Impaired DNA Double-Strand Break Repair in Irradiated Sheep Lung Fibroblasts: Late Effects of Previous Irradiation of the Spinal Thecal Sac
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sheep Treatment
- -
- Low-dose (LD) region: area of the sheep that received a total dose lower than 2 Gy.
- -
- High-dose (HD) region: area of the sheep that received a total dose higher than 70% of the total dose to the thecal sac, i.e., more than 18 Gy.
2.2. Sheep Euthanasia and Lung Tissue Sampling
2.3. Primary Cell Culture
2.4. Cell Line Irradiation
2.5. Trypan Blue Exclusion Assay
2.6. Immunofluorescence
2.7. Cell Survival Clonogenic Assay
2.8. Statistical Analysis
3. Results
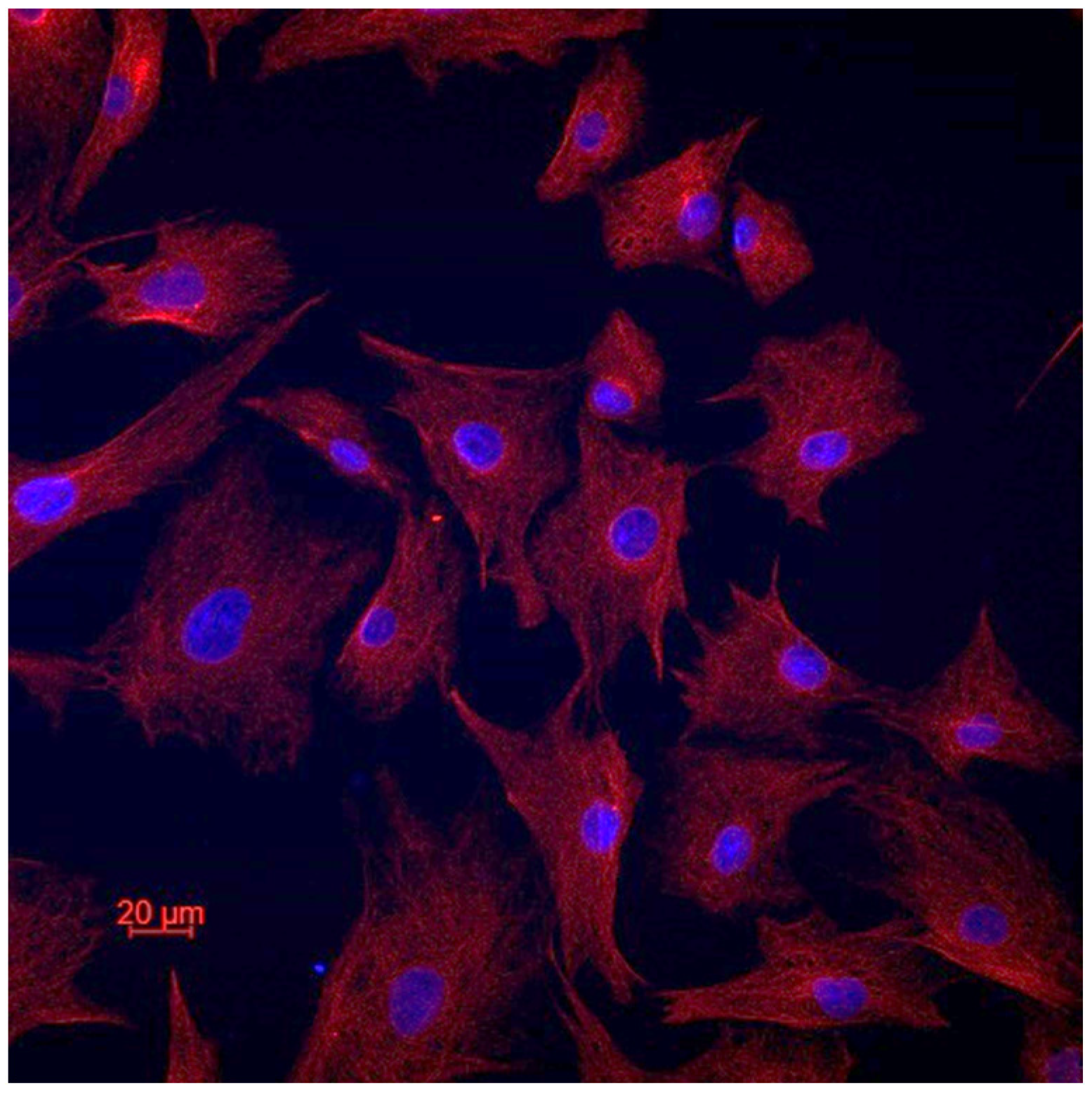
3.1. Fibroblastic Nature of the Established Cell Lines
