Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. Global variations in lung cancer incidence by histological subtype in 2020: A population-based study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Myall, N.J.; Yu, H.; Soltys, S.G.; Wakelee, H.A.; Pollom, E. Management of brain metastases in lung cancer: Evolving roles for radiation and systemic treatment in the era of targeted and immune therapies. Neurooncol. Adv. 2021, 3, v52–v62. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Ciftci, R.; Kilic, L.; Karabulut, S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol. Lett. 2013, 6, 1507–1513. [Google Scholar] [CrossRef]
- Lamba, N.; Kearney, R.B.; Catalano, P.J.; Hassett, M.J.; Wen, P.Y.; Haas-Kogan, D.A.; Aizer, A.A. Population-based estimates of survival among elderly patients with brain metastases. Neuro Oncol. 2021, 23, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Li, R.; Tian, Y.; Zhang, J.; Lin, J.; Liang, Y.; Xu, A.; Zheng, R.; Liu, M.; et al. Tumor Primary Site and Histology Subtypes Role in Radiotherapeutic Management of Brain Metastases. Front. Oncol. 2020, 10, 781. [Google Scholar] [CrossRef]
- Souza, V.G.P.; de Araújo, R.P.; Santesso, M.R.; Seneda, A.L.; Minutentag, I.W.; Felix, T.F.; Hamamoto Filho, P.T.; Pewarchuk, M.E.; Brockley, L.J.; Marchi, F.A.; et al. Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers 2023, 15, 722. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Prim. 2015, 1, 15009. [Google Scholar] [CrossRef]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef]
- Li, N.; Chu, Y.; Song, Q. Brain Metastasis in Patients with Small Cell Lung Cancer. Int. J. Gen. Med. 2021, 14, 10131–10139. [Google Scholar] [CrossRef]
- Schiff, D.; Alyahya, M. Neurological and Medical Complications in Brain Tumor Patients. Curr. Neurol. Neurosci. Rep. 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Noé, J.; Lovejoy, A.; Ou, S.I.; Yaung, S.J.; Bordogna, W.; Klass, D.M.; Cummings, C.A.; Shaw, A.T. ALK Mutation Status Before and After Alectinib Treatment in Locally Advanced or Metastatic ALK-Positive NSCLC: Pooled Analysis of Two Prospective Trials. J. Thorac. Oncol. 2020, 15, 601–608. [Google Scholar] [CrossRef]
- Le, X.; Elamin, Y.Y.; Zhang, J. New Actions on Actionable Mutations in Lung Cancers. Cancers 2023, 15, 2917. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Ahluwalia, M.S. Targeted therapy of brain metastases: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Blumenthal, G.M.; Jiang, X.; He, K.; Boyd, K.; Murgo, A.; Justice, R.; Keegan, P.; Pazdur, R.U.S. Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist 2014, 19, 774–779. [Google Scholar] [CrossRef]
- Pruitt, A.A. Epidemiology, Treatment, and Complications of Central Nervous System Metastases. Continuum 2017, 23, 1580–1600. [Google Scholar] [CrossRef]
- Ene, C.I.; Ferguson, S.D. Surgical Management of Brain Metastasis: Challenges and Nuances. Front. Oncol. 2022, 12, 847110. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- May, L.; Shows, K.; Nana-Sinkam, P.; Li, H.; Landry, J.W. Sex Differences in Lung Cancer. Cancers 2023, 15, 3111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Dmukauskas, M.; Cioffi, G.; Waite, K.A.; Sloan, A.E.; Neff, C.; Price, M.; Ostrom, Q.T.; Barnholtz-Sloan, J.S. Sex differences in adverse events in Medicare individuals ≥66 years of age post glioblastoma treatment. J. Neurooncol. 2024, 168, 111–123. [Google Scholar] [CrossRef]
- Merchant, T.E.; Pollack, I.F.; Loeffler, J.S. Brain tumors across the age spectrum: Biology, therapy, and late effects. Semin. Radiat. Oncol. 2010, 20, 58–66. [Google Scholar] [CrossRef]
- Carr, M.T.; Hochheimer, C.J.; Rock, A.K.; Dincer, A.; Ravindra, L.; Zhang, F.L.; Opalak, C.F.; Poulos, N.; Sima, A.P.; Broaddus, W.C. Comorbid Medical Conditions as Predictors of Overall Survival in Glioblastoma Patients. Sci. Rep. 2019, 9, 20018. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; LeBlanc, M.; Minasian, L.M.; Gotay, C.C.; Henry, N.L.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 1474–1486. [Google Scholar] [CrossRef]
- Edward, R.; Berchick, E.H.; Barnett, J.C. Health Insurance Coverage in the United States: 2017; United States Census Bureau: Suitland, MA, USA, 2018.
