Correlation of Immunomodulatory Cytokines with Tumor Volume and Cerebrospinal Fluid in Vestibular Schwannoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
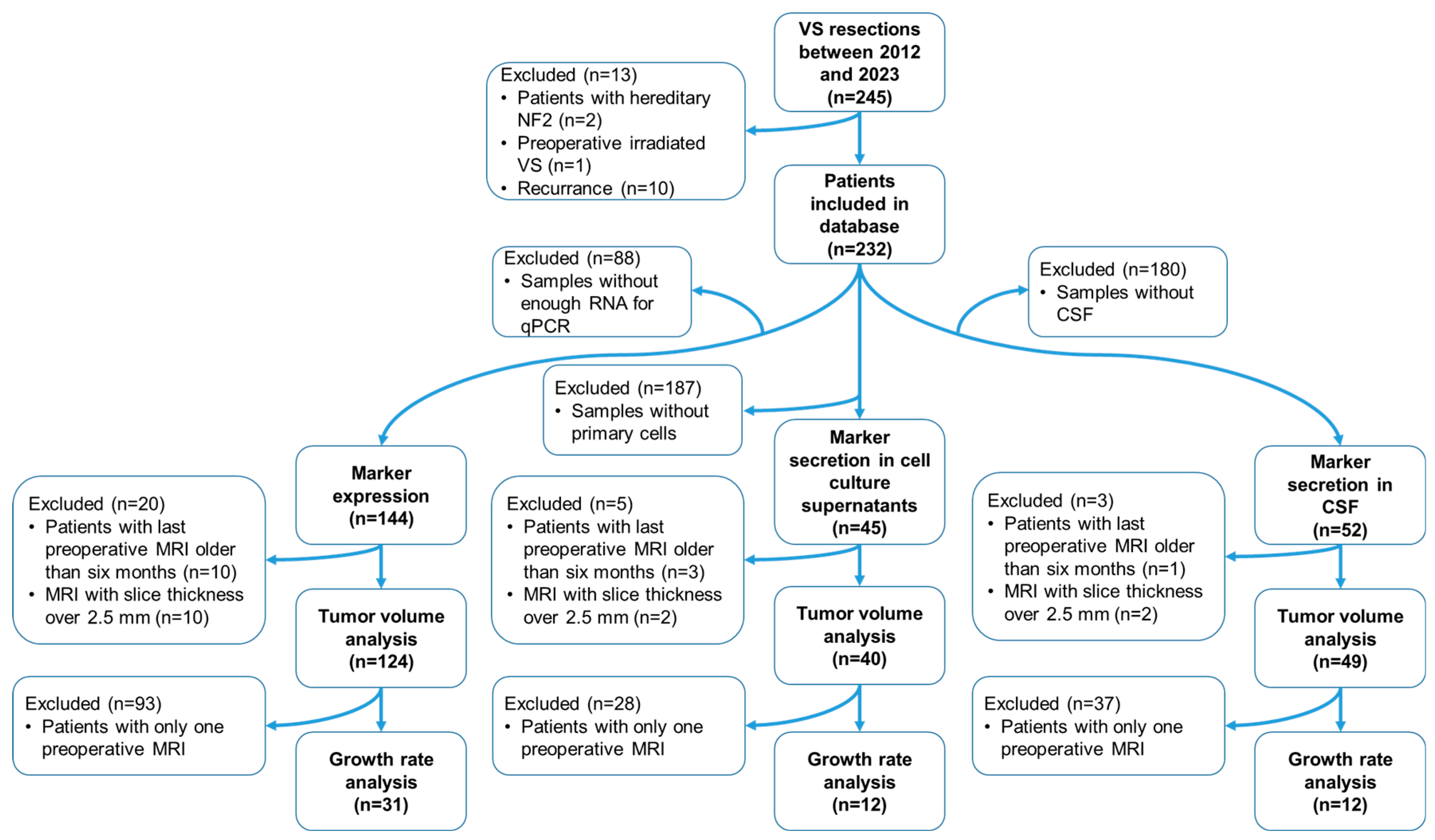
2. Materials and Methods
2.1. Study Design, Ethics, and Tumor Volumetry
2.2. Nerve Samples, DNA Extraction, and Genotyping
2.3. CSF Samples
2.4. RNA Extraction from Tumors and Corresponding Nerve Samples and Reverse Transcription into cDNA
2.5. RNAseq Analysis of Tumor and Corresponding Nerve Samples
2.6. Isolation of Primary Cultures from Cavitron Ultrasonic Surgical Aspirator Filter-Obtained Tissue
2.7. Immunofluorescence Staining
2.8. Obtaining Cell Culture Supernatants from Primary Cultures and RNA Isolation from Primary Cells
2.9. Quantitative Real-Time PCR
2.10. Enzyme-Linked Immunosorbent Assay
2.11. Statistical Analysis
3. Results
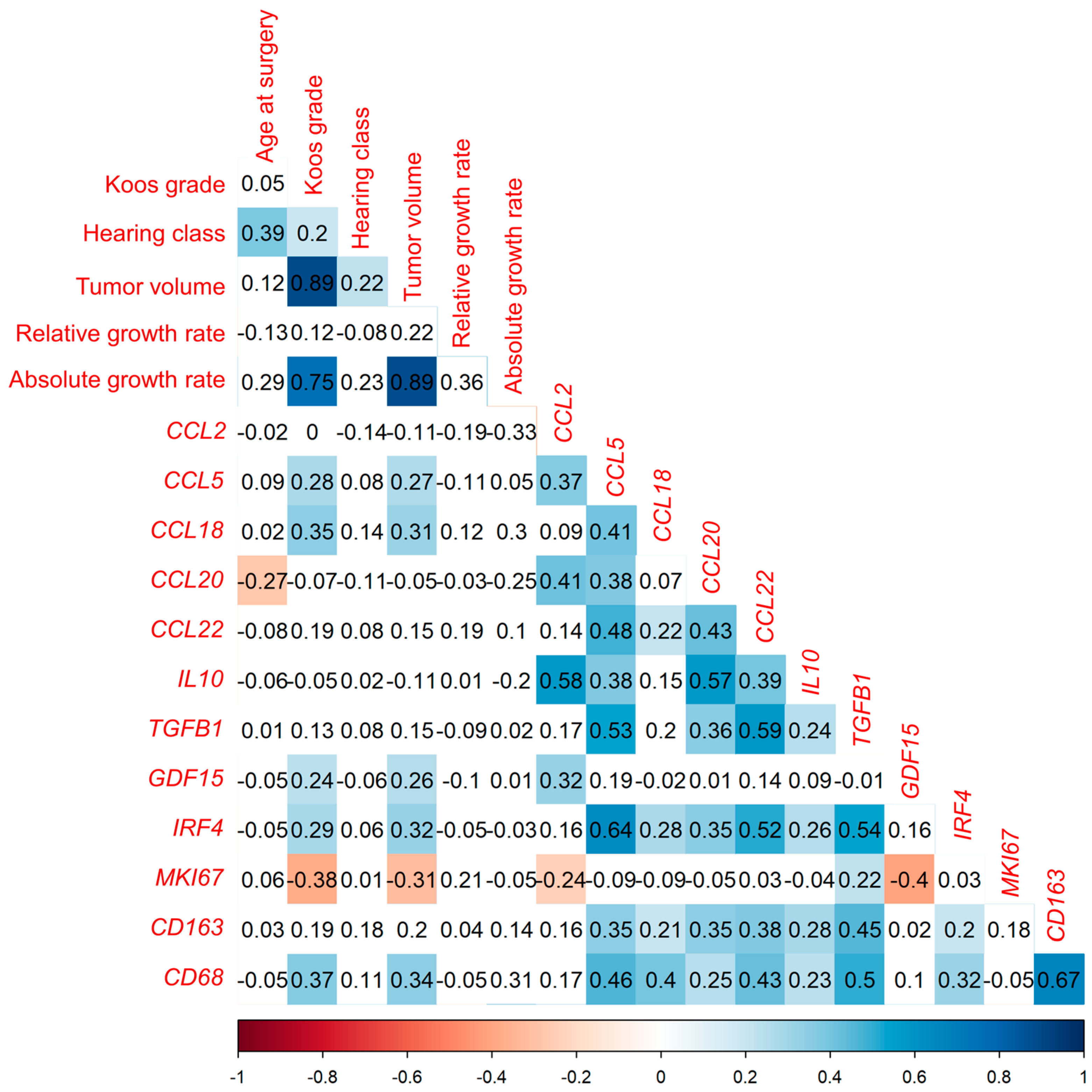
3.1. Cytokine mRNA Analyzed by qPCR Correlates Positively with Tumor Volume and Macrophage Markers but Not with Tumor Growth Rate
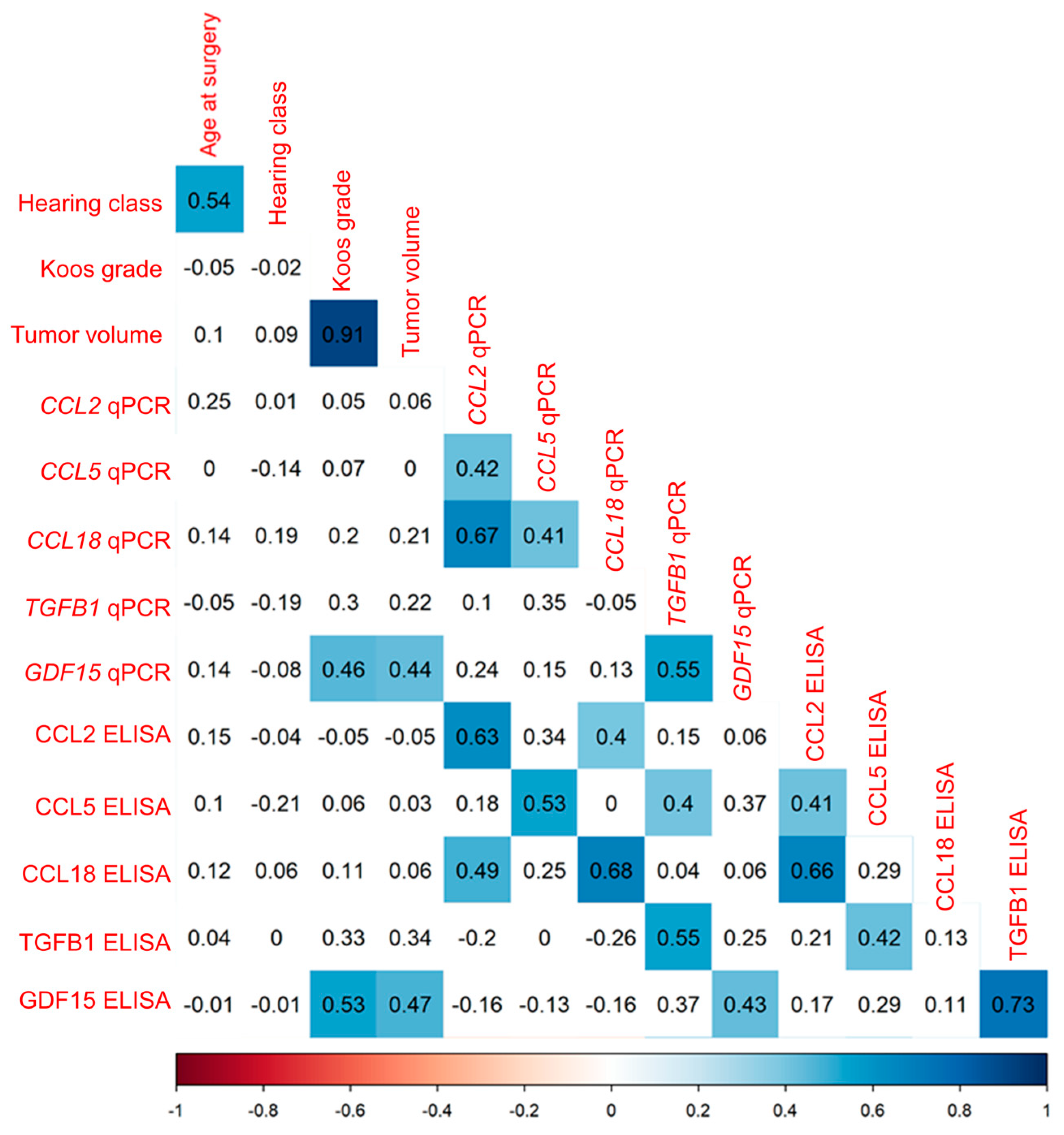
3.1.1. Correlation Analysis of Investigated Markers with Tumor Volume in Tumor Samples from 144 VS Patients
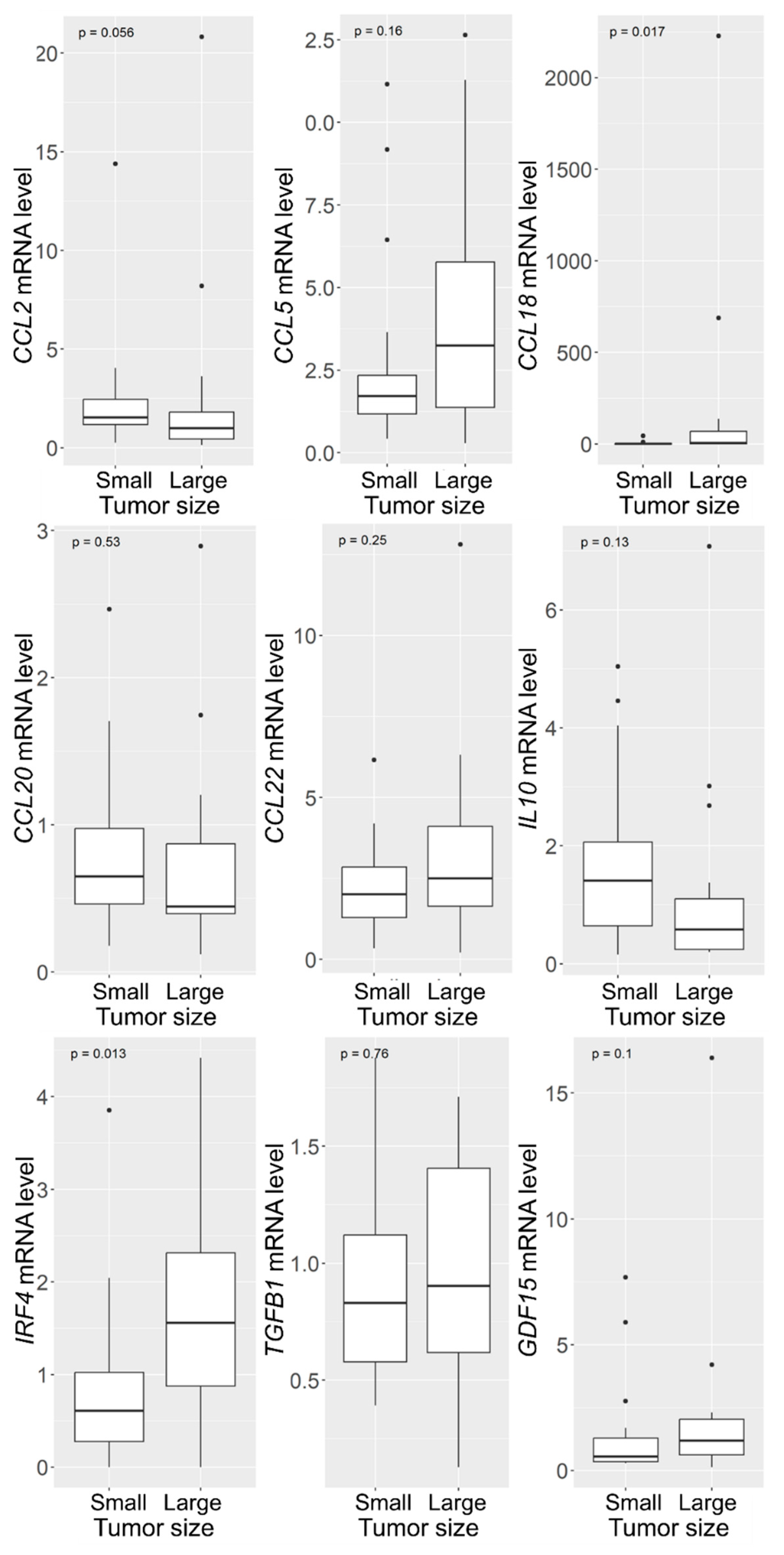
3.1.2. Increased CCL18 and IRF4 mRNA Levels in VSs with Large Tumor Size
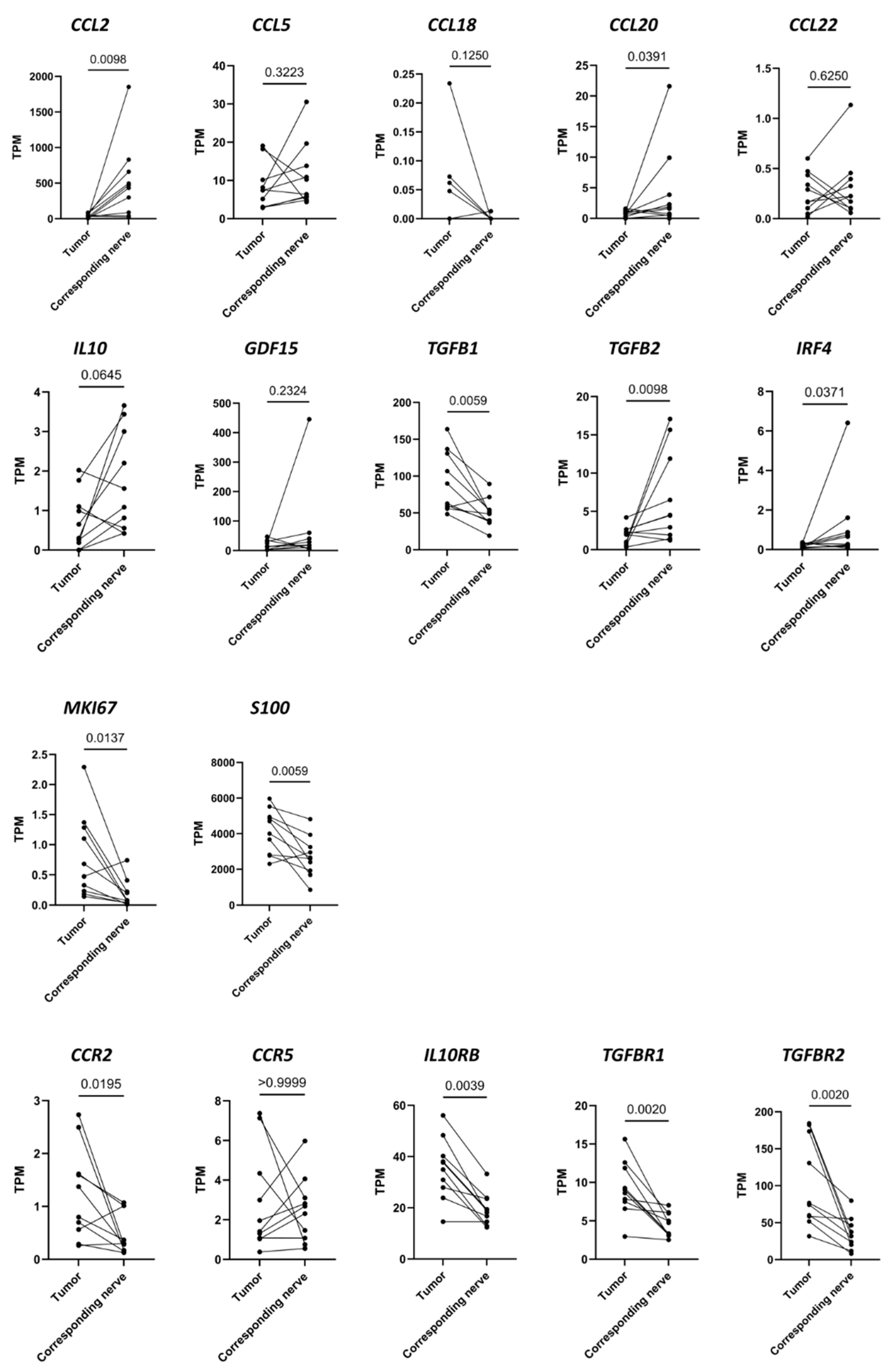
3.2. Differences in RNA Transcription Levels Investigated Using RNAseq between 10 VS Tumors and the Corresponding Vestibular Nerves
3.3. TGFB1 and GDF15 Concentration in CCS Correlates Positively with Tumor Volume
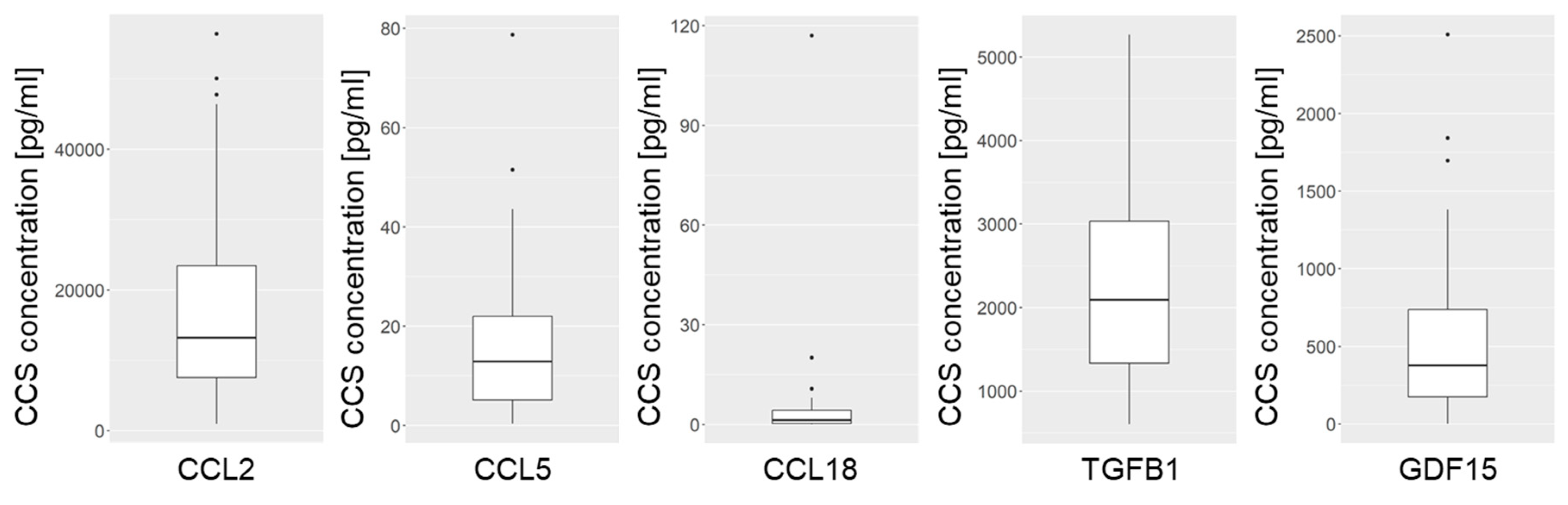
3.3.1. Concentrations of the Investigated Cytokines in CCS of VS Primary Cultures
3.3.2. Correlation Analysis of Cytokine mRNA Levels and Secretion in VS Primary Cultures
3.4. Distinct Cytokine Concentrations in CSF of VS Patients Compared to Control Group
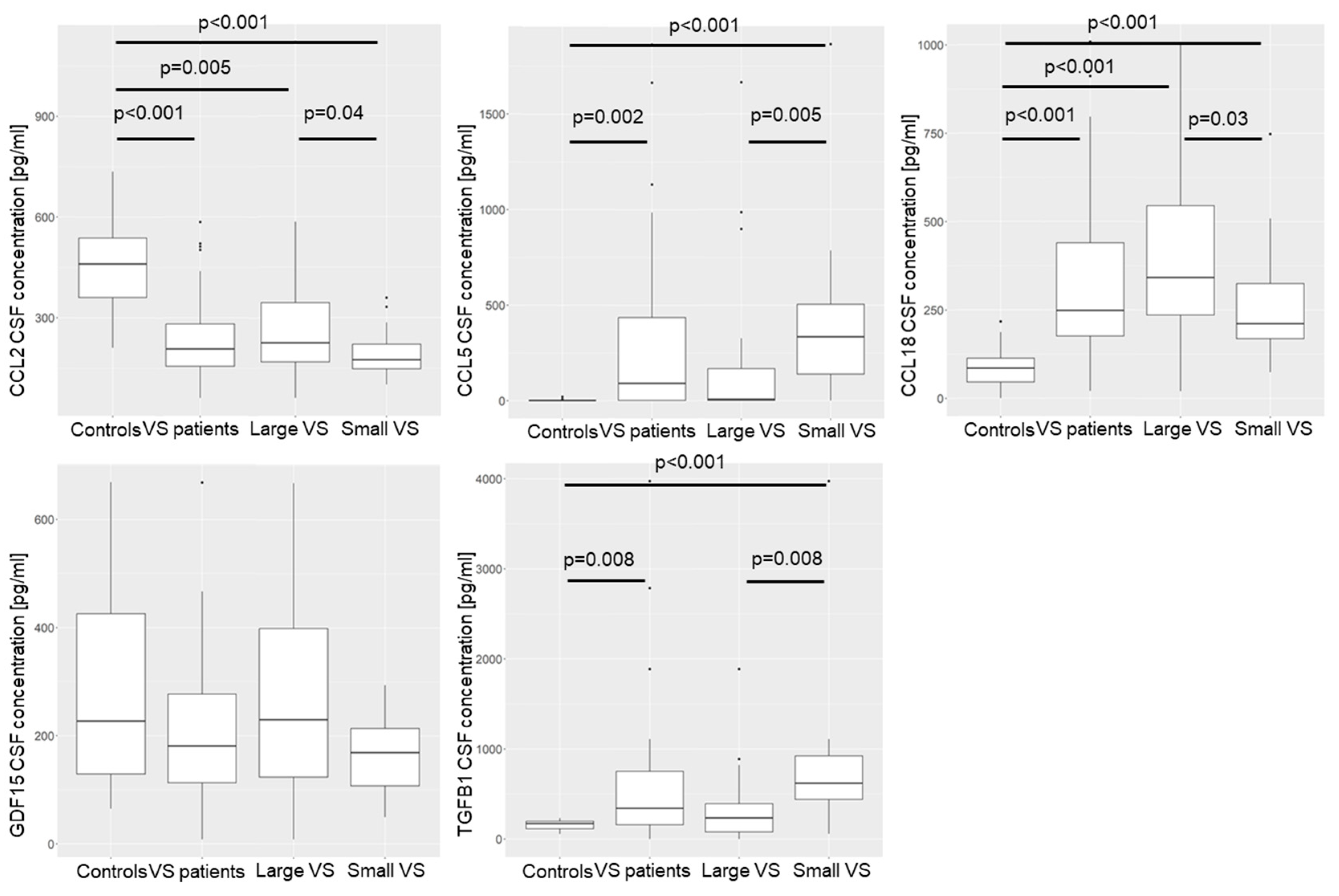
3.4.1. Concentration of Cytokines in CSF of VS Patients Compared to Control Group
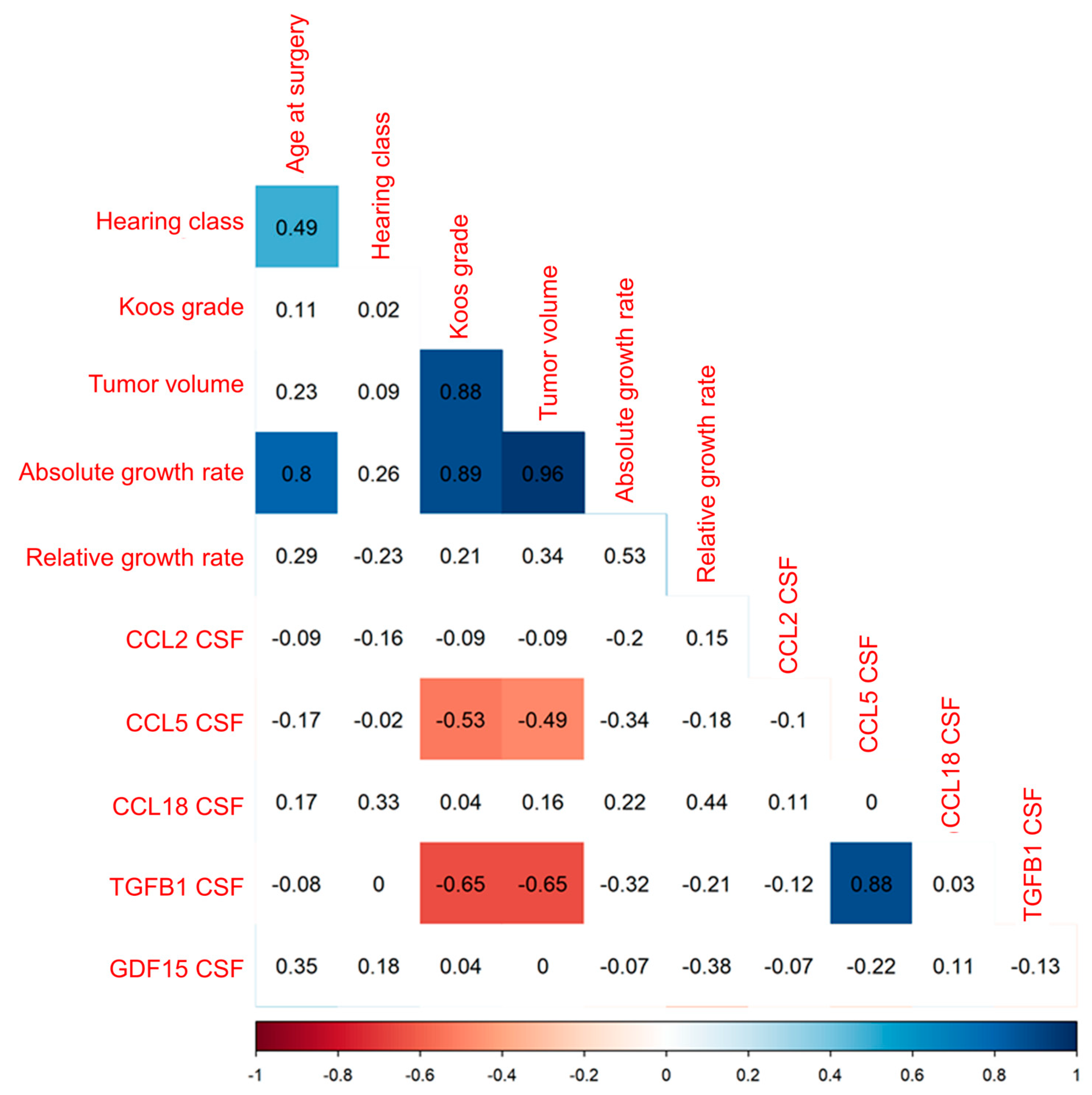
3.4.2. Decreased CCL5 and TGFB1 CSF Concentration with Increasing Tumor Volume of VS
