Recurrence Rates and Patterns after Radical Resection of Lung Carcinoids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
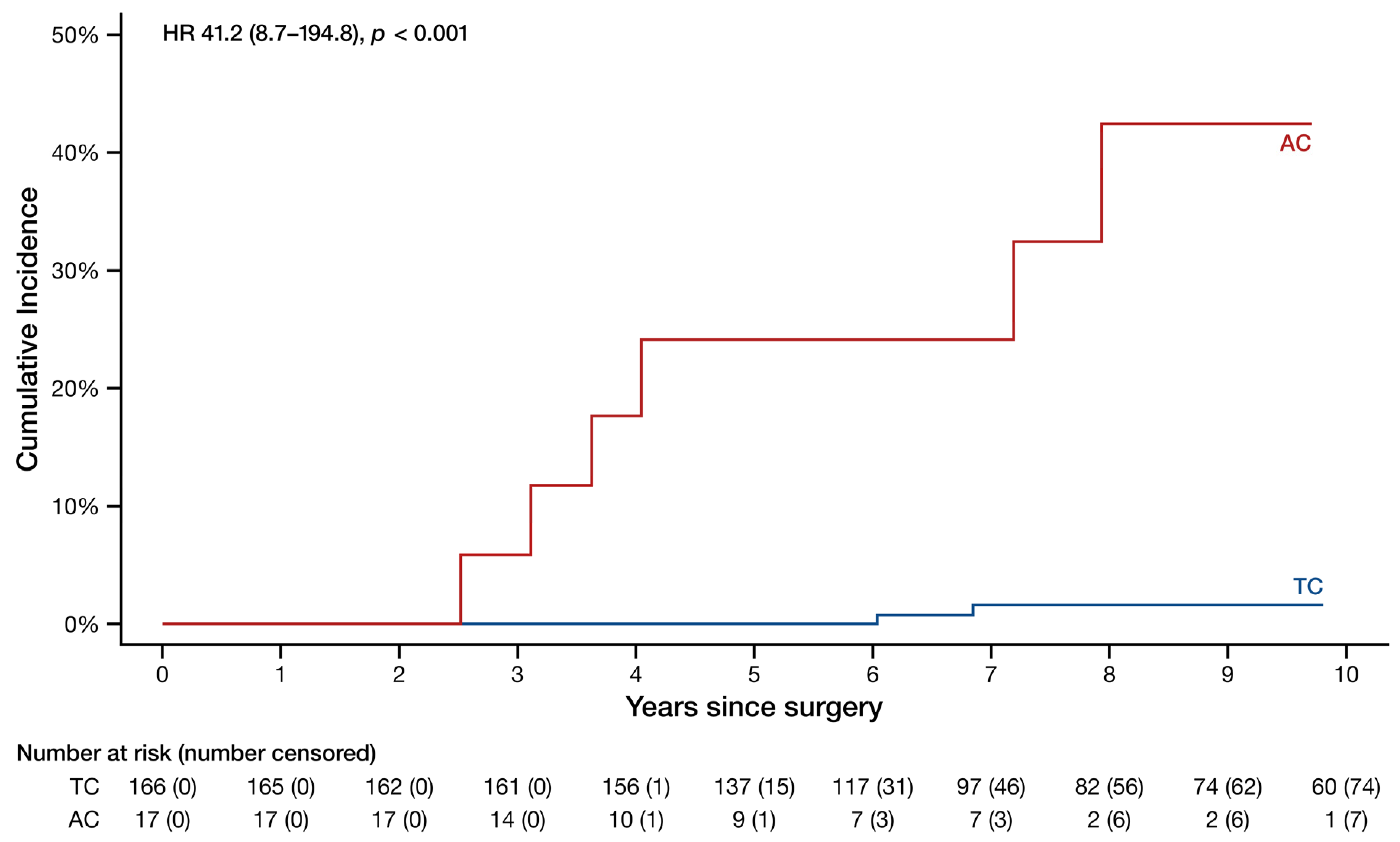
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Soldath, P.; Petersen, R.H. The Surgical Management of Lung Neuroendocrine Neoplasms. Cancers 2023, 15, 1695. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Horsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.J.; Petersen, R.H. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach—The Copenhagen experience. Ann. Cardiothorac. Surg. 2012, 1, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P.; International Association for the Study of Lung Cancer (IASLC) Staging Committee. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Fink, G.; Krelbaum, T.; Yellin, A.; Bendayan, D.; Saute, M.; Glazer, M.; Kramer, M.R. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001, 119, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Yap, Y.K.; De Stavola, B.L.; Nicholson, A.G.; Goldstraw, P. The impact of stage and cell type on the prognosis of pulmonary neuroendocrine tumors. J. Thorac. Cardiovasc. Surg. 2005, 130, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Yuste, M.; Matilla, J.M.; Cueto, A.; Paniagua, J.M.; Ramos, G.; Canizares, M.A.; Muguruza, I. Typical and atypical carcinoid tumours: Analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur. J. Cardiothorac. Surg. 2007, 31, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rea, F.; Rizzardi, G.; Zuin, A.; Marulli, G.; Nicotra, S.; Bulf, R.; Schiavon, M.; Sartori, F. Outcome and surgical strategy in bronchial carcinoid tumors: Single institution experience with 252 patients. Eur. J. Cardiothorac. Surg. 2007, 31, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yan, T.D.; Kennedy, C.; Hendel, N.; Bannon, P.G.; McCaughan, B.C. Bronchopulmonary carcinoid tumors: Long-term outcomes after resection. Ann. Thorac. Surg. 2011, 91, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Ibrahim, M.; Andreetti, C.; D’Andrilli, A.; Ciccone, A.M.; Pomes, L.M.; Menna, C.; Pellegrini, M.; Venuta, F.; Rendina, E.A. Long-term results after resection of bronchial carcinoid tumour: Evaluation of survival and prognostic factors. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Okereke, I.C.; Taber, A.M.; Griffith, R.C.; Ng, T.T. Outcomes after surgical resection of pulmonary carcinoid tumors. J. Cardiothorac. Surg. 2016, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Florisson, D.; Telianidis, S.; Yaftian, N.; Lee, J.; Knight, S.; Barnett, S.; Seevanayagam, S.; Antippa, P.; Alam, N.; et al. Pulmonary carcinoid tumours: A multi-centre analysis