- Ascha, M.S.; Ostrom, Q.T.; Wright, J.; Kumthekar, P.; Bordeaux, J.S.; Sloan, A.E.; Schumacher, F.R.; Kruchko, C.; Barnholtz-Sloan, J.S. Lifetime Occurrence of Brain Metastases Arising from Lung, Breast, and Skin Cancers in the Elderly: A SEER-Medicare Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity Measures for Use with Administrative Data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Amsbaugh, M.J.; Kim, C.S. Brain Metastasis; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Tewarie, I.A.; Jessurun, C.A.C.; Hulsbergen, A.F.C.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D. Leptomeningeal disease in neurosurgical brain metastases patients: A systematic review and meta-analysis. Neurooncol. Adv. 2021, 3, vdab162. [Google Scholar] [CrossRef]
- Cooper, M.R.; Chim, H.; Chan, H.; Durand, C. Ceritinib: A new tyrosine kinase inhibitor for non-small-cell lung cancer. Ann. Pharmacother. 2015, 49, 107–112. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Xie, L.; Nagpal, S.; Wakelee, H.A.; Li, G.; Soltys, S.G.; Neal, J.W. Osimertinib for EGFR-Mutant Lung Cancer with Brain Metastases: Results from a Single-Center Retrospective Study. Oncologist 2019, 24, 836–843. [Google Scholar] [CrossRef]
- Tomasini, P.; Egea, J.; Souquet-Bressand, M.; Greillier, L.; Barlesi, F. Alectinib in the treatment of ALK-positive metastatic non-small cell lung cancer: Clinical trial evidence and experience with a focus on brain metastases. Ther. Adv. Respir. Dis. 2019, 13, 1753466619831906. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Eldredge, C.; Cho, C.C.; Cisler, R.A. Population Analysis of Adverse Events in Different Age Groups Using Big Clinical Trials Data. JMIR Med. Inform. 2016, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Pike, C.J. Sex hormones, aging, and Alzheimer’s disease. Front. Biosci. Elite Ed. 2012, 4, 976–997. