FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Search and Selection Strategy
- (1)
- HNSCC was defined as squamous cell carcinoma in the oral cavity, oropharynx, hypopharynx, and larynx. Locations such as unknown primary, nasopharynx, salivary glands, head and neck skin, paranasal sinuses, and ear were excluded.
- (2)
- Primary HNSCC proven histologically.
- (3)
- No synchronous oncological disease.
- (4)
- Clinically N0 neck (cN0).
- (5)
- No prior oncologic treatment.
- (6)
- Histological analysis of a neck dissection specimen used as a gold standard.
- (7)
- PET combined with both non-contrast-enhanced (NCE) or contrast-enhanced CT (CECT).
- (8)
- PET-CT acquisitions after the year 2000.
- (9)
- Available or retrievable data for true positive (TP), true negative (TN), false positive (FP), and false negative (FN) evaluations.
2.2. Data Extraction
2.3. Data Analysis
3. Results
3.1. Systematic Review
3.1.1. Search Results
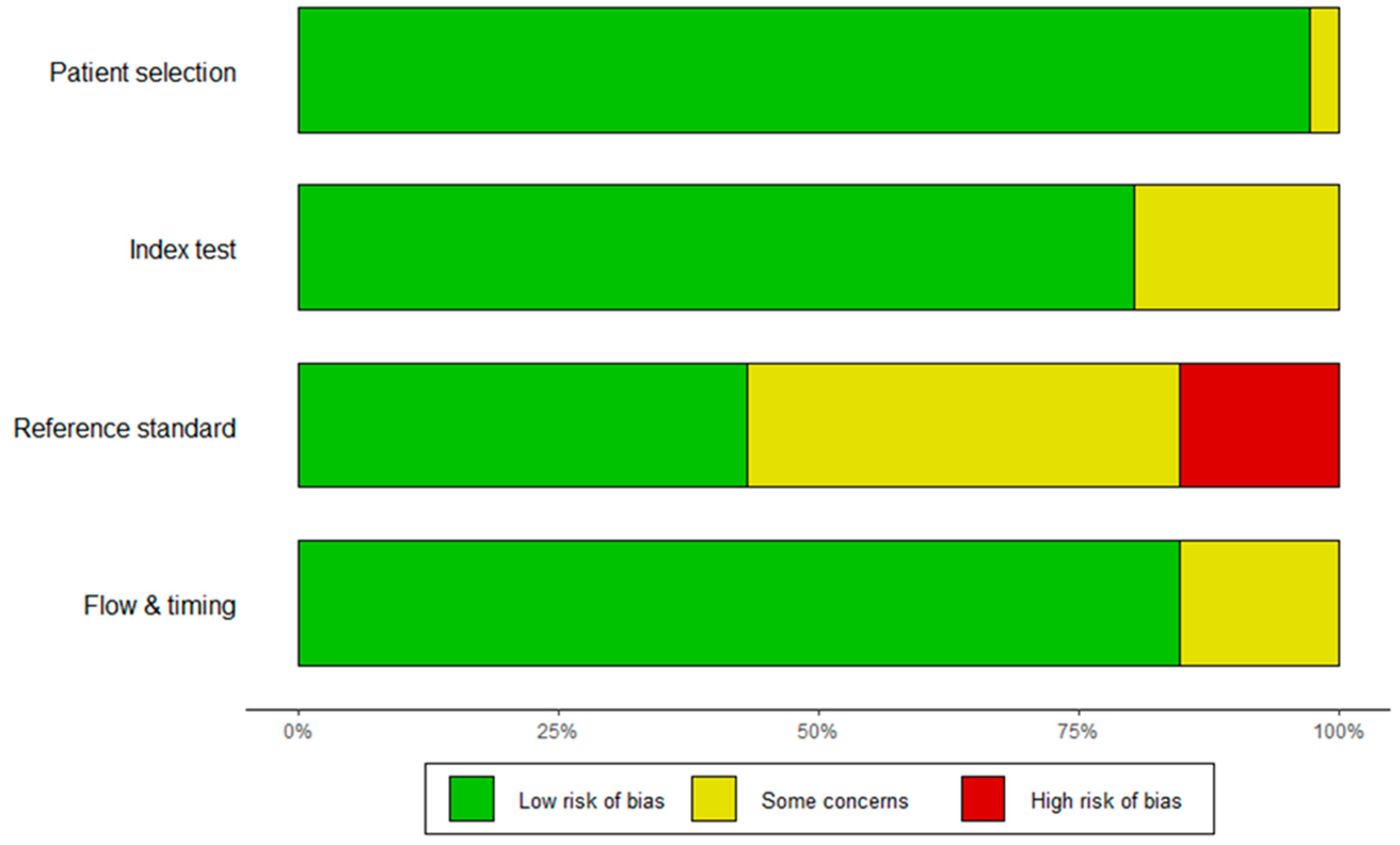
3.1.2. Study Characteristics and Quality Assessment
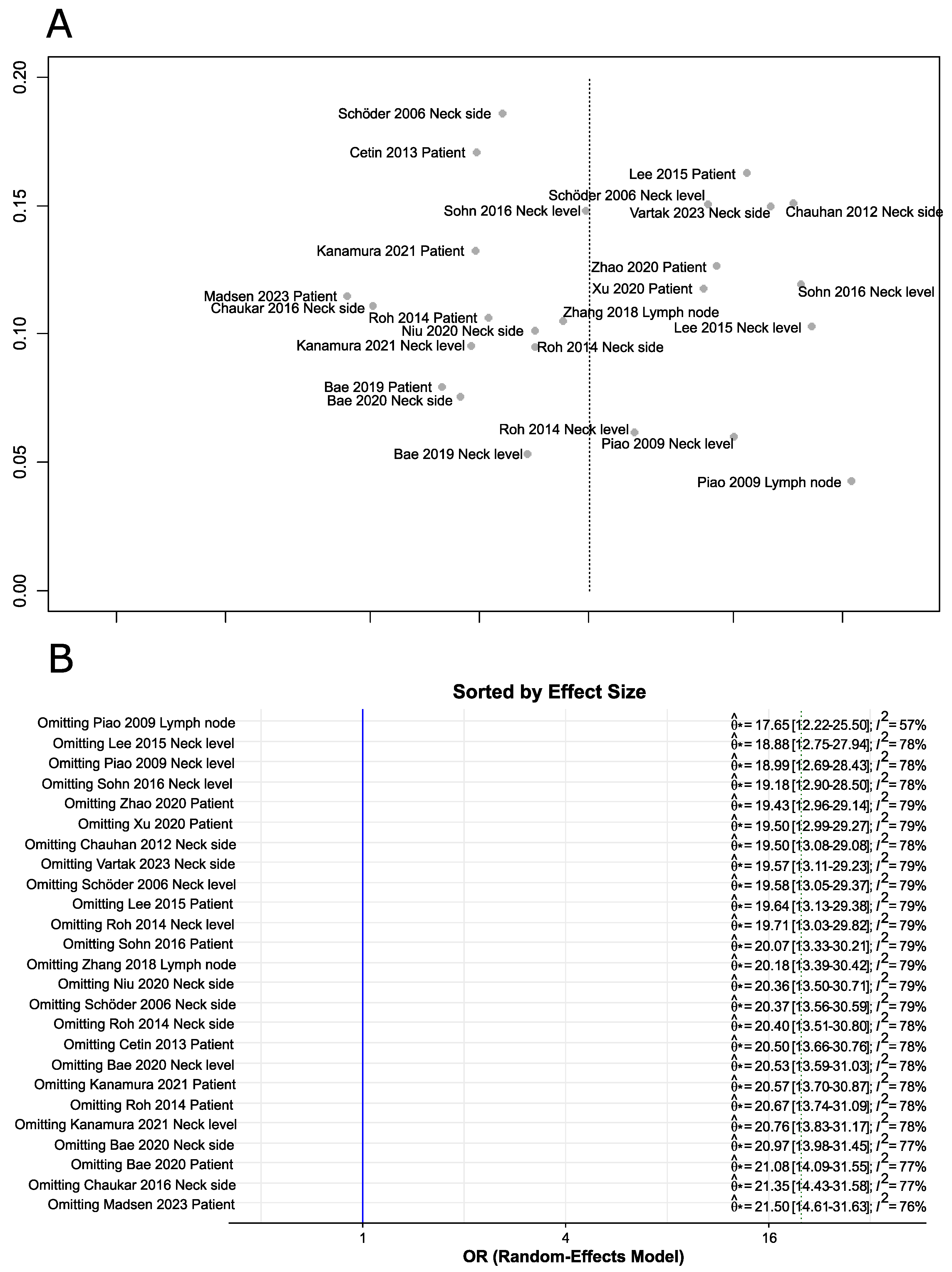
3.1.3. Publication Bias and Sensitivity Analysis
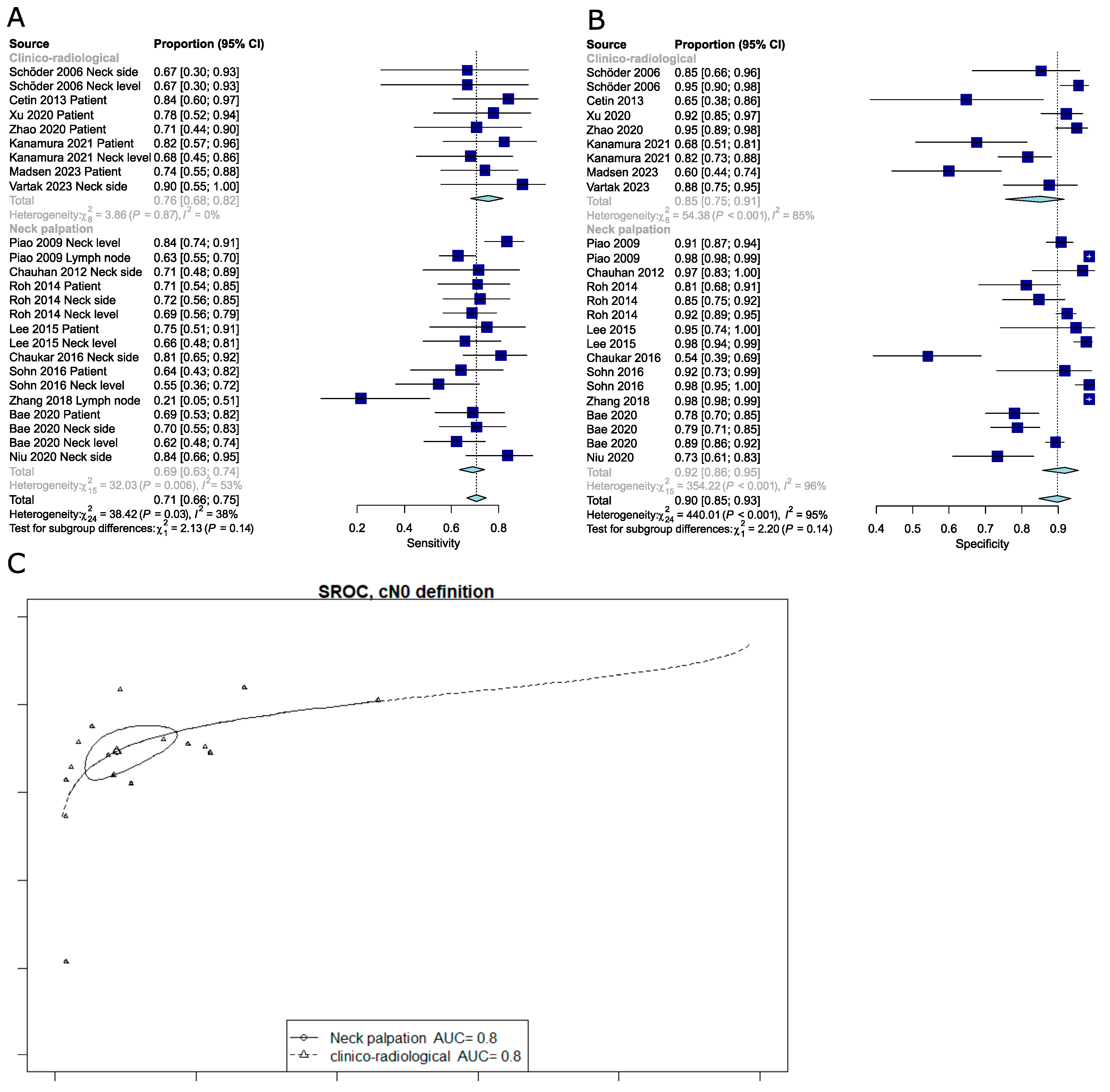
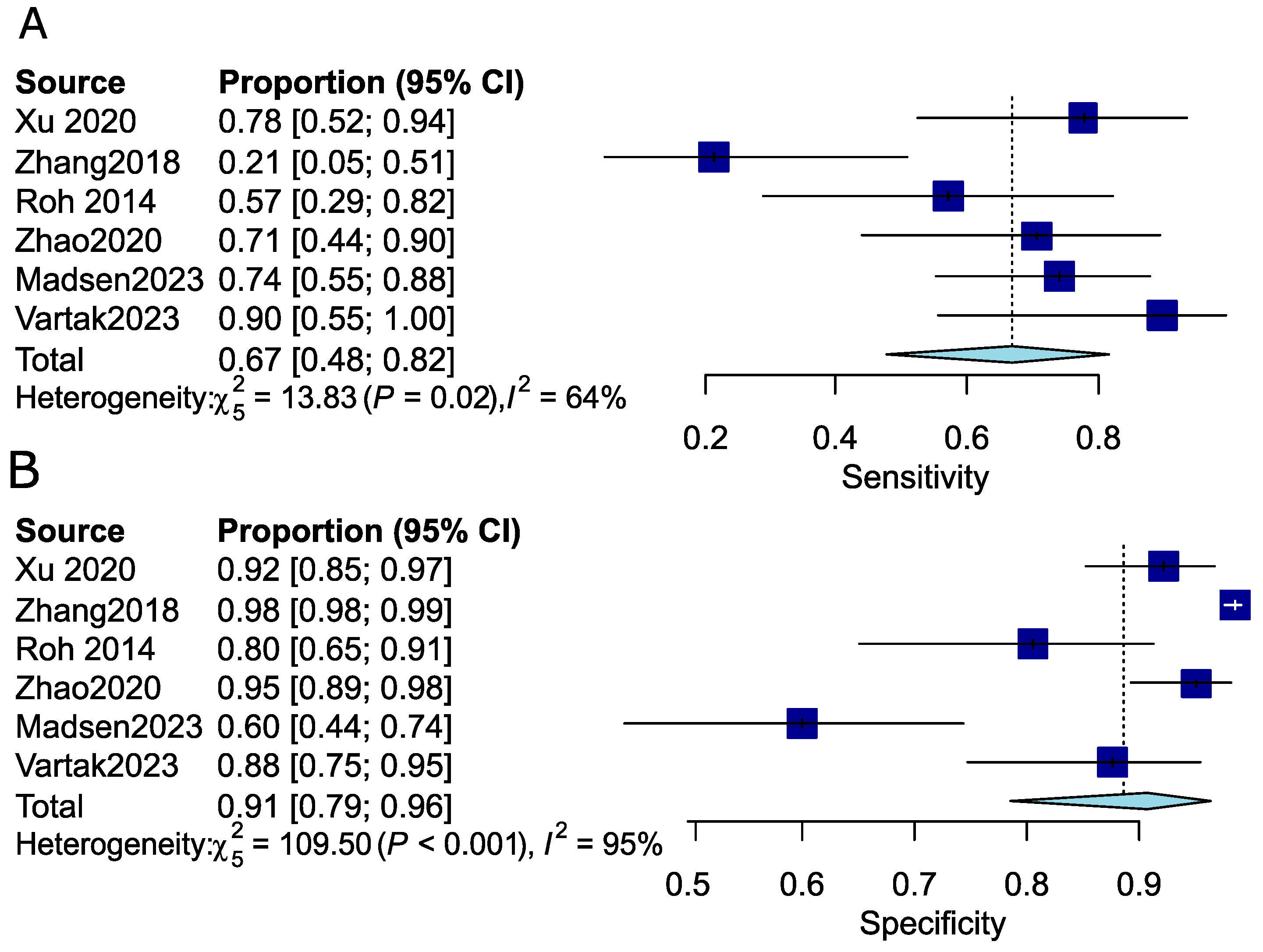
3.1.4. Pooled Analysis of the Diagnostic Performance of PET-CT for the Detection of Occult Lymph Node Metastasis (OLNM)
3.1.5. Meta-Regression Analysis Reveals Sources of Heterogeneity
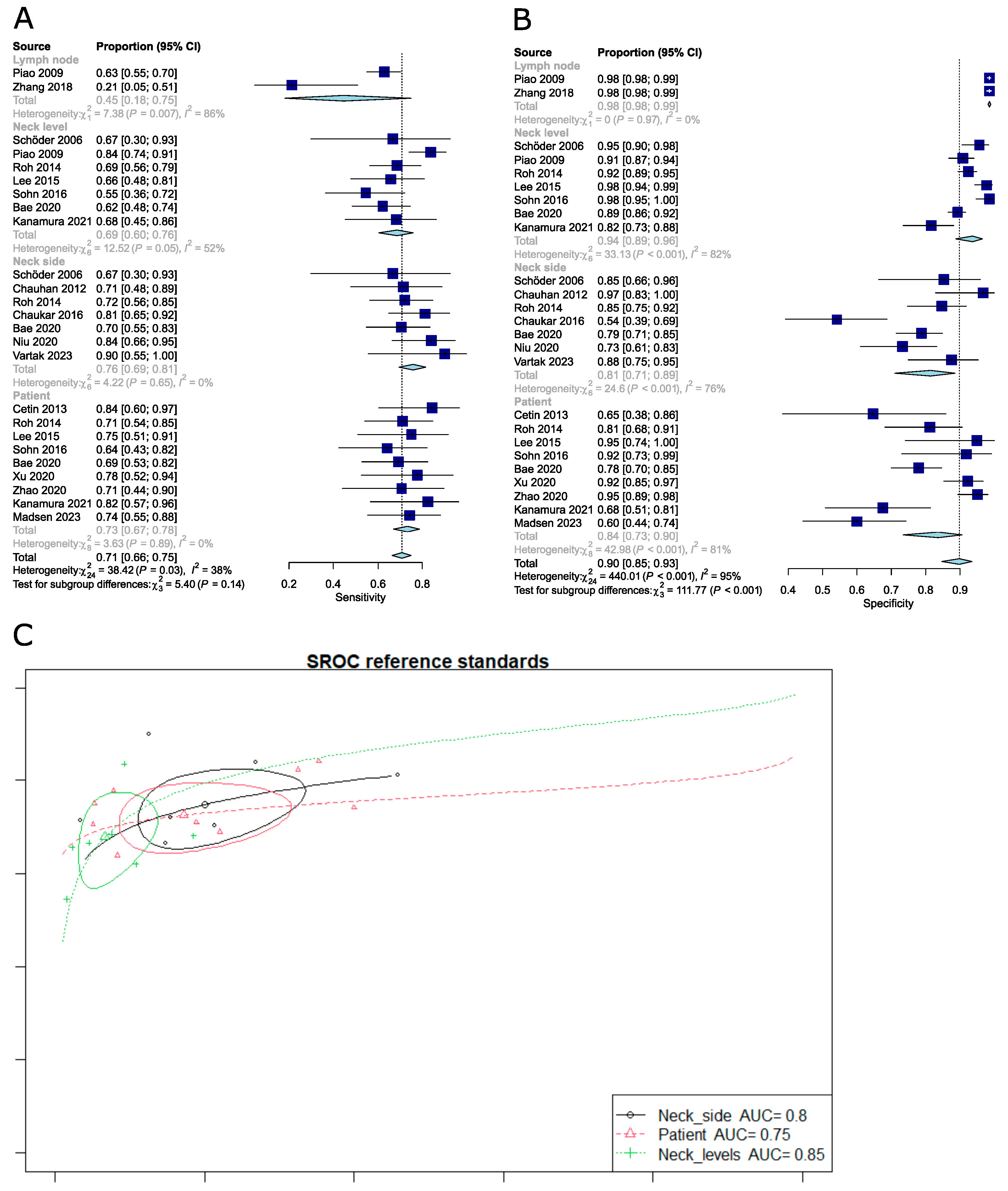
3.1.6. Comparison of the Diagnostic Performance of FDG PET-CT Depending on Different Reference Standards
3.1.7. Pooled Analysis of the Diagnostic Performance of PET-CT for the Detection of Occult Lymph Node Metastasis (OLNM) in the Early-Stage Disease Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
PubMed Database Research Equation 8 July 2024
Appendix B
EMBASE Database Research Equation 8 July 2024
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Psychogios, G.; Mantsopoulos, K.; Bohr, C.; Koch, M.; Zenk, J.; Iro, H. Incidence of occult cervical metastasis in head and neck carcinomas: Development over time. J. Surg. Oncol. 2013, 107, 384–387. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Garrel, R.; Poissonnet, G.; Moyà Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy Versus Neck Node Dissection in Operable T1-T2N0 Oral and Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R.; Petruzelli, G.; Gourin, C.G.; Wong, R.J.; Ferris, R.L.; et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: Results of a prospective multi-institutional trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef]