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, V.M.; Suhm, E.M.; Ries, V.; Skardelly, M.; Tabatabai, G.; Tatagiba, M.; Schittenhelm, J.; Behling, F. Macrophage and Lymphocyte Infiltration Is Associated with Volumetric Tumor Size but Not with Volumetric Growth in the Tübingen Schwannoma Cohort. Cancers 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, T.S.; Shahrestani, S.; Borrelli, M.; Raskin, J.; Hopp, M.L.; Wu, A.W.; Miller, M.E.; Wong, Y.T. Analysis of readmissions data among frail and non-frail patients presenting for acoustic neuroma. J. Clin. Neurosci. 2022, 99, 82–88. [Google Scholar] [CrossRef]
- Gupta, V.K.; Thakker, A.; Gupta, K.K. Vestibular Schwannoma: What We Know and Where We are Heading. Head Neck Pathol. 2020, 14, 1058–1066. [Google Scholar] [CrossRef]
- Moffat, D.A.; Kasbekar, A.; Axon, P.R.; Lloyd, S.K. Growth characteristics of vestibular schwannomas. Otol. Neurotol. 2012, 33, 1053–1058. [Google Scholar] [CrossRef]
- Lewis, D.; Roncaroli, F.; Agushi, E.; Mosses, D.; Williams, R.; Li, K.L.; Zhu, X.; Hinz, R.; Atkinson, R.; Wadeson, A.; et al. Inflammation and vascular permeability correlate with growth in sporadic vestibular schwannoma. Neuro Oncol. 2019, 21, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Macielak, R.J.; Wallerius, K.P.; Lawlor, S.K.; Lohse, C.M.; Marinelli, J.P.; Neff, B.A.; Van Gompel, J.J.; Driscoll, C.L.W.; Link, M.J.; Carlson, M.L. Defining clinically significant tumor size in vestibular schwannoma to inform timing of microsurgery during wait-and-scan management: Moving beyond minimum detectable growth. J. Neurosurg. 2021, 136, 1289–1297. [Google Scholar] [CrossRef]
- de Vries, W.M.; Briaire-de Bruijn, I.H.; van Benthem, P.P.G.; van der Mey, A.G.L.; Hogendoorn, P.C.W. M-CSF and IL-34 expression as indicators for growth in sporadic vestibular schwannoma. Virchows Arch. 2019, 474, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Leisz, S.; Klause, C.H.; Vital Dos Santos, T.; Haenel, P.; Scheer, M.; Simmermacher, S.; Mawrin, C.; Strauss, C.; Scheller, C.; Rampp, S. Vestibular Schwannoma Volume and Tumor Growth Correlates with Macrophage Marker Expression. Cancers 2022, 14, 4429. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Briaire-de Bruijn, I.; Malessy, M.J.; de Bruïne, S.F.; van der Mey, A.G.; Hogendoorn, P.C. Tumor-associated macrophages are related to volumetric growth of vestibular schwannomas. Otol. Neurotol. 2013, 34, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Frankenberger, C.; Rabe, D.; Bainer, R.; Sankarasharma, D.; Chada, K.; Krausz, T.; Gilad, Y.; Becker, L.; Rosner, M.R. Metastasis Suppressors Regulate the Tumor Microenvironment by Blocking Recruitment of Prometastatic Tumor-Associated Macrophages. Cancer Res. 2015, 75, 4063–4073. [Google Scholar] [CrossRef]