of survival and predictors of outcome following sublobar, lobar, and extended pulmonary resections. Asian Cardiovasc. Thorac. Ann. 2021, 29, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Machuca, T.N.; Cardoso, P.F.; Camargo, S.M.; Signori, L.; Andrade, C.F.; Moreira, A.L.; Moreira Jda, S.; Felicetti, J.C.; Camargo, J.J. Surgical treatment of bronchial carcinoid tumors: A single-center experience. Lung Cancer 2010, 70, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Soldath, P.; Binderup, T.; Kjaer, A.; Federspiel, B.; Langer, S.W.; Knigge, U.; Petersen, R.H. Long-term survival and recurrence after resection of bronchopulmonary carcinoids: A single-center cohort study of 236 patients. Lung Cancer 2021, 156, 109–116. [Google Scholar] [CrossRef]
- Lee, P.C.; Osakwe, N.C.; Narula, N.; Port, J.L.; Paul, S.; Stiles, B.M.; Andrews, W.G.; Nasar, A.; Altorki, N.K. Predictors of Disease-free Survival and Recurrence in Patients with Resected Bronchial Carcinoid Tumors. Thorac. Cardiovasc. Surg. 2016, 64, 159–165. [Google Scholar] [CrossRef]
- Lou, F.; Sarkaria, I.; Pietanza, C.; Travis, W.; Roh, M.S.; Sica, G.; Healy, D.; Rusch, V.; Huang, J. Recurrence of pulmonary carcinoid tumors after resection: Implications for postoperative surveillance. Ann. Thorac. Surg. 2013, 96, 1156–1162. [Google Scholar] [CrossRef]
- Filosso, P.L.; Oliaro, A.; Ruffini, E.; Bora, G.; Lyberis, P.; Asioli, S.; Delsedime, L.; Sandri, A.; Guerrera, F. Outcome and prognostic factors in bronchial carcinoids: A single-center experience. J. Thorac. Oncol. 2013, 8, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Sigel, K.; Martin, J.; Jordan, R.; Beasley, M.B.; Smith, C.; Kaufman, A.; Wisnivesky, J.; Kim, M.K. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, J.; Zheng, Q.; Wu, D.; Lu, T.; Lin, M.; Pu, Q.; Mei, J.; Liu, L. A Competing Risk Model Nomogram to Predict the Long-Term Prognosis of Lung Carcinoid. Ann. Surg. Oncol. 2023, 30, 5830–5839. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.S.; Gronbaek, H.; Tabaksblat, E.M.; Dahl Baunwall, S.M.; Dam, G. Prognosis of Patients with Bronchopulmonary Neuroendocrine Neoplasms in a Tertiary Neuroendocrine Tumor Centre of Excellence. Neuroendocrinology 2022, 112, 1214–1224. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall N = 217 1 | TC N = 194 1 | AC N = 23 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 64 (53–71) | 63 (52–70) | 70 (64–73) | 0.016 |
| Sex | >0.99 | |||
| Female | 151 (70) | 135 (70) | 16 (70) | |
| Male | 66 (30) | 59 (30) | 7 (30) | |
| Smoking history | 0.96 | |||
| Current | 34 (16) | 30 (15) | 4 (17) | |
| Former | 99 (46) | 89 (46) | 10 (43) | |
