Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
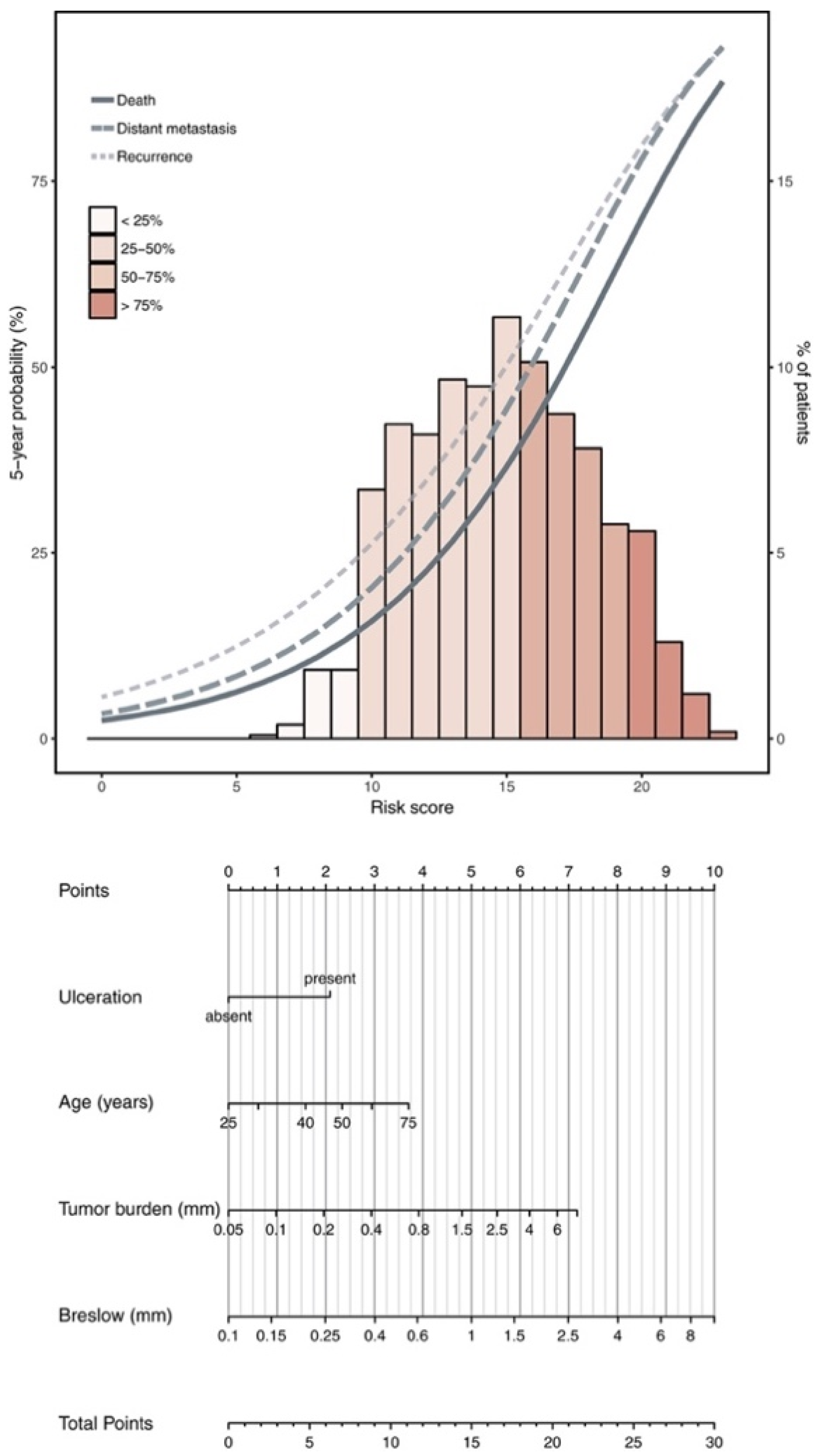
2. Evidence for Radiation Therapy’s Role in Regional Recurrence Risk Reduction
3. Current Adjuvant Systemic Therapies
4. Current Systemic Therapy Strategies and Radiation Therapy as an Adjuvant Therapy
5. Use of Adjuvant Radiation Therapy after Poor Response to Neoadjuvant Immunotherapy or MAPK Pathway Inhibitors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, L.; Bertsch, H.P.; Zapf, A.; Mitteldorf, C.; Satzger, I.; Thoms, K.M.; Völker, B.; Schön, M.P.; Gutzmer, R.; Starz, H. Nodal Basin Recurrence after Sentinel Lymph Node Biopsy for Melanoma: A Retrospective Multicenter Study in 2653 Patients. Medicine 2015, 94, e1433. [Google Scholar] [CrossRef] [PubMed]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D.; et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef]
- Perez, M.C.; Tanabe, K.K.; Ariyan, C.E.; Miura, J.T.; Mutabdzic, D.; Farma, J.M.; Zager, J.S. Local and Recurrent Regional Metastases of Melanoma. Cutan. Melanoma 2019, 705–737. [Google Scholar] [CrossRef]
- Weitman, E.S.; Perez, M.; Thompson, J.F.; Andtbacka, R.H.I.; Dalton, J.; Martin, M.L.; Miller, T.; Gwaltney, C.; Sarson, D.; Wachter, E.; et al. Quality of life patient-reported outcomes for locally advanced cutaneous melanoma. Melanoma Res. 2018, 28, 134–142. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Strom, T.; Torres-Roca, J.F.; Parekh, A.; Naghavi, A.O.; Caudell, J.J.; Oliver, D.E.; Messina, J.L.; Khushalani, N.I.; Zager, J.S.; Sarnaik, A.; et al. Regional Radiation Therapy Impacts Outcome for Node-Positive Cutaneous Melanoma. J. Natl. Compr. Cancer Netw. 2017, 15, 473–482. [Google Scholar] [CrossRef]