- Byrne, S.N.; Knox, M.C.; Halliday, G.M. TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunol. Cell Biol. 2008, 86, 92–97. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Saji, H.; Koike, M.; Yamori, T.; Saji, S.; Seiki, M.; Matsushima, K.; Toi, M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 2001, 92, 1085–1091. [Google Scholar] [CrossRef]
- Svensson, S.; Abrahamsson, A.; Rodriguez, G.V.; Olsson, A.K.; Jensen, L.; Cao, Y.; Dabrosin, C. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin. Cancer Res. 2015, 21, 3794–3805. [Google Scholar] [CrossRef]
- Ahyi, A.-N.N.; Chang, H.-C.; Dent, A.L.; Nutt, S.L.; Kaplan, M.H. IFN Regulatory Factor 4 Regulates the Expression of a Subset of Th2 Cytokines1. J. Immunol. 2009, 183, 1598–1606. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Urakawa, N.; Utsunomiya, S.; Nishio, M.; Shigeoka, M.; Takase, N.; Arai, N.; Kakeji, Y.; Koma, Y.; Yokozaki, H. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab. Investig. 2015, 95, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef]
- Tap, W.D.; Wainberg, Z.A.; Anthony, S.P.; Ibrahim, P.N.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Staddon, A.P.; Cohn, A.L.; Shapiro, G.I.; et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N. Engl. J. Med. 2015, 373, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef]
- Moisan, F.; Francisco, E.B.; Brozovic, A.; Duran, G.E.; Wang, Y.C.; Chaturvedi, S.; Seetharam, S.; Snyder, L.A.; Doshi, P.; Sikic, B.I. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol. Oncol. 2014, 8, 1231–1239. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Papadopoulos, K.; Fong, P.C.; Patnaik, A.; Messiou, C.; Olmos, D.; Wang, G.; Tromp, B.J.; Puchalski, T.A.; Balkwill, F.; et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 1041–1050. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). Otolaryngol. Head Neck Surg. 1995, 113, 179–180. [Google Scholar] [CrossRef]
- Rahne, T.; Plontke, S.K.; Vordermark, D.; Strauss, C.; Scheller, C. Hearing classification in patients with vestibular schwannoma using German-language test procedures. HNO 2021, 69, 750–758. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.; Schwarz, T.; Engel, K.; Volkmer, I.; Krüger, A.; Tarabuko, A.; Junghans, J.; Kornhuber, M.E.; Hoffmann, F.; Staege, M.S.; et al. Epstein-Barr Virus-Induced Genes and Endogenous Retroviruses in Immortalized B Cells from Patients with Multiple Sclerosis. Cells 2022, 11, 3619. [Google Scholar] [CrossRef]