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Napolitano, L.; Abate, M.; Cirillo, L.; Reccia, P.; Passaro, F.; Turco, C.; Morra, S.; Mastrangelo, F.; Scarpato, A.; et al. The Role of Testosterone in the Elderly: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3535. [Google Scholar] [CrossRef]
- Jackson, S.S.; Marks, M.A.; Katki, H.A.; Cook, M.B.; Hyun, N.; Freedman, N.D.; Kahle, L.L.; Castle, P.E.; Graubard, B.I.; Chaturvedi, A.K. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer 2022, 128, 3531–3540. [Google Scholar] [CrossRef]
- Seute, T.; Leffers, P.; ten Velde, G.P.M.; Twijnstra, A. Leptomeningeal metastases from small cell lung carcinoma. Cancer 2005, 104, 1700–1705. [Google Scholar] [CrossRef]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. 2021, 23, 1447–1456. [Google Scholar] [CrossRef]
- Li, Q.; Lin, Z.; Hong, Y.; Fu, Y.; Chen, Y.; Liu, T.; Zheng, Y.; Tian, J.; Liu, C.; Pu, W.; et al. Brain parenchymal and leptomeningeal metastasis in non-small cell lung cancer. Sci. Rep. 2022, 12, 22372. [Google Scholar] [CrossRef]
- Rybarczyk-Kasiuchnicz, A.; Ramlau, R.; Stencel, K. Treatment of Brain Metastases of Non-Small Cell Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 593. [Google Scholar] [CrossRef]
- Blanco, R.; Maestu, I.; de la Torre, M.G.; Cassinello, A.; Nuñez, I. A review of the management of elderly patients with non-small-cell lung cancer. Ann. Oncol. 2015, 26, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Bellur, S.; Khosla, A.A.; Ozair, A.; Kotecha, R.; McDermott, M.W.; Ahluwalia, M.S. Management of Brain Metastases: A Review of Novel Therapies. Semin. Neurol. 2023, 43, 845–858. [Google Scholar] [CrossRef]