- Huang, S.H.; Hwang, D.; Lockwood, G.; Goldstein, D.P.; O’Sullivan, B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: A meta-analysis of reported studies. Cancer 2009, 115, 1489–1497. [Google Scholar] [CrossRef]
- Mermod, M.; Bongiovanni, M.; Petrova, T.; Goun, E.; Simon, C.; Tolstonog, G.; Monnier, Y. Prediction of Occult Lymph Node Metastasis in Head and Neck Cancer with CD31 Vessel Quantification. Otolaryngol. Head. Neck Surg. 2019, 160, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mermod, M.; Jourdan, E.F.; Gupta, R.; Bongiovanni, M.; Tolstonog, G.; Simon, C.; Clark, J.; Monnier, Y. Development and validation of a multivariable prediction model for the identification of occult lymph node metastasis in oral squamous cell carcinoma. Head. Neck 2020, 42, 1811–1820. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-Analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007, 8, 239–251. [Google Scholar] [CrossRef]
- Doebler, P.; Holling, H. Meta-Analysis of Diagnostic Accuracy and ROC Curves with Covariate Adjusted Semiparametric Mixtures. Psychometrika 2015, 80, 1084–1104. [Google Scholar] [CrossRef]
- Schwarzer, G. Meta: An R package for meta-Analysis. R J. 2007, 7, 40–45. [Google Scholar]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Schöder, H.; Carlson, D.L.; Kraus, D.H.; Stambuk, H.E.; Gönen, M.; Erdi, Y.E.; Yeung, H.W.; Huvos, A.G.; Shah, J.P.; Larson, S.M.; et al. 18F-FDG PET-CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J. Nucl. Med. 2006, 47, 755–762. [Google Scholar] [PubMed]
- Piao, Y.; Bold, B.; Tayier, A.; Ishida, R.; Omura, K.; Okada, N.; Shibuya, H. Evaluation of 18F-FDG PET-CT for diagnosing cervical nodal metastases in patients with oral cavity or oropharynx carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 933–938. [Google Scholar] [CrossRef]
- Chauhan, A.; Kulshrestha, P.; Kapoor, S.; Singh, H.; Jacob, M.; Patel, M.; Ganguly, M. Comparison of PET/CT with conventional imaging modalities (USG, CECT) in evaluation of N0 neck in head and neck squamous cell carcinoma. Med J. Armed Forces India 2012, 68, 322–327. [Google Scholar] [CrossRef]