- Leisz, S.; Klause, C.H.; Becker, A.L.; Scheer, M.; Simmermacher, S.; Strauss, C.; Scheller, C. Establishment of vestibular schwannoma primary cell cultures obtained from cavitron ultrasonic surgical aspirator tissue material. J. Neurosci. Methods 2023, 397, 109955. [Google Scholar] [CrossRef]
- Mechtersheimer, G.; Staudter, M.; Möller, P. Expression of the natural killer cell-associated antigens CD56 and CD57 in human neural and striated muscle cells and in their tumors. Cancer Res. 1991, 51, 1300–1307. [Google Scholar] [PubMed]
- Jaiswal, P.; Cd, A.; John, J.J. A Spectrum of Histomorphological and Immunohistochemical Expression Profiles of S-100, CD56 and Calretinin in Benign Peripheral Nerve Sheath Tumours. Cureus 2023, 15, e40751. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing, 4.0.5; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hannan, C.J.; Lewis, D.; O’Leary, C.; Waqar, M.; Brough, D.; Couper, K.N.; Dyer, D.P.; Vail, A.; Heal, C.; Macarthur, J.; et al. Increased Circulating Chemokines and Macrophage Recruitment in Growing Vestibular Schwannomas. Neurosurgery 2023, 92, 581–589. [Google Scholar] [CrossRef]
- Luput, L.; Licarete, E.; Sesarman, A.; Patras, L.; Alupei, M.C.; Banciu, M. Tumor-associated macrophages favor C26 murine colon carcinoma cell proliferation in an oxidative stress-dependent manner. Oncol. Rep. 2017, 37, 2472–2480. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Li, X.; Wu, X.; Tang, M.; Wang, X. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol. Rep. 2018, 39, 818–826. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, H.; Guo, M.; Chen, J.; Wu, W.; Li, W.; Xu, X.; Lin, X.; Fu, W.; Yao, Y.; et al. Breast Phyllodes Tumors Recruit and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to Promote Malignant Progression, Which Can Be Inhibited by CCR5 Inhibition Therapy. Clin. Cancer Res. 2019, 25, 3873–3886. [Google Scholar] [CrossRef]
- Li, F.; Kitajima, S.; Kohno, S.; Yoshida, A.; Tange, S.; Sasaki, S.; Okada, N.; Nishimoto, Y.; Muranaka, H.; Nagatani, N.; et al. Retinoblastoma Inactivation Induces a Protumoral Microenvironment via Enhanced CCL2 Secretion. Cancer Res. 2019, 79, 3903–3915. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhao, X.; Yuan, B.; Zeng, Z.; Chen, Y. Blocking the CCL5-CCR5 Axis Using Maraviroc Promotes M1 Polarization of Macrophages Cocultured with Irradiated Hepatoma Cells. J. Hepatocell. Carcinoma 2021, 8, 599–611. [Google Scholar] [CrossRef]