3.2. Previous Irradiation Does Not Affect Cellular Viability
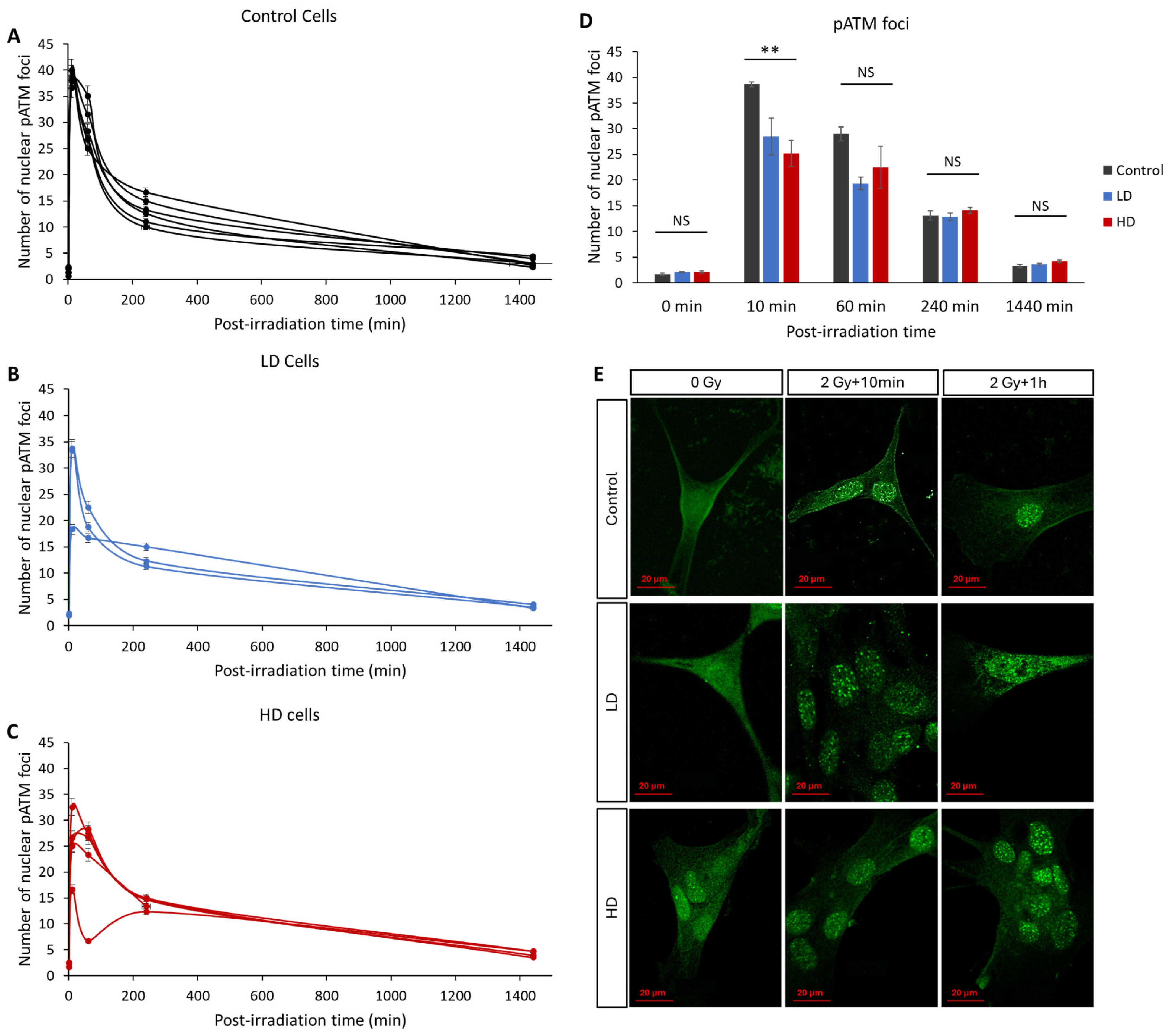
3.3. Previous Irradiation Impairs DNA DSB Signaling and Repair
3.4. ATM Nucleoshuttling Is Delayed in Previously Treated Cells
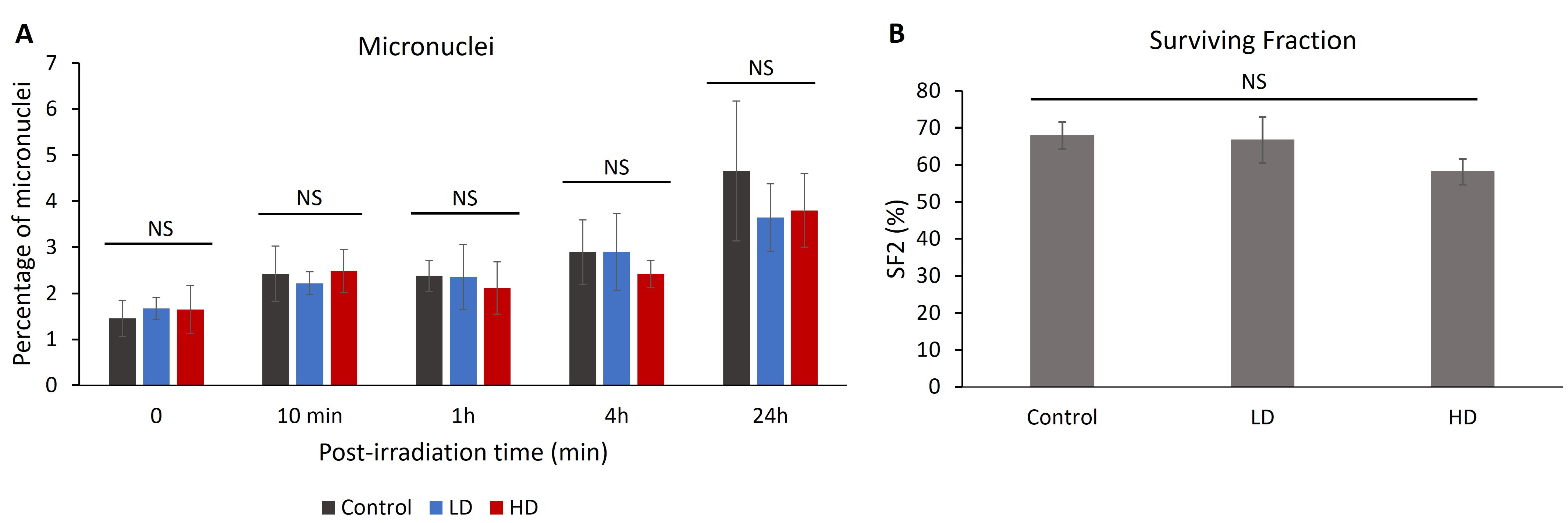
3.5. Previous Irradiation Did Not Affect the Percentage of Radio-Induced Micronuclei or Cell Survival
4. Discussion
4.1. Pediatric Radiotherapy: Long-Term Consequences
- Animal size: This study required an animal model the size of a very young human, in order to have the most relevant treatment plan. It is noteworthy that the bone structure and composition of sheep are very similar to those of the human body [40].
- Life expectancy: This study required an animal model with a life expectancy long enough to observe any effects, but not too long for technical considerations. The life expectancy of sheep ranges between 8 and 10 years, which made them ideal for this project.
- Adult age: This study required an animal that reaches adulthood at ages 2–4 years in order to assess the effect of early pediatric treatment at adulthood.
- Radiosensitivity and DNA repair: The non-homologous end-joining and the homologous recombination repair pathways, which involved ATM and H2AX, are assumed to be the two DSB repair pathways present in mammalian cells [41,42]. Specifically, γH2AX is detected in all tested mammalian cells [43,44,45]. This justifies our focus on these two proteins. Moreover, as highlighted in [15], and as confirmed in our results, the cellular and molecular radiosensitivity of sheep was shown to be comparable to that of humans.