- Hines, J.B.; Bowar, B.; Levine, E.; Esposito, A.; Garassino, M.C.; Bestvina, C.M. Targeted Toxicities: Protocols for Monitoring the Adverse Events of Targeted Therapies Used in the Treatment of Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2023, 24, 9429. [Google Scholar] [CrossRef] [PubMed]
- Sacks, P.; Rahman, M. Epidemiology of Brain Metastases. Neurosurg. Clin. N. Am. 2020, 31, 481–488. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Qi, Y.; Xiu, J.; Li, R.; Han, M. Distribution Of Brain Metastasis From Lung Cancer. Cancer Manag. Res. 2019, 11, 9331–9338. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Mittal, S.; Rostami, P.; Tavassoli, F.; Jabbari, B. Brain metastasis in breast cancer: A comprehensive literature review. J. Neuro-Oncol. 2016, 127, 407–414. [Google Scholar] [CrossRef]
- Delaruelle, Z.; Ivanova, T.A.; Khan, S.; Negro, A.; Ornello, R.; Raffaelli, B.; Terrin, A.; Mitsikostas, D.D.; Reuter, U. Male and female sex hormones in primary headaches. J. Headache Pain 2018, 19, 117. [Google Scholar] [CrossRef]
- Ferrari, A.; Spaccapelo, L.; Gallesi, D.; Sternieri, E. Focus on headache as an adverse reaction to drugs. J. Headache Pain 2009, 10, 235–239. [Google Scholar] [CrossRef]


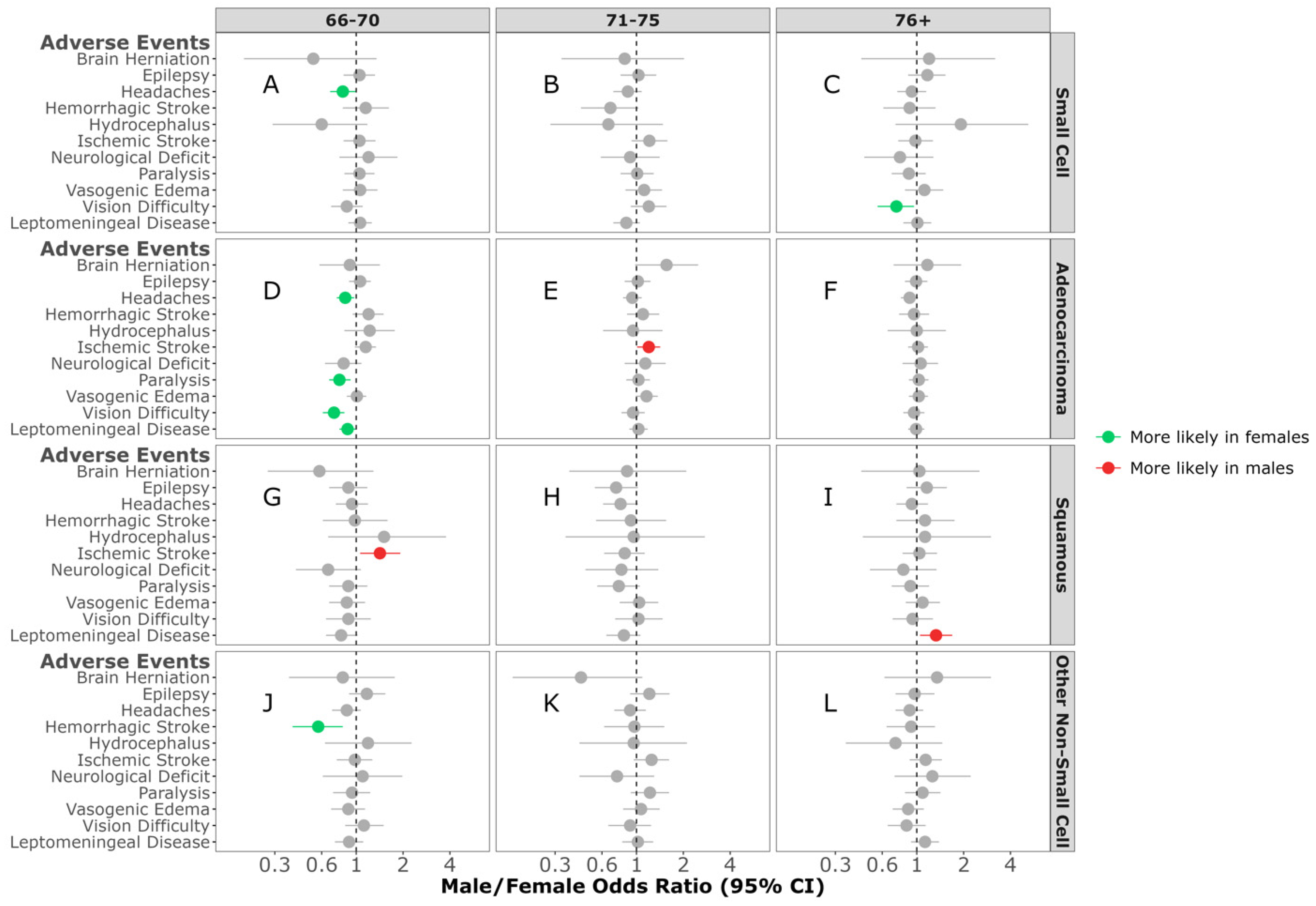
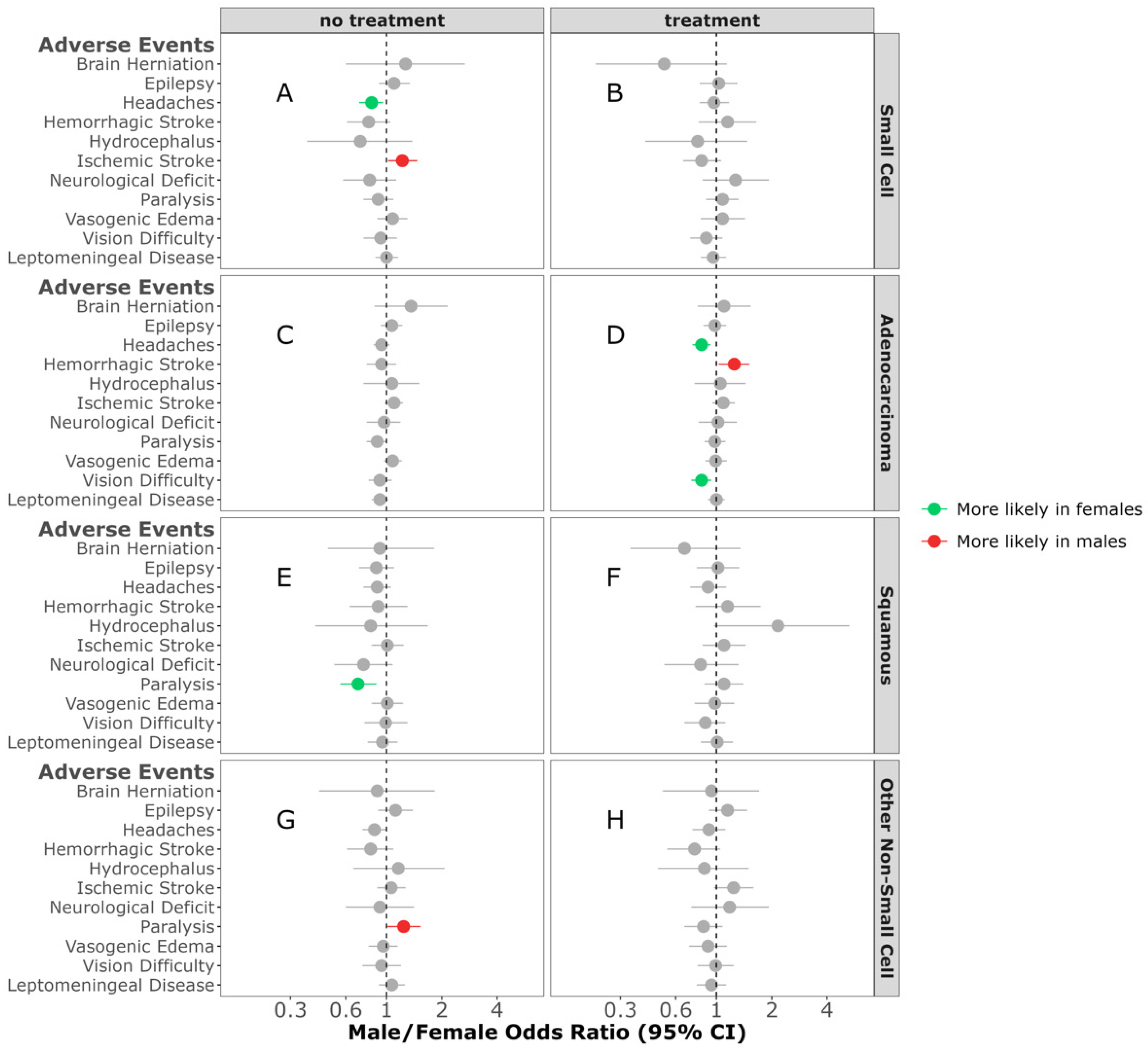

| Small-Cell | Adenocarcinoma | Squamous | Other Non-Small-Cell | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female, n = 3646 (54%) 1 | Male, n = 3166 (46%) 1 | p-Value 2 | Female, n = 7860 (55%) 1 | Male, n = 6343 (45%) 1 | p-Value 2 | Female, n = 1760 (41%) 1 | Male, n = 2490 (59%) 1 | p-Value 2 | Female, n = 2423 (49%) 1 | Male, n = 2542 (51%) 1 | p-Value 2 | |
| Race | <0.001 | 0.016 | 0.4 | 0.073 | ||||||||
| White | 3340 (92%) | 2815 (89%) | 6745 (86%) | 5351 (84%) | 1530 (87%) | 2140 (86%) | 2154 (89%) | 2217 (87%) | ||||
| Non-White | 306 (8.4%) | 351 (11%) | 1115 (14%) | 992 (16%) | 230 (13%) | 350 (14%) | 269 (11%) | 325 (13%) | ||||