| Never | 84 (39) | 75 (39) | 9 (39) | |
| FEV1 | 88 (76–101) | 89 (76–101) | 88 (75–107) | 0.91 |
| Unknown | 4 | 4 | 0 | |
| DLCO | 80 (66–89) | 79 (65–89) | 83 (69–88) | 0.46 |
| Unknown | 82 | 77 | 5 | |
| Tumor location | 0.63 | |||
| Central | 76 (35) | 69 (36) | 7 (30) | |
| Peripheral | 141 (65) | 125 (64) | 16 (70) | |
| Surgical approach | 0.35 | |||
| Thoracotomy | 55 (25) | 51 (26) | 4 (17) | |
| VATS | 162 (75) | 143 (74) | 19 (83) | |
| Surgical resection | 0.92 | |||
| Bilobectomy | 8 (3.7) | 8 (4.1) | 0 (0) | |
| Bronchial sleeve | 5 (2.3) | 5 (2.6) | 0 (0) | |
| Lobectomy | 150 (69) | 132 (68) | 18 (78) | |
| Pneumonectomy | 1 (0.5) | 1 (0.5) | 0 (0) | |
| Segmentectomy | 6 (2.8) | 5 (2.6) | 1 (4.3) | |
| Sleeve lobectomy | 27 (12) | 25 (13) | 2 (8.7) | |
| Wedge | 20 (9.2) | 18 (9.3) | 2 (8.7) | |
| Pathological T-category | 0.43 | |||
| 1a | 30 (14) | 29 (15) | 1 (4.3) | |
| 1b | 90 (41) | 76 (39) | 14 (61) | |
| 1c | 35 (16) | 33 (17) | 2 (8.7) | |
| 2a | 42 (19) | 38 (20) | 4 (17) | |
| 2b | 6 (2.8) | 6 (3.1) | 0 (0) | |
| 3 | 12 (5.5) | 10 (5.2) | 2 (8.7) | |
| 4 | 2 (0.9) | 2 (1.0) | 0 (0) | |
| Pathological N-category | 0.03 | |||
| 0 | 183 (84) | 166 (86) | 17 (74) | |
| 1 | 10 (4.6) | 7 (3.6) | 3 (13) | |
| 2 | 11 (5.1) | 8 (4.1) | 3 (13) | |
| Unknown | 13 (6.0) | 13 (6.7) | 0 (0) | |
| Resection margin | 0.17 | |||
| Less than 2 cm | 139 (66) | 121 (64) | 18 (78) | |
| More than 2 cm | 73 (34) | 68 (36) | 5 (22) | |
| Unknown | 5 | 5 | 0 | |
| Ki-67 | 4.0 (2.0–6.0) | 4.0 (2.0–5.0) | 14.0 (10.0–20.0) | <0.001 |
| Unknown | 6 | 6 | 0 |
| TC | AC | |||
|---|---|---|---|---|
| Category | Clinical, N = 194 1 | Pathological, N = 194 1 | Clinical, N = 23 1 | Pathological, N = 23 1 |
| T-category | ||||
| T1 | 115 (59) | 138 (71) | 17 (74) | 17 (74) |
| T2 | 53 (27) | 44 (23) | 5 (22) | 4 (17) |
| T3 | 13 (6.7) | 10 (5.2) | 1 (4.3) | 2 (8.7) |
| T4 | 9 (4.6) | 2 (1.0) | ||
| Unknown | 4 (2.1) | |||
| N-category | ||||
| N0 | 166 (86) | 166 (86) | 20 (87) | 17 (74) |
| N1 | 12 (6.2) | 7 (3.6) | 3 (13) | 3 (13) |
| N2 | 11 (5.7) | 8 (4.1) | 3 (13) | |
| Unknown | 5 (2.6) | 13 (6.7) | ||
| Subtype | Resection Type | Tumor Location | Pathological T-Category | Pathological N-Category | Resection Margin | Ki-67 | Recurrence Type | Recurrence Site | Recurrence Diagnosis | Histological Confirmation | Years to Recurrence | Recurrence Treatment | Status | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | Lobectomy | Peripheral | 1b | N0 | Below 2 cm | 3 | Distant | Contralateral lung | Coughing | Yes | 6.0 | Segmentectomy | Alive | — |