- Cetin, B.; Atasever, T.; Akdemir, U.O.; Senturk, S.; Tufan, G.; Turan, N.; Buyukberber, S.; Coskun, U.; Benekli, M. The role of positron emission tomography with 18F-fluorodeoxyglucose in nodal staging of clinical and radiological N0 head and neck cancers. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Park, J.P.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. 18F fluorodeoxyglucose PET-CT in head and neck squamous cell carcinoma with negative neck palpation findings: A prospective study. Radiology 2014, 271, 153–161. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.; Woo, H.Y.; Kang, W.J.; Lee, J.H.; Koh, Y.W. 18F-FDG PET-CT as a supplement to CT/MRI for detection of nodal metastasis in hypopharyngeal SCC with palpably negative neck. Laryngoscope 2015, 125, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Sohn, B.; Koh, Y.W.; Kang, W.J.; Lee, J.H.; Shin, N.Y.; Kim, J. Is there an additive value of 18 F-FDG PET-CT to CT/MRI for detecting nodal metastasis in oropharyngeal squamous cell carcinoma patients with palpably negative neck? Acta Radiol. 2016, 57, 1352–1359. [Google Scholar] [CrossRef]
- Chaukar, D.; Dandekar, M.; Kane, S.; Arya, S.; Purandare, N.; Rangarajan, V.; Deshmukh, A.; Pai, P.; Chaturvedi, P.; D’Cruz, A. Relative value of ultrasound, computed tomography and positron emission tomography imaging in the clinically node-negative neck in oral cancer. Asia-Pacific J. Clin. Oncol. 2014, 12, e332–e338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Seikaly, H.; Biron, V.L.; Jeffery, C.C. Utility of PET-CT in detecting nodal metastasis in cN0 early stage oral cavity squamous cell carcinoma. Oral Oncol. 2018, 80, 89–92. [Google Scholar] [CrossRef]
- Niu, L.; Zheng, D.; Wang, D.; Zhang, J.; Fei, J.; Guo, C. Accuracy of 18 F-FDG PET/CT in Detection of Neck Metastases of Oral Squamous Cell Carcinoma in Patients Without Large Palpable Lymph Nodes. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 418–426. [Google Scholar] [CrossRef]
- Bae, M.R.; Roh, J.L.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. (18)F-FDG PET-CT versus CT/MR imaging for detection of neck lymph node metastasis in palpably node-negative oral cavity cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 237–244. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Seng, D.; Liu, F. Significance of SUV Max for Predicting Occult Lymph Node Metastasis and Prognosis in Early-Stage Tongue Squamous Cell Carcinoma. J. Oncol. 