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Ethnicity | 0.3 | 0.4 | 0.018 | 0.023 | ||||||||
| Non-Hispanic | 3533 (97%) | 3052 (96%) | 7513 (96%) | 6045 (95%) | 1694 (96%) | 2358 (95%) | 2328 (96%) | 2408 (95%) | ||||
| Hispanic | 113 (3.1%) | 114 (3.6%) | 347 (4.4%) | 298 (4.7%) | 66 (3.8%) | 132 (5.3%) | 95 (3.9%) | 134 (5.3%) | ||||
| Elixhauser Score | 0.014 | 0.15 | 0.2 | 0.12 | ||||||||
| 0–3 | 956 (26%) | 882 (28%) | 3084 (39%) | 2435 (38%) | 522 (30%) | 804 (32%) | 857 (35%) | 971 (38%) | ||||
| 4–6 | 1397 (38%) | 1275 (40%) | 3067 (39%) | 2447 (39%) | 737 (42%) | 980 (39%) | 975 (40%) | 964 (38%) | ||||
| 7–9 | 941 (26%) | 751 (24%) | 1345 (17%) | 1120 (18%) | 359 (20%) | 525 (21%) | 453 (19%) | 448 (18%) | ||||
| 10+ | 352 (9.7%) | 258 (8.1%) | 364 (4.6%) | 341 (5.4%) | 142 (8.1%) | 181 (7.3%) | 138 (5.7%) | 159 (6.3%) | ||||
| Age | 0.15 | <0.001 | <0.001 | 0.024 | ||||||||
| 66–70 | 1392 (38%) | 1261 (40%) | 2626 (33%) | 2277 (36%) | 551 (31%) | 918 (37%) | 790 (33%) | 922 (36%) | ||||
| 71–75 | 1133 (31%) | 998 (32%) | 2257 (29%) | 1859 (29%) | 507 (29%) | 738 (30%) | 732 (30%) | 733 (29%) | ||||
| 76+ | 1121 (31%) | 907 (29%) | 2977 (38%) | 2207 (35%) | 702 (40%) | 834 (33%) | 901 (37%) | 887 (35%) | ||||
| Treatment | 0.6 | 0.5 | 0.14 | 0.7 | ||||||||
| No Treatment | 1929 (53%) | 1654 (52%) | 3809 (48%) | 3036 (48%) | 969 (55%) | 1314 (53%) | 1313 (54%) | 1392 (55%) | ||||
| Treatment | 1717 (47%) | 1512 (48%) | 4051 (52%) | 3307 (52%) | 791 (45%) | 1176 (47%) | 1110 (46%) | 1150 (45%) | ||||
| Year of Diagnosis | 0.068 | >0.9 | 0.6 | 0.4 | ||||||||
| 2007–2013 | 2401 (66%) | 2151 (68%) | 4965 (63%) | 4006 (63%) | 1179 (67%) | 1647 (66%) | 1846 (76%) | 1961 (77%) | ||||
| 2014–2017 | 1245 (34%) | 1015 (32%) | 2895 (37%) | 2337 (37%) | 581 (33%) | 843 (34%) | 577 (24%) | 581 (23%) | ||||