| TC | Sleeve lobectomy | Central | 3 | N0 | Below 2 cm | 5 | Local, regional, and distant | Ipsilateral lung, mediastinum, and bones | Dyspnea | No | 11.9 | None | Deceased | Disease related |
| TC | Bilobectomy | Central | 1c | N0 | Below 2 cm | 8 | Distant | Liver | Scheduled scan | Yes | 6.8 | PRRT and SSA | Alive | — |
| TC | Wedge | Peripheral | 1a | Unknown | Below 2 cm | 3 | Local | Ipsilateral lung | Scheduled scan | No | 1.9 | None | Alive | — |
| TC | Wedge | Peripheral | 1a | Unknown | Below 2 cm | 5 | Local | Ipsilateral lung | Scheduled scan | Yes | 0.6 | Wedge resection and SSA | Alive | — |
| AC | Lobectomy | Central | 1b | N0 | Below 2 cm | 13 | Distant | Liver | Scheduled scan | Yes | 7.9 | Microwave ablation | Alive | — |
| AC | Lobectomy | Central | 3 | N0 | Below 2 cm | 13 | Distant | Ileum | Ileus | Yes | 7.2 | Bowel resection | Alive | — |
| AC | Lobectomy | Peripheral | 1a | N0 | Below 2 cm | 10 | Distant | Liver | Scheduled scan | No | 3.6 | SSA | Deceased | Disease related |
| AC | Lobectomy | Peripheral | 1b | N0 | Below 2 cm | 15 | Local and distant | Both lungs and right adrenal gland | Scheduled scan | Yes | 2.5 | SSA | Deceased | Disease related |
| AC | Lobectomy | Peripheral | 1b | N0 | Above 2 cm | 20 | Local, regional, and distant | Mediastinum, liver, and bones | Scheduled scan | No | 4.0 | PRRT and SSA | Deceased | Disease related |
| AC | Lobectomy | Peripheral | 2a | N0 | Below 2 cm | 16 | Distant | Pancreas and brain | Headache | Yes | 3.1 | Brain resection and WBRT | Deceased | Disease related |
| AC | Lobectomy | Peripheral | 2a | N1 | Below 2 cm | 10 | Distant | Liver | Scheduled scan | Yes | 14.2 | SSA | Deceased | Disease related |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askildsen, E.; Soldath, P.; Langer, S.W.; Andreassen, M.; Knigge, U.; Petersen, R.H. Recurrence Rates and Patterns after Radical Resection of Lung Carcinoids. Cancers 2024, 16, 2978. https://doi.org/10.3390/cancers16172978
Askildsen E, Soldath P, Langer SW, Andreassen M, Knigge U, Petersen RH. Recurrence Rates and Patterns after Radical Resection of Lung Carcinoids. Cancers. 2024; 16(17):2978. https://doi.org/10.3390/cancers16172978
Chicago/Turabian StyleAskildsen, Erika, Patrick Soldath, Seppo W. Langer, Mikkel Andreassen, Ulrich Knigge, and René Horsleben Petersen. 2024. "Recurrence Rates and Patterns after Radical Resection of Lung Carcinoids" Cancers 16, no. 17: 2978. https://doi.org/10.3390/cancers16172978
APA StyleAskildsen, E., Soldath, P., Langer, S. W., Andreassen, M., Knigge, U., & Petersen, R. H. (2024). Recurrence Rates and Patterns after Radical Resection of Lung Carcinoids. Cancers, 16(17), 2978. https://doi.org/10.3390/cancers16172978