- Adema, G.J.; Hartgers, F.; Verstraten, R.; de Vries, E.; Marland, G.; Menon, S.; Foster, J.; Xu, Y.; Nooyen, P.; McClanahan, T.; et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997, 387, 713–717. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yu, H.; Yin, Q.; Li, M.; Shi, L.; Zhang, W.; Li, D.; Li, L. CCL18 from tumor-cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol. Carcinog. 2016, 55, 1688–1699. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bhattacharya-Chatterjee, M.; O’Malley, B.W., Jr.; Chatterjee, S.K. Tumor metastasis in an orthotopic murine model of head and neck cancer: Possible role of TGF-beta 1 secreted by the tumor cells. J. Cell. Biochem. 2006, 97, 1036–1051. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, A.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhao, Y.C.; Chen, C.; Xie, M. CCL5 secreted by luminal B breast cancer cells induces polarization of M2 macrophages through activation of MEK/STAT3 signaling pathway via CCR5. Gene 2022, 812, 146100. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Q.; Li, L.; Chen, K.; Yang, J.; Dixit, D.; Gimple, R.C.; Ci, S.; Lu, C.; Hu, L.; et al. β2-Microglobulin Maintains Glioblastoma Stem Cells and Induces M2-like Polarization of Tumor-Associated Macrophages. Cancer Res. 2022, 82, 3321–3334. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Bach, C.; Böttcher, M.; Beier, F.; Röhner, L.; Stoll, A.; Rehli, M.; Gebhard, C.; Lischer, C.; Eberhardt, M.; et al. The IKZF1-IRF4/IRF5 Axis Controls Polarization of Myeloma-Associated Macrophages. Cancer Immunol. Res. 2021, 9, 265–278. [Google Scholar] [CrossRef]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Nisenbaum, E.; Wiefels, M.; Telischi, J.; Marasigan, M.; Kanumuri, V.; Pena, S.; Thielhelm, T.; Bracho, O.; Bhatia, R.; Scaglione, T.; et al. Cytokine Profiling of Cyst Fluid and Tumor-Associated Macrophages in Cystic Vestibular Schwannoma. Otol. Neurotol. 2023, 44, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Yaguchi, T.; Ohmura, G.; Ohta, S.; Kobayashi, A.; Kawamura, N.; Fujita, T.; Nakano, H.; Shimada, T.; Takahashi, T.; et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int. J. Cancer 2013, 132, 2755–2766. [Google Scholar] [CrossRef]