- Pidhorecky, I.; Lee, R.J.; Proulx, G.; Kollmorgen, D.R.; Jia, C.; Driscoll, D.L.; Kraybill, W.G.; Gibbs, J.F. Risk factors for nodal recurrence after lymphadenectomy for melanoma. Ann. Surg. Oncol. 2001, 8, 109–115. [Google Scholar] [CrossRef]
- Lee, R.J.; Gibbs, J.F.; Proulx, G.M.; Kollmorgen, D.R.; Jia, C.; Kraybill, W.G. Nodal basin recurrence following lymph node dissection for melanoma: Implications for adjuvant radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. United States 2000, 46, 467–474. [Google Scholar] [CrossRef]
- Ballo, M.T.; Bonnen, M.D.; Garden, A.S.; Myers, J.N.; Gershenwald, J.E.; Zagars, G.K.; Schechter, N.R.; Morrison, W.H.; Ross, M.I.; Kian Ang, K. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer 2003, 97, 1789–1796. [Google Scholar] [CrossRef]
- Ballo, M.T.; Zagars, G.K.; Gershenwald, J.E.; Lee, J.E.; Mansfield, P.F.; Kim, K.B.; Camacho, L.H.; Hwu, P.; Ross, M.I. A Critical Assessment of Adjuvant Radiotherapy for Inguinal Lymph Node Metastases from Melanoma. Ann. Surg. Oncol. 2004, 11, 1079–1084. [Google Scholar] [CrossRef]
- Ballo, M.T.; Strom, E.A.; Zagars, G.K.; Bedikian, A.Y.; Prieto, V.G.; Mansfield, P.F.; Lee, J.E.; Gershenwald, J.E.; Ross, M.I. Adjuvant irradiation for axillary metastases from malignant melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 964–972. [Google Scholar] [CrossRef]
- Uppal, A.; Stern, S.; Thompson, J.F.; Foshag, L.; Mizzollo, N.; Nieweg, O.E.; Hoekstra, H.J.; Roses, D.F.; Sondak, V.K.; Kashani-Sabet, M.; et al. Regional Node Basin Recurrence in Melanoma Patients: More Common after Node Dissection for Macroscopic Rather than Clinically Occult Nodal Disease. Ann. Surg. Oncol. 2020, 27, 1970–1977. [Google Scholar] [CrossRef]
