUS in combination with MB | BPA-Fr 250 mg/kg intravenous over 2 h | 558 kHz transducer Peak rarefaction pressure = 0.4 MPa A single sonication treatment duration = 20 s, | US increased the accumulation of 10B in the tumors |
| [77] 2014 | Human oral cancer, nude mouse tumor | Delivery of BPA-Fr to tumors | 18F-FBPA-Fr intravenously injected immediately after pulsed HIFU | Two min pulsed HIFU was applied to tumors | Higher tumor 10B in pulsed HIFU-treated mice |
| [78] 2014 | Rat glioma, brain tumor model | Delivery of 18F-FBPA-Fr | Intravenous injection of 18F-FBPA-Fr | FUS prior to BPA administration | The tumor-to-contralateral brain ratio was 1.75-fold higher in sonicated tumors than in control tumors |
| [79] 2015 | Human oral cancer | Delivery of BPA and BSH into SCC cells with MB | BPA, BSH 50 ppm in culture | Cells were incubated with BPA or BSH for 2 h before USMB Frequency = 1 MHz Intensity = 1 W/cm2 DC = 20%, 10 s | USMB increased the accumulation of BPA and BSH and BNCT decreased cell viability |
| [80] 2019 | Rat brain tumor | Delivery of a boron polymer | Self- assembled boron- containing nanoparticles | Frequency = 1 MHz, Pressure = 0.3–0.7 MPa, DC = 0.5%, Sonication = 1 min | The T/M ratio was increased 3-fold by FUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yura, Y.; Fujita, Y.; Hamada, M. Ultrasound Combination to Improve the Efficacy of Current Boron Neutron Capture Therapy for Head and Neck Cancer. Cancers 2024, 16, 2770. https://doi.org/10.3390/cancers16152770
Yura Y, Fujita Y, Hamada M. Ultrasound Combination to Improve the Efficacy of Current Boron Neutron Capture Therapy for Head and Neck Cancer. Cancers. 2024; 16(15):2770. https://doi.org/10.3390/cancers16152770
Chicago/Turabian StyleYura, Yoshiaki, Yusei Fujita, and Masakazu Hamada. 2024. "Ultrasound Combination to Improve the Efficacy of Current Boron Neutron Capture Therapy for Head and Neck Cancer" Cancers 16, no. 15: 2770. https://doi.org/10.3390/cancers16152770