2020, 2020, 6241637. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, J.; Ba, K.; Zhang, Y. Significance of PET-CT for Detecting Occult Lymph Node Metastasis and Affecting Prognosis in Early-Stage Tongue Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Kanamura, R.; Suzuki, M.; Otozai, S.; Yoshii, T.; Okamoto, H.; Kitamura, Y.; Abe, K.; Fujii, T.; Takeda, N. A new combined index of SUVmax of lymph node in PET / CT by a weighting coefficient plus its maximum minor axis in CECT to evaluate occult lymph node metastasis in clinical N0 patients with tongue cancer. J. Med. Invest. 2021, 68, 154–158. [Google Scholar] [CrossRef]
- Vartak, A.; Malhotra, M.; Jaiswal, P.; Talwar, R.; Tyagi, A.; Kishore, B. Role of (18)F-FDG PET-CT in Guiding Surgical Management of Clinically Node Negative Neck (cN0) in Carcinoma Oral Cavity. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 1799–1805. [Google Scholar] [CrossRef]
- Madsen, C.B.; Rohde, M.; Gerke, O.; Godballe, C.; Sørensen, J.A. Diagnostic Accuracy of Up-Front PET-CT and MRI for Detecting Cervical Lymph Node Metastases in T1-T2 Oral Cavity Cancer-A Prospective Cohort Study. Diagnostics 2023, 13, 3414. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Coatesworth, A.P.; MacLennan, K. Cervical metastases in upper aerodigestive tract squamous cell carcinoma: Histopathologic analysis and reporting. Head Neck 2003, 25, 194–197. [Google Scholar] [CrossRef]
- Goldstein, D.P.; Ringash, J.; Bissada, E.; Jaquet, Y.; Irish, J.; Chepeha, D.; Davis, A.M. Scoping review of the literature on shoulder impairments and disability after neck dissection. Head Neck 2014, 36, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hanai, N.; Eba, J.; Mizusawa, J.; Asakage, T.; Homma, A.; Kiyota, N.; Fukuda, H.; Hayashi, R. Randomized phase III study to evaluate the value of omission of prophylactic neck dissection for stage I/II tongue cancer: Japan Clinical Oncology Group study (JCOG1601, RESPOND). Jpn. J. Clin. Oncol. 2018, 48, 1105–1108. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Saitoh, M.; Notani, K.; Tei, K.; Totsuka, Y.; Takinami, S.; Kanegae, K.; Inubushi, M.; Tamaki, N.; Kitagawa, Y. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann. Nucl. Med. 2008, 22, 177–184. [Google Scholar] [CrossRef]