4.2. Previous Irradiation Can Radiosensitize Cells by Impairing DNA DSB Signaling and Repair
- Group I: radioresistant. Cells from this group show efficient DNA DSB repair and fast ATM nucleoshuttling. The usual number of residual γH2AX foci in this group ranges between 0 and 2, and the number of pATM foci at 10 min is higher than 35.
- Group II: moderately radiosensitive. Cells show less efficient DNA DSB repair and slower ATM nucleoshuttling. These can be radiosensitive and/or with high cancer proneness. The number of residual γH2AX foci is between 2 and 8 and the number of pATM foci is between 25 and 35.
- Group III: hyper-radiosensitive. These usually include DNA repair genetic mutation, such as that present in ataxia telangiectasia. This group has a high risk of cancer and more than eight residual γH2AX foci.
- A small but significant increase in the number of basal DNA DSBs was detected, as highlighted by the number of γH2AX foci without irradiation (Figure 3). Although the increase was small, the significant difference shows that the previously irradiated cells can continuously have remaining DNA DSBs, even without any exposure to genotoxic stress. Many factors contribute to this phenomenon, for example, the unrepaired DSBs from environmental stress, or an increase in the activity of reactive oxygen species. Studies have shown that this can also be a sign of cell aging [46].
- A significant decrease in the number of recognized DSBs, highlighted by the number of γH2AX foci 10 min after irradiation. Knowing that the number of radio-induced DSBs should be the same, this shows that LD and HD cells might have a DSB signaling problem.
- A significant decrease in the ATM activity 10 min after irradiation. Knowing that ATM acts early after exposure to genotoxic stress, multiple studies have shown that any decrease or delay in ATM activity can lead to genomic instability and increased radiosensitivity.
- A significant increase in the number of residual γH2AX foci 24 h after irradiation. This means that previous irradiation might have affected the capacity of the treated cells to repair their DNA DSBs upon re-irradiation.
4.3. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Ajithkumar, T.; Horan, G.; Padovani, L.; Thorp, N.; Timmermann, B.; Alapetite, C.; Gandola, L.; Ramos, M.; Van Beek, K.; Christiaens, M.; et al. SIOPE—Brain tumor group consensus guideline on craniospinal target volume delineation for high-precision radiotherapy. Radiother. Oncol. 2018, 128, 192–197. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model*. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.; Lane, D. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Jones, R.G.; Thompson, C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef]
- Iggo, R.; Bartek, J.; Lane, D.; Gatter, K.; Harris, A.L.; Bartek, J. Increased expression of mutant forms of p53 oncogene in primary lung cancer. The Lancet 1990, 335, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Granzotto, A.; Devic, C.; Bodgi, L.; Ferlazzo, M.; Peaucelle, C.; Bajard, M.; Giraud, J.Y.; Balosso, J.; Hérault, J.; et al. Influence of Linear Energy Transfer on the Nucleo-shuttling of the ATM Protein: A Novel Biological Interpretation Relevant for Particles and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 709–718. [Google Scholar] [CrossRef]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef]
- Le Reun, E.; Granzotto, A.; Pêtre, A.; Bodgi, L.; Beldjoudi, G.; Lacornerie, T.; Vallet, V.; Bouchet, A.; Al-Choboq, J.; Bourguignon, M.; et al. Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy. Cancers 2023, 15, 3979. [Google Scholar] [CrossRef]