- Ploeg, A.P.T.v.d.; Akkooi, A.C.J.v.; Rutkowski, P.; Nowecki, Z.I.; Michej, W.; Mitra, A.; Newton-Bishop, J.A.; Cook, M.; Ploeg, I.M.C.v.d.; Nieweg, O.E.; et al. Prognosis in Patients with Sentinel Node–Positive Melanoma Is Accurately Defined by the Combined Rotterdam Tumor Load and Dewar Topography Criteria. J. Clin. Oncol. 2011, 29, 2206–2214. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final Trial Report of Sentinel-Node Biopsy versus Nodal Observation in Melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Verver, D.; Rekkas, A.; Garbe, C.; van Klaveren, D.; van Akkooi, A.C.J.; Rutkowski, P.; Powell, B.W.E.M.; Robert, C.; Testori, A.; van Leeuwen, B.L.; et al. The EORTC-DeCOG nomogram adequately predicts outcomes of patients with sentinel node–positive melanoma without the need for completion lymph node dissection. Eur. J. Cancer 2020, 134, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Multicenter Selective Lymphadenectomy Trials Study Group. Therapeutic Value of Sentinel Lymph Node Biopsy in Patients with Melanoma: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 835–842. [Google Scholar] [CrossRef]
- Broman, K.K.; Hughes, T.; Dossett, L.; Sun, J.; Kirichenko, D.; Carr, M.J.; Sharma, A.; Bartlett, E.K.; Nijhuis, A.A.G.; Thompson, J.F.; et al. Active surveillance of patients who have sentinel node positive melanoma: An international, multi-institution evaluation of adoption and early outcomes after the Multicenter Selective Lymphadenectomy Trial II (MSLT-2). Cancer 2021, 127, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.-W.; Lehmann, P.; et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 757–767. [Google Scholar] [CrossRef]
- Agrawal, S.; Kane, J.M., 3rd; Guadagnolo, B.A.; Kraybill, W.G.; Ballo, M.T. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer 2009, 115, 5836–5844. [Google Scholar] [CrossRef]
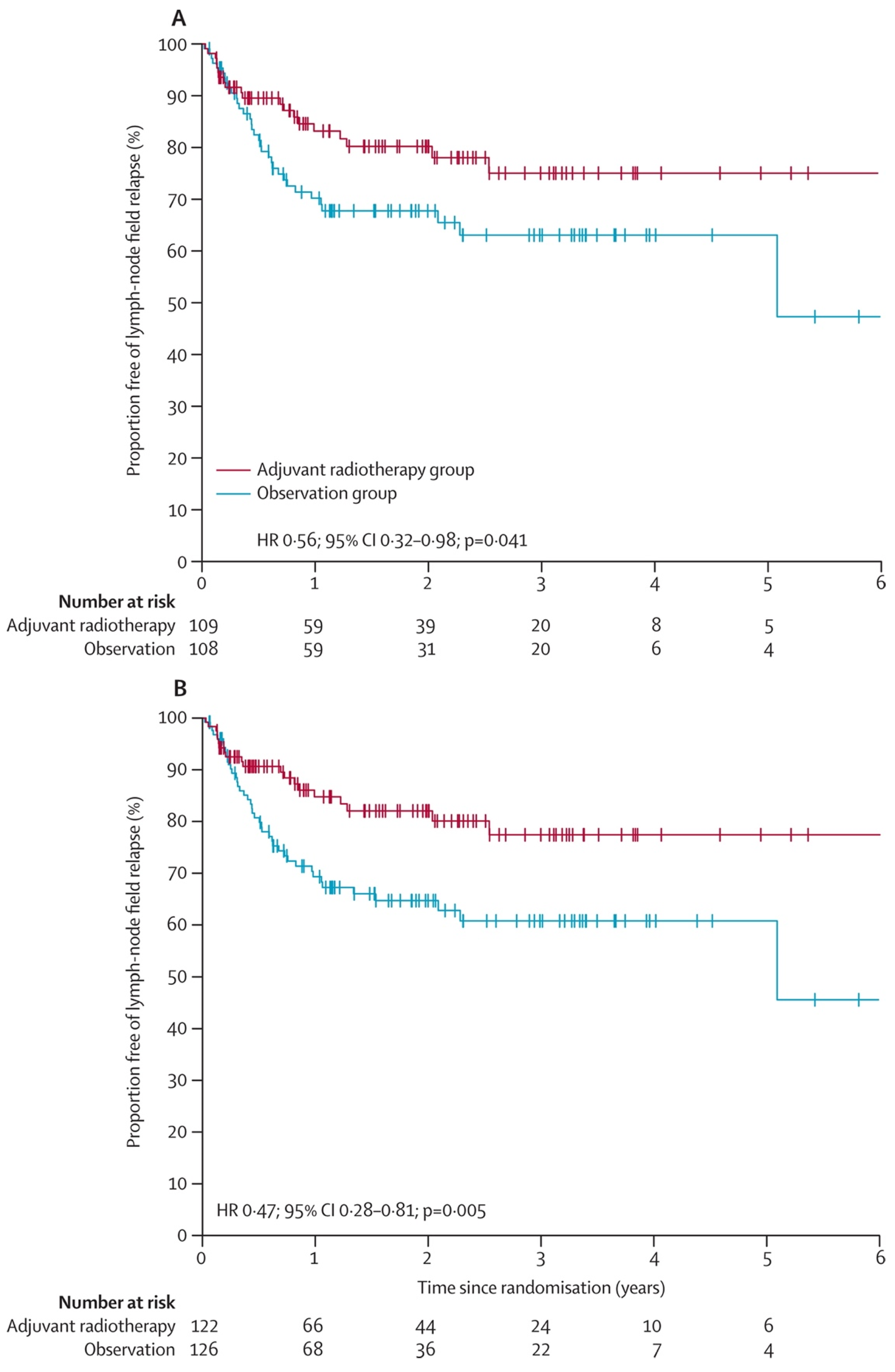