- Bhargava, P.; Rahman, S.; Wendt, J. Atlas of confounding factors in head and neck PET-CT imaging. Clin. Nucl. Med. 2011, 36, 20–29. [Google Scholar] [CrossRef]
- Paidpally, V.; Chirindel, A.; Lam, S.; Agrawal, N.; Quon, H.; Subramaniam, R.M. FDG-PET-CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012, 4, 633–647. [Google Scholar] [CrossRef]
- Purohit, B.S.; Ailianou, A.; Dulguerov, N.; Becker, C.D.; Ratib, O.; Becker, M. FDG-PET-CT pitfalls in oncological head and neck imaging. Insights Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Katirtzidou, E.; Rager, O.; Varoquaux, A.D.; Poncet, A.; Lenoir, V.; Dulguerov, N.; Platon, A.; Garibotto, V.; Zaidi, H.; Becker, M. Detection of distant metastases and distant second primary cancers in head and neck squamous cell carcinoma: Comparison of [(18)F]FDG PET/MRI and [(18)F]FDG PET-CT. Insights Imaging 2022, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, P.A.; Evangelou, E.; Denaxa-Kyza, D.; Ioannidis, J.P. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: A meta-Analysis. J. Natl. Cancer Inst. 2008, 100, 712–720. [Google Scholar] [CrossRef]
- Liao, L.J.; Lo, W.C.; Hsu, W.L.; Wang, C.T.; Lai, M.S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-A meta-Analysis comparing different imaging modalities. BMC Cancer 2012, 12, 236. [Google Scholar] [CrossRef]
- Kim, S.J.; Pak, K.; Kim, K. Diagnostic accuracy of F-18 FDG PET or PET-CT for detection of lymph node metastasis in clinically node negative head and neck cancer patients; A systematic review and meta-analysis. Am. J. Otolaryngol. 2019, 40, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; van den Brekel, M.M.W.; Maroldi, R. ESR Bridges: Imaging and treatment of extranodal spread in head and neck cancer-a multidisciplinary view. Eur. Radiol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Giannitto, C.; Mercante, G.; Ammirabile, A.; Cerri, L.; De Giorgi, T.; Lofino, L.; Vatteroni, G.; Casiraghi, E.; Marra, S.; Esposito, A.A.; et al. Radiomics-Based machine learning for the diagnosis of lymph node metastases in patients with head and neck cancer: Systematic review. Head Neck 2023, 45, 482–491. [Google Scholar] [CrossRef]
- Becker, M.; de Vito, C.; Dulguerov, N.; Zaidi, H. PET/MR Imaging in Head and Neck Cancer. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 539–564. [Google Scholar] [CrossRef]