- Wu, Y.C.; Shen, Y.C.; Chang, J.W.; Hsieh, J.J.; Chu, Y.; Wang, C.H. Autocrine CCL5 promotes tumor progression in esophageal squamous cell carcinoma in vitro. Cytokine 2018, 110, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Morales, R.T.; Qian, W.; Wang, H.; Gagner, J.P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M.; et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 2018, 161, 164–178. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Zhang, L.; Zhang, J.; Xiao, Y.; Wu, Q.; Wang, Y.; Zhan, Q. Tumor-associated macrophage (TAM)-derived CCL22 induces FAK addiction in esophageal squamous cell carcinoma (ESCC). Cell. Mol. Immunol. 2022, 19, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhou, Q.; Zheng, S.; Li, G.; Lin, Q.; Wei, L.; Fu, Z.; Zhang, B.; Liu, Y.; Li, Z.; et al. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 453. [Google Scholar] [CrossRef]
- Wei, Y.T.; Wang, X.R.; Yan, C.; Huang, F.; Zhang, Y.; Liu, X.; Wen, Z.F.; Sun, X.T.; Zhang, Y.; Chen, Y.Q.; et al. Thymosin α-1 Reverses M2 Polarization of Tumor-Associated Macrophages during Efferocytosis. Cancer Res. 2022, 82, 1991–2002. [Google Scholar] [CrossRef]
- Ye, X.Z.; Xu, S.L.; Xin, Y.H.; Yu, S.C.; Ping, Y.F.; Chen, L.; Xiao, H.L.; Wang, B.; Yi, L.; Wang, Q.L.; et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, H.; Wei, T.; Lin, A.; Sun, Y.; Luo, P.; Zhang, J. Single-Cell RNA Sequencing Reveals the Heterogeneity of Tumor-Associated Macrophage in Non-Small Cell Lung Cancer and Differences between Sexes. Front. Immunol. 2021, 12, 756722. [Google Scholar] [CrossRef]
- Shen, C.; Liu, J.; Jiao, W.; Zhang, X.; Zhao, X.; Yang, X.; Wang, Y. A feed-forward loop based on aerobic glycolysis and TGF-β between tumor-associated macrophages and bladder cancer cells promoted malignant progression and immune escape. J. Cancer Res. Clin. Oncol. 2023, 149, 12867–12880. [Google Scholar] [CrossRef] [PubMed]
- Löttrich, M.; Mawrin, C.; Chamaon, K.; Kirches, E.; Dietzmann, K.; Freigang, B. Expression of transforming growth factor-β receptor type 1 and type 2 in human sporadic vestibular Schwannoma. Pathol.-Res. Pract. 2007, 203, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Brieger, J.; Bedavanija, A.; Lehr, H.A.; Maurer, J.; Mann, W.J. Expression of angiogenic growth factors in acoustic neurinoma. Acta Otolaryngol. 