- Le Reun, E.; Bodgi, L.; Granzotto, A.; Sonzogni, L.; Ferlazzo, M.L.; Al-Choboq, J.; El-Nachef, L.; Restier-Verlet, J.; Berthel, E.; Devic, C.; et al. Quantitative Correlations between Radiosensitivity Biomarkers Show That the ATM Protein Kinase Is Strongly Involved in the Radiotoxicities Observed after Radiotherapy. Int. J. Mol. Sci. 2022, 23, 10434. [Google Scholar] [CrossRef]
- Vogin, G.; Bastogne, T.; Bodgi, L.; Gillet-Daubin, J.; Canet, A.; Pereira, S.; Foray, N. The Phosphorylated ATM Immunofluorescence Assay: A High-performance Radiosensitivity Assay to Predict Postradiation Therapy Overreactions. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Bodgi, L.; Duclos, M.; Canet, A.; Ferlazzo, M.L.; Devic, C.; Granzotto, A.; Deneuve, S.; Vogin, G.; Foray, N. Fast and Binary Assay for Predicting Radiosensitivity Based on the Theory of ATM Nucleo-Shuttling: Development, Validation, and Performance. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Sarukhanov, V.J.; Kolganov, I.M.; Epimahov, V.G. The Comparative Estimation of Animal Radiosensitivity. Radiats. Biol. Radioecol. 2016, 56, 475–480. [Google Scholar]
- DeNunzio, N.J.; Yock, T.I. Modern Radiotherapy for Pediatric Brain Tumors. Cancers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Seidel, C.; Viehweger, C.; Kortmann, R.-D. Is There an Indication for First Line Radiotherapy in Primary CNS Lymphoma? Cancers 2021, 13, 2580. [Google Scholar] [CrossRef]
- Foray, N.; Marot, D.; Gabriel, A.; Randrianarison, V.; Carr, A.M.; Perricaudet, M.; Ashworth, A.; Jeggo, P. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 2003, 22, 2860–2871. [Google Scholar] [CrossRef]
- Renier, W.; Joubert, A.; Bencokova, Z.; Gastaldo, J.; Massart, C.; Foray, N. Consequences of the bleed-through phenomenon in immunofluorescence of proteins forming radiation-induced nuclear foci. Int. J. Radiat. Biol. 2007, 83, 543–549. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Dsouza, K.; Thomas, V.; Mirov, S.; Pérez-Sala, D.; Athar, M.; Thannickal, V.J.; et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 2019, 4, e123253. [Google Scholar] [CrossRef]
- Rothkamm, K.; Kruger, I.; Thompson, L.H.; Lobrich, M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 2003, 23, 5706–5715. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Bodgi, L.; Granzotto, A.; Devic, C.; Vogin, G.; Lesne, A.; Bottollier-Depois, J.F.; Victor, J.M.; Maalouf, M.; Fares, G.; Foray, N. A single formula to describe radiation-induced protein relocalization: Towards a mathematical definition of individual radiosensitivity. J. Theor. Biol. 2013, 333, 135–145. [Google Scholar] [CrossRef]
- Francis, M.; Ahmad, A.; Bodgi, L.; Azzam, P.; Youssef, T.; Abou Daher, A.; Eid, A.A.; Fornoni, A.; Pollack, A.; Marples, B. SMPDL3b modulates radiation-induced DNA damage response in renal podocytes. FASEB J. 2022, 36, e22545. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Panda, S.; Gekara, N.O. Comet and micronucleus assays for analyzing DNA damage and genome integrity. Methods Enzymol. 2019, 625, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Huang, W.Y.; Li, X.S.; Lin, J.S.; Cai, X.K.; Lian, K.H.; Zhou, H.J. Prediction value of radiosensitivity of hepatocarcinoma cells for apoptosis and micronucleus assay. World J. Gastroenterol. 2005, 11, 7036–7039. [Google Scholar] [CrossRef] [PubMed]
- Champion, A.R.; Hanson, J.A.; Court, J.B.; Venables, S.E. The micronucleus assay: An evaluation of its use in determining radiosensitivity in vitro. Mutagenesis 1995, 10, 203–208. [Google Scholar] [CrossRef]
- Puck, T.T.; Markus, P.I. Action of X-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef]
- Deschavanne, P.J.; Debieu, D.; Fertil, B.; Malaise, E.P. Re-evaluation of in vitro radiosensitivity of human fibroblasts of different genetic origins. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 279–293. [Google Scholar] [CrossRef]