| Country | Type of Study | Patients (n) | Reference Standard | cN0 Definition | TP | TN | FP | FN | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | T stages Included | Histological Analysis | PET/CT Indicating Positive Nodes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schöder 2006 [20] | United states | Prospective | 31 | Neck level (142) | Clinical + CT/MRI/US | 6 | 127 | 6 | 3 | 67 | 95 | 94 | 50 | 98 | T1/T2/T3/T4 | Serial analysis, H&E staining | Non-quantitative, visual focal uptake > background |
| Neck side (36) | Clinical + CT/MRI/US | 6 | 23 | 4 | 3 | 67 | 85 | 80 | 60 | 88 | |||||||
| Piao 2009 [21] | Japan | Retrospective | 56 | Neck level (345) | Neck palpation | 71 | 236 | 24 | 14 | 83.5 | 90.8 | 89 | 74.7 | 94.4 | Not mentionned | Not mentionned | SUV > 2.5 |
| Lymph nodes (2705) | Neck palpation | 103 | 2501 | 40 | 61 | 62.8 | 98.4 | 96.3 | 72 | 97.6 | |||||||
| Chauhan 2012 [22] | India | Prospective | 49 | Neck side (51) | Neck palpation | 15 | 29 | 1 | 6 | 71.4 | 96.7 | 86.3 | 93.5 | 82.9 | T1/T2/T3/T4 | Standard analysis H&E staining | Non-quantitative, visual focal uptake > background |
| Cetin 2013 [23] | Turkey | Retrospective | 36 | Patient | Clinical + CT/MRI/US | 16 | 11 | 6 | 3 | 84.2 | 76.5 | 75 | 72.7 | 78.6 | T1/T2/T3/T4 | Standard analysis H&E staining | Non-quantitative, visual focal uptake > background |
| Roh 2014 [24] | Korea | Prospective | 91 | Patient | Neck palpation | 27 | 43 | 10 | 11 | 71 | 81 | 77 | 73 | 80 | T1/T2/T3/T4 | Standard analysis H&E staining | Non-quantitative, visual focal uptake > background |
| Neck side (121) | Neck palpation | 31 | 66 | 12 | 12 | 72 | 85 | 80 | 72 | 85 | |||||||
| Neck level (466) | Neck palpation | 48 | 366 | 30 | 22 | 69 | 92 | 89 | 62 | 94 | |||||||
| Lee 2015 [25] | Korea | Retrospective | 39 | Patient | Neck palpation | 15 | 18 | 1 | 5 | 75 | 94.7 | 84.6 | 93.8 | 78.3 | T1/T2/T3/T4 | Standard analysis H&E staining | SUVmax > 2.5 |
| Neck level (210) | Neck palpation | 23 | 171 | 4 | 12 | 65.7 | 97.7 | 92.4 | 85.2 | 93.4 | |||||||
| Sohn 2016 [26] | Korea | Retrospective | 49 | Patient | Neck palpation | 16 | 22 | 2 | 9 | 64 | 91.7 | 77.6 | 88.9 | 71 | T1/T2/T3/T4 | Standard analysis H&E staining | SUVmax ≥ 2.5 |
| Neck level (162) | Neck palpation | 18 | 127 | 2 | 15 | 54.6 | 98.5 | 89.5 | 90 | 89.4 | |||||||