| Synchronous Metastases | 0.006 | <0.001 | 0.4 | <0.001 | ||||||||
| Asynchronous | 1820 (50%) | 1475 (47%) | 3255 (41%) | 2354 (37%) | 788 (45%) | 1085 (44%) | 926 (38%) | 852 (34%) | ||||
| Synchronous | 1826 (50%) | 1691 (53%) | 4605 (59%) | 3989 (63%) | 972 (55%) | 1405 (56%) | 1497 (62%) | 1690 (66%) | ||||
| Surgery | 147 (4.0%) | 107 (3.4%) | 0.2 | 818 (10%) | 688 (11%) | 0.4 | 171 (9.7%) | 255 (10%) | 0.6 | 255 (11%) | 231 (9.1%) | 0.089 |
| Radiation | 300 (8.2%) | 241 (7.6%) | 0.3 | 951 (12%) | 777 (12%) | 0.8 | 228 (13%) | 313 (13%) | 0.7 | 297 (12%) | 293 (12%) | 0.4 |
| Chemotherapy | 1404 (39%) | 1287 (41%) | 0.071 | 2246 (29%) | 2038 (32%) | <0.001 | 465 (26%) | 737 (30%) | 0.023 | 671 (28%) | 747 (29%) | 0.2 |
| Targeted | -- | -- | 0.8 | 911 (12%) | 597 (9.4%) | <0.001 | 55 (3.1%) | 58 (2.3%) | 0.11 | 129 (5.3%) | 102 (4.0%) | 0.028 |
| Immunotherapy | -- | -- | 0.5 | 320 (4.1%) | 247 (3.9%) | 0.6 | 59 (3.4%) | 89 (3.6%) | 0.7 | 52 (2.1%) | 50 (2.0%) | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmukauskas, M.; Cioffi, G.; Waite, K.A.; Mammoser, A.G.; Sloan, A.E.; Ma, P.C.; Barnholtz-Sloan, J.S. Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age. Cancers 2024, 16, 2986. https://doi.org/10.3390/cancers16172986
Dmukauskas M, Cioffi G, Waite KA, Mammoser AG, Sloan AE, Ma PC, Barnholtz-Sloan JS. Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age. Cancers. 2024; 16(17):2986. https://doi.org/10.3390/cancers16172986
Chicago/Turabian StyleDmukauskas, Mantas, Gino Cioffi, Kristin A. Waite, Aaron G. Mammoser, Andrew E. Sloan, Patrick C. Ma, and Jill S. Barnholtz-Sloan. 2024. "Sex Difference in Disease-Related Adverse Events Post-Diagnosis of Lung Cancer Brain Metastases in Medicare Individuals ≥ 66 Years of Age" Cancers 16, no. 17: 2986. https://doi.org/10.3390/cancers16172986