- Fertil, B.; Malaise, E.P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Bodgi, L.; Canet, A.; Pujo-Menjouet, L.; Lesne, A.; Victor, J.M.; Foray, N. Mathematical models of radiation action on living cells: From the target theory to the modern approaches. A historical and critical review. J. Theor. Biol. 2016, 394, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.J.; Khater, N.; Zhang, R.; Geara, F.B.; Mahajan, A.; Jalbout, W.; Pérez-Andújar, A.; Youssef, B.; Newhauser, W.D. Inter-institutional comparison of personalized risk assessments for second malignant neoplasms for a 13-year-old girl receiving proton versus photon craniospinal irradiation. Cancers 2015, 7, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.J.; Mahajan, A.; Mirkovic, D.; Zhang, R.; Giebeler, A.; Kornguth, D.; Harvey, M.; Woo, S.; Newhauser, W.D. Predicted risks of second malignant neoplasm incidence and mortality due to secondary neutrons in a girl and boy receiving proton craniospinal irradiation. Phys. Med. Biol. 2010, 55, 7067. [Google Scholar] [CrossRef]
- Taddei, P.J.; Mirkovic, D.; Fontenot, J.D.; Giebeler, A.; Zheng, Y.; Kornguth, D.; Mohan, R.; Newhauser, W.D. Stray radiation dose and second cancer risk for a pediatric patient receiving craniospinal irradiation with proton beams. Phys. Med. Biol. 2009, 54, 2259. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Fontenot, J.D.; Mahajan, A.; Kornguth, D.; Stovall, M.; Zheng, Y.; Taddei, P.J.; Mirkovic, D.; Mohan, R.; Cox, J.D. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys. Med. Biol. 2009, 54, 2277. [Google Scholar] [CrossRef]
- Larson, D.L.; Kroll, S.; Jaffe, N.; Serure, A.; Geopfert, H. Long-term effects of radiotherapy in childhood and adolescence. Am. J. Surg. 1990, 160, 348–351. [Google Scholar] [CrossRef]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- Bhatia, S.; Sklar, C. Second cancers in survivors of childhood cancer. Nat. Rev. Cancer 2002, 2, 124–132. [Google Scholar] [CrossRef]
- Rehman, I.; Smith, R.; Hench, L.L.; Bonfield, W. Structural evaluation of human and sheep bone and comparison with synthetic hydroxyapatite by FT-Raman spectroscopy. J. Biomed. Mater. Res. 1995, 29, 1287–1294. [Google Scholar] [CrossRef]
- Zhao, B.; Rothenberg, E.; Ramsden, D.A.; Lieber, M.R. The molecular basis and disease relevance of non-homologous DNA end joining. Nat. Rev. Mol. Cell Biol. 2020, 21, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, M.; Löbrich, M. One end to rule them all: Non-homologous end-joining and homologous recombination at DNA double-strand breaks. Br. J. Radiol. 2020, 93, 20191054. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Kovalchuk, I.; Koturbash, I.; Kolb, B.; Martin, O.A.; Kovalchuk, O. Induction and persistence of radiation-induced DNA damage is more pronounced in young animals than in old animals. Aging 2011, 3, 609. [Google Scholar] [CrossRef]
- Redon, C.E.; Nakamura, A.J.; Gouliaeva, K.; Rahman, A.; Blakely, W.F.; Bonner, W.M. Q(γ-H2AX), an analysis method for partial-body radiation exposure using γ-H2AX in nonhuman primate lymphocytes. Radiat. Meas. 2011, 46, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Melo, L.; Freitas, V.; Salamone, D.F. Phosphorylated H2AX in parthenogenetically activated, in vitro fertilized and cloned bovine embryos. Zygote 2015, 23, 485–493. [Google Scholar] [CrossRef]
- Turinetto, V.; Giachino, C. Multiple facets of histone variant H2AX: A DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA damage foci: Meaning and significance. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef]