| Chaukar 2016 [27] | India | Prospective | 70 | Neck side (85) | Neck palpation | 30 | 26 | 22 | 7 | 82 | 54 | 66 | 57 | 79 | Not mentionned | Not mentionned | SUV > 2.5 |
| Zhang 2018 [28] | Canada | Retrospective | 32 | Lymph nodes | Neck palpation | 3 | 1237 | 20 | 11 | 21.4 | 98.4 | 97.6 | 13 | 99.1 | T1/T2 | Not mentionned | SUVmax > 2.5 |
| Niu 2020 [29] | China | Prospective | 78 | Neck side (98) | Neck palpation | 26 | 49 | 18 | 5 | 83.9 | 73.1 | 76.5 | 59.1 | 90.7 | T1/T2/T3/T4 | Serial analysis H&E staining | Non-quantitative with defined criteria * |
| Bae 2020 [30] | Korea | Prospective | 178 | Patient | Neck palpation | 29 | 106 | 30 | 13 | 69.1 | 77.9 | 75.8 | 49.2 | 89.1 | T1/T2/T3/T4 | Serial analysis H&E staining | Non-quantitative, visual focal uptake > background |
| Neck side (199) | Neck palpation | 31 | 122 | 33 | 13 | 70.5 | 78.7 | 76.9 | 48.4 | 90.4 | |||||||
| Neck level (678) | Neck palpation | 36 | 553 | 67 | 22 | 62.1 | 89.2 | 86.9 | 35.0 | 96.2 | |||||||
| Xu 2020 [31] | China | Prospective | 120 | Patient | Clinical + CT/MRI/US | 14 | 94 | 8 | 4 | 77.8 | 92.2 | 90 | 63.6 | 95.9 | T1/T2 | Not mentionned | SUVmax > 2.5 |
| Zhao 2020 [32] | China | Prospective | 135 | Patient | Clinical + CT/MRI/US | 12 | 112 | 6 | 5 | 70.6 | 94.9 | 91.9 | 66.7 | 95.7 | T1/T2 | Not mentionned | SUVmax > 2.5 |
| Kanamura 2021 [33] | Japan | Retrospective | 57 | Patient | Clinical + CT | 14 | 27 | 13 | 3 | 82.4 | 67.5 | 72 | 51.9 | 90 | Not mentionned | Not mentionned | SUVmax > 2.0 with combined index |
| Neck level (141) | Clinical + CT | 15 | 119 | 22 | 7 | 68.2 | 84.4 | 82.2 | 40.5 | 94.4 | |||||||
| Vartak 2023 [34] | India | Prospective | 51 | Neck side (58) | Clinical + CT/MRI/US | 9 | 42 | 6 | 1 | 90 | 87.5 | 87.9 | 60 | 97.7 | T1/T2 | Serial H&E staining | SUV max calculated by the inbuilt software of the PET |
| Madsen 2023 [35] | Denmark | Prospective | 76 | Patient | Clinical + US+/- FNAC | 23 | 27 | 18 | 8 | 74 | 60 | 66 | 56 | 77 | T1/T2 | Not mentionned | Subjective |
| Variables | Diagnostic Estimate | Regression | SE | z | p | 95%CI | |
|---|---|---|---|---|---|---|---|
| Type of study (Retrospective vs. Prospective) | Sensitivity | 0.001 | 0.201 | 0.007 | 0.995 | −0.392 | 0.395 |
| False positive rate | −0.924 | 0.464 | −1.993 | 0.046 | −1.834 | −0.015 | |