2003, 123, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Taurone, S.; Bianchi, E.; Attanasio, G.; Di Gioia, C.; Ierinó, R.; Carubbi, C.; Galli, D.; Pastore, F.S.; Giangaspero, F.; Filipo, R.; et al. Immunohistochemical profile of cytokines and growth factors expressed in vestibular schwannoma and in normal vestibular nerve tissue. Mol. Med. Rep. 2015, 12, 737–745. [Google Scholar] [CrossRef]
- Bizzarri, M.; Filipo, R.; Valente, M.G.; Bernardeschi, D.; Ronchetti, F.; Monini, S.; Chiappini, I.; Barbara, M. Release of transforming growth factor beta-1 in a vestibular schwannoma cell line. Acta Otolaryngol. 2002, 122, 785–787. [Google Scholar] [CrossRef]
- Weerda, H.G.; Gamberger, T.I.; Siegner, A.; Gjuric, M.; Tamm, E.R. Effects of transforming growth factor-beta1 and basic fibroblast growth factor on proliferation of cell cultures derived from human vestibular nerve schwannoma. Acta Otolaryngol. 1998, 118, 337–343. [Google Scholar] [CrossRef]
- Stankovic, K.M.; Batts, S.; Welling, D.B.; Vasilijic, S. Immune Profiling of Secreted Factors from Human Vestibular Schwannoma Cells and Tumor-associated Macrophages. Laryngoscope 2023, 134, S1–S14. [Google Scholar] [CrossRef]
- Dilwali, S.; Lysaght, A.; Roberts, D.; Barker, F.G., 2nd; McKenna, M.J.; Stankovic, K.M. Sporadic vestibular schwannomas associated with good hearing secrete higher levels of fibroblast growth factor 2 than those associated with poor hearing irrespective of tumor size. Otol. Neurotol. 2013, 34, 748–754. [Google Scholar] [CrossRef]
- Borysenko, O.; Melnykov, O.; Prilutskaya, A.; Buracovschi, M. Immunological Analysis of Vestibular Schwannoma Patients. J. Int. Adv. Otol. 2023, 19, 1–4. [Google Scholar] [CrossRef]
- Vasilijic, S.; Atai, N.A.; Hyakusoku, H.; Worthington, S.; Ren, Y.; Sagers, J.E.; Sahin, M.I.; Fujita, T.; Landegger, L.D.; Lewis, R.; et al. Identification of Immune-Related Candidate Biomarkers in Plasma of Patients with Sporadic Vestibular Schwannoma: Candidate Plasma Biomarkers in Vestibular Schwannoma. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kuo, T.C.; Jackler, R.K.; Wong, K.; Blevins, N.H.; Pitts, L.H. Are acoustic neuromas encapsulated tumors? Otolaryngol. Head Neck Surg. 1997, 117, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