- Banáth, J.P.; Klokov, D.; MacPhail, S.H.; Banuelos, C.A.; Olive, P.L. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer 2010, 10, 4. [Google Scholar] [CrossRef]
- Burma, S.; Chen, B.P.; Murphy, M.; Kurimasa, A.; Chen, D.J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001, 276, 42462–42467. [Google Scholar] [CrossRef]
- Colin, C.; Granzotto, A.; Devic, C.; Viau, M.; Maalouf, M.; Vogin, G.; Joubert, A.; Thomas, C.; Foray, N. MRE11 and H2AX biomarkers in the response to low-dose exposure: Balance between individual susceptibility to radiosensitivity and to genomic instability. Int. J. Low Radiat. 2011, 8, 96–106. [Google Scholar] [CrossRef]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Beaton, L.A.; Marro, L.; Malone, S.; Samiee, S.; Grimes, S.; Malone, K.; Wilkins, R.C. Investigating γH2AX as a Biomarker of Radiosensitivity Using Flow Cytometry Methods. ISRN Radiol. 2013, 2013, 704659. [Google Scholar] [CrossRef]
- Goutham, H.V.; Mumbrekar, K.D.; Vadhiraja, B.M.; Fernandes, D.J.; Sharan, K.; Kanive Parashiva, G.; Kapaettu, S.; Bola Sadashiva, S.R. DNA double-strand break analysis by gamma-H2AX foci: A useful method for determining the overreactors to radiation-induced acute reactions among head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e607–e612. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Höhne, K.; von Neubeck, C.; Thames, H.D.; Yaromina, A.; Dahm-Daphi, J.; Baumann, M.; Krause, M. Residual γH2AX foci predict local tumour control after radiotherapy. Radiother. Oncol. 2013, 108, 434–439. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, S.; Banath, J.; Yu, T.; Chu, E.; Lambur, H.; Olive, P. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int. J. Radiat. Biol. 2003, 79, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Meneceur, S.; Löck, S.; Gudziol, V.; Hering, S.; Bütof, R.; Rehm, M.; Baumann, M.; Krause, M.; von Neubeck, C. Residual gammaH2AX foci in head and neck squamous cell carcinomas as predictors for tumour radiosensitivity: Evaluation in pre-clinical xenograft models and clinical specimens. Radiother. Oncol. 2019, 137, 24–31. [Google Scholar] [CrossRef]
- Menegakis, A.; von Neubeck, C.; Yaromina, A.; Thames, H.; Hering, S.; Hennenlotter, J.; Scharpf, M.; Noell, S.; Krause, M.; Zips, D.; et al. γH2AX assay in ex vivo irradiated tumour specimens: A novel method to determine tumour radiation sensitivity in patient-derived material. Radiother. Oncol. 2015, 116, 473–479. [Google Scholar] [CrossRef]
- Philouze, P.; Gauthier, A.; Lauret, A.; Malesys, C.; Muggiolu, G.; Sauvaigo, S.; Galmiche, A.; Ceruse, P.; Alphonse, G.; Wozny, A.-S.; et al. CD44, γ-H2AX, and p-ATM Expressions in Short-Term Ex Vivo Culture of Tumour Slices Predict the Treatment Response in Patients with Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 877. [Google Scholar] [CrossRef]
- Pouliliou, S.E.; Lialiaris, T.S.; Dimitriou, T.; Giatromanolaki, A.; Papazoglou, D.; Pappa, A.; Pistevou, K.; Kalamida, D.; Koukourakis, M.I. Survival Fraction at 2 Gy and gammaH2AX Expression Kinetics in Peripheral Blood Lymphocytes From Cancer Patients: Relationship With Acute Radiation-Induced Toxicities. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 667–674. [Google Scholar] [CrossRef]
- Widjaja, L.; Werner, R.A.; Krischke, E.; Christiansen, H.; Bengel, F.M.; Bogdanova, N.; Derlin, T. Individual radiosensitivity reflected by γ-H2AX and 53BP1 foci predicts outcome in PSMA-targeted radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 602–612. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tatin, X.; Muggiolu, G.; Sauvaigo, S.; Breton, J. Evaluation of DNA double-strand break repair capacity in human cells: Critical overview of current functional methods. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108388. [Google Scholar] [CrossRef]