| Sample size | Sensitivity | −0.001 | 0.002 | −0.613 | 0.540 | −0.005 | 0.003 |
| False positive rate | 0.007 | 0.005 | 1.285 | 0.199 | −0.003 | 0.017 | |
| Definition of cN0 (clinico-radiological vs. Clinical) | Sensitivity | −0.232 | 0.232 | −0.999 | 0.318 | −0.687 | 0.223 |
| False positive rate | −0.644 | 0.494 | −1.303 | 0.193 | −1.614 | 0.325 | |
| Year | Sensitivity | −0.021 | 0.023 | −0.899 | 0.369 | −0.066 | 0.025 |
| False positive rate | 0.089 | 0.047 | 1.902 | 0.057 | −0.003 | 0.180 | |
| Histological processing (Routine vs. Serial) | Sensitivity | 0.18 | 0.334 | 0.54 | 0.589 | -0.474 | 0.835 |
| False positive rate | −0.09 | 0.669 | −0.134 | 0.893 | −1.401 | 1.221 | |
| Reference standard (Neck Level vs. Neck Side and Whole neck) | Sensitivity | 0.485 | 0.325 | 1.491 | 0.136 | −0.152 | 1.122 |
| False positive rate | 1.511 | 0.629 | 2.402 | 0.016 | 0.278 | 2.744 | |
| Reference standard (Neck Side vs. Neck Level and Patient) | Sensitivity | 0.833 | 0.345 | 2.412 | 0.016 | 0.156 | 1.510 |
| False positive rate | 2.725 | 0.635 | 4.288 | 0.0000 | 1.479 | 3.970 | |
| Reference standard (Patient vs. Neck Level and Neck Side) | Sensitivity | 0.715 | 0.331 | 2.158 | 0.031 | 0.066 | 1.365 |
| False positive rate | 2.590 | 0.619 | 4.182 | 0.0000 | 1.376 | 3.804 | |
| Localization (Other vs. Oral cavity) | Sensitivity | 0.105 | 0.208 | 0.504 | 0.614 | −0.303 | 0.513 |
| False positive rate | −0.813 | 0.473 | −1.72 | 0.085 | −1.74 | 0.113 | |
| Contrast (Non-injected vs. Injected) | Sensitivity | 0.318 | 0.311 | 1.023 | 0.306 | −0.291 | 0.928 |
| False positive rate | −0.493 | 0.647 | −0.762 | 0.446 | −1.762 | 0.775 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedj, D.; Neveü, S.; Becker, M.; Mermod, M. FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2954. https://doi.org/10.3390/cancers16172954
Guedj D, Neveü S, Becker M, Mermod M. FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(17):2954. https://doi.org/10.3390/cancers16172954
Chicago/Turabian StyleGuedj, Danaé, Sophie Neveü, Minerva Becker, and Maxime Mermod. 2024. "FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis" Cancers 16, no. 17: 2954. https://doi.org/10.3390/cancers16172954
APA StyleGuedj, D., Neveü, S., Becker, M., & Mermod, M. (2024). FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Cancers, 16(17), 2954. https://doi.org/10.3390/cancers16172954