- Djukic, M.; Lange, P.; Erbguth, F.; Nau, R. Spatial and temporal variation of routine parameters: Pitfalls in the cerebrospinal fluid analysis in central nervous system infections. J. Neuroinflamm. 2022, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-Oncol. 2019, 22, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tiruthani, K.; Li, S.; Hu, M.; Zhong, G.; Tang, Y.; Roy, S.; Zhang, L.; Tan, J.; Liao, C.; et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv. Mater. 2021, 33, e2007603. [Google Scholar] [CrossRef]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef]
- Pozzi, S.; Scomparin, A.; Ben-Shushan, D.; Yeini, E.; Ofek, P.; Nahmad, A.D.; Soffer, S.; Ionescu, A.; Ruggiero, A.; Barzel, A.; et al. MCP-1/CCR2 axis inhibition sensitizes the brain microenvironment against melanoma brain metastasis progression. JCI Insight 2022, 7, e154804. [Google Scholar] [CrossRef]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Pervaiz, A.; Zepp, M.; Mahmood, S.; Ali, D.M.; Berger, M.R.; Adwan, H. CCR5 blockage by maraviroc: A potential therapeutic option for metastatic breast cancer. Cell. Oncol. 2019, 42, 93–106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, A.-L.; Scholle, L.; Klause, C.H.; Staege, M.S.; Strauss, C.; Otto, M.; Rampp, S.; Scheller, C.; Leisz, S. Correlation of Immunomodulatory Cytokines with Tumor Volume and Cerebrospinal Fluid in Vestibular Schwannoma Patients. Cancers 2024, 16, 3002. https://doi.org/10.3390/cancers16173002
Becker A-L, Scholle L, Klause CH, Staege MS, Strauss C, Otto M, Rampp S, Scheller C, Leisz S. Correlation of Immunomodulatory Cytokines with Tumor Volume and Cerebrospinal Fluid in Vestibular Schwannoma Patients. Cancers. 2024; 16(17):3002. https://doi.org/10.3390/cancers16173002
Chicago/Turabian StyleBecker, Anna-Louisa, Leila Scholle, Clara Helene Klause, Martin Sebastian Staege, Christian Strauss, Markus Otto, Stefan Rampp, Christian Scheller, and Sandra Leisz. 2024. "Correlation of Immunomodulatory Cytokines with Tumor Volume and Cerebrospinal Fluid in Vestibular Schwannoma Patients" Cancers 16, no. 17: 3002. https://doi.org/10.3390/cancers16173002
APA StyleBecker, A. -L., Scholle, L., Klause, C. H., Staege, M. S., Strauss, C., Otto, M., Rampp, S., Scheller, C., & Leisz, S. (2024). Correlation of Immunomodulatory Cytokines with Tumor Volume and Cerebrospinal Fluid in Vestibular Schwannoma Patients. Cancers, 16(17), 3002. https://doi.org/10.3390/cancers16173002