- Bodgi, L.; Pujo-Menjouet, L.; Bouchet, A.; Bourguignon, M.; Foray, N. Seventy Years of Dose-response Models: From the Target Theory to the Use of Big Databases Involving Cell Survival and DNA Repair. Radiat. Res. 2024, 202, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Devic, C.; Bodgi, L.; Sonzogni, L.; Pilleul, F.; Ribot, H.; De Charry, C.; Le Moigne, F.; Paul, D.; Carbillet, F.; Munier, M.; et al. Influence of cellular models and individual factor in the biological response to head CT scan exams. Eur. Radiol. Exp. 2022, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Devic, C.; Bodgi, L.; Sonzogni, L.; Pilleul, F.; Ribot, H.; Charry, C.; Le Moigne, F.; Paul, D.; Carbillet, F.; Munier, M.; et al. Influence of cellular models and individual factor in the biological response to chest CT scan exams. Eur. Radiol. Exp. 2022, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Viau, M.; Sonzogni, L.; Ferlazzo, M.L.; Berthel, E.; Pereira, S.; Bodgi, L.; Granzotto, A.; Devic, C.; Fervers, B.; Charlet, L.; et al. DNA Double-Strand Breaks Induced in Human Cells by Twelve Metallic Species: Quantitative Inter-Comparisons and Influence of the ATM Protein. Biomolecules 2021, 11, 1462. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A. Role of oncogenes and tumor-suppressor genes in carcinogenesis: A review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Yeh, J.M.; Ward, Z.J.; Chaudhry, A.; Liu, Q.; Yasui, Y.; Armstrong, G.T.; Gibson, T.M.; Howell, R.; Hudson, M.M.; Krull, K.R.; et al. Life Expectancy of Adult Survivors of Childhood Cancer Over 3 Decades. JAMA Oncol. 2020, 6, 350–357. [Google Scholar] [CrossRef]
- Elzahhar, P.A.; Nematalla, H.A.; Al-Koussa, H.; Abrahamian, C.; El-Yazbi, A.F.; Bodgi, L.; Bou-Gharios, J.; Azzi, J.; Al Choboq, J.; Labib, H.F.; et al. Inclusion of Nitrofurantoin into the Realm of Cancer Chemotherapy via Biology-Oriented Synthesis and Drug Repurposing. J. Med. Chem. 2023, 66, 4565–4587. [Google Scholar] [CrossRef]
- Bodgi, L.; Bou-Gharios, J.; Azzi, J.; Challita, R.; Feghaly, C.; Baalbaki, K.; Kharroubi, H.; Chhade, F.; Geara, F.; Abou-Kheir, W.; et al. Effect of bisphosphonates and statins on the in vitro radiosensitivity of breast cancer cell lines. Pharmacol. Rep. 2023, 76, 171–184. [Google Scholar] [CrossRef]
- Azzi, J.; Waked, A.; Bou-Gharios, J.; Al Choboq, J.; Geara, F.; Bodgi, L.; Maalouf, M. Radiosensitizing Effect of Curcumin on Human Bladder Cancer Cell Lines: Impact on DNA Repair Mechanisms. Nutr. Cancer 2022, 74, 2207–2221. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, B.; Feghaly, C.; Al Choboq, J.; Bou-Gharios, J.; Challita, R.; Azzi, J.; Bou Hadir, H.; Abi Antoun, F.; Araji, T.; Taddei, P.J.; et al. Impaired DNA Double-Strand Break Repair in Irradiated Sheep Lung Fibroblasts: Late Effects of Previous Irradiation of the Spinal Thecal Sac. Cancers 2024, 16, 2968. https://doi.org/10.3390/cancers16172968
Youssef B, Feghaly C, Al Choboq J, Bou-Gharios J, Challita R, Azzi J, Bou Hadir H, Abi Antoun F, Araji T, Taddei PJ, et al. Impaired DNA Double-Strand Break Repair in Irradiated Sheep Lung Fibroblasts: Late Effects of Previous Irradiation of the Spinal Thecal Sac. Cancers. 2024; 16(17):2968. https://doi.org/10.3390/cancers16172968
Chicago/Turabian StyleYoussef, Bassem, Charbel Feghaly, Joelle Al Choboq, Jolie Bou-Gharios, Rafka Challita, Joyce Azzi, Hanine Bou Hadir, Fabienne Abi Antoun, Tarek Araji, Phillip J. Taddei, and et al. 2024. "Impaired DNA Double-Strand Break Repair in Irradiated Sheep Lung Fibroblasts: Late Effects of Previous Irradiation of the Spinal Thecal Sac" Cancers 16, no. 17: 2968. https://doi.org/10.3390/cancers16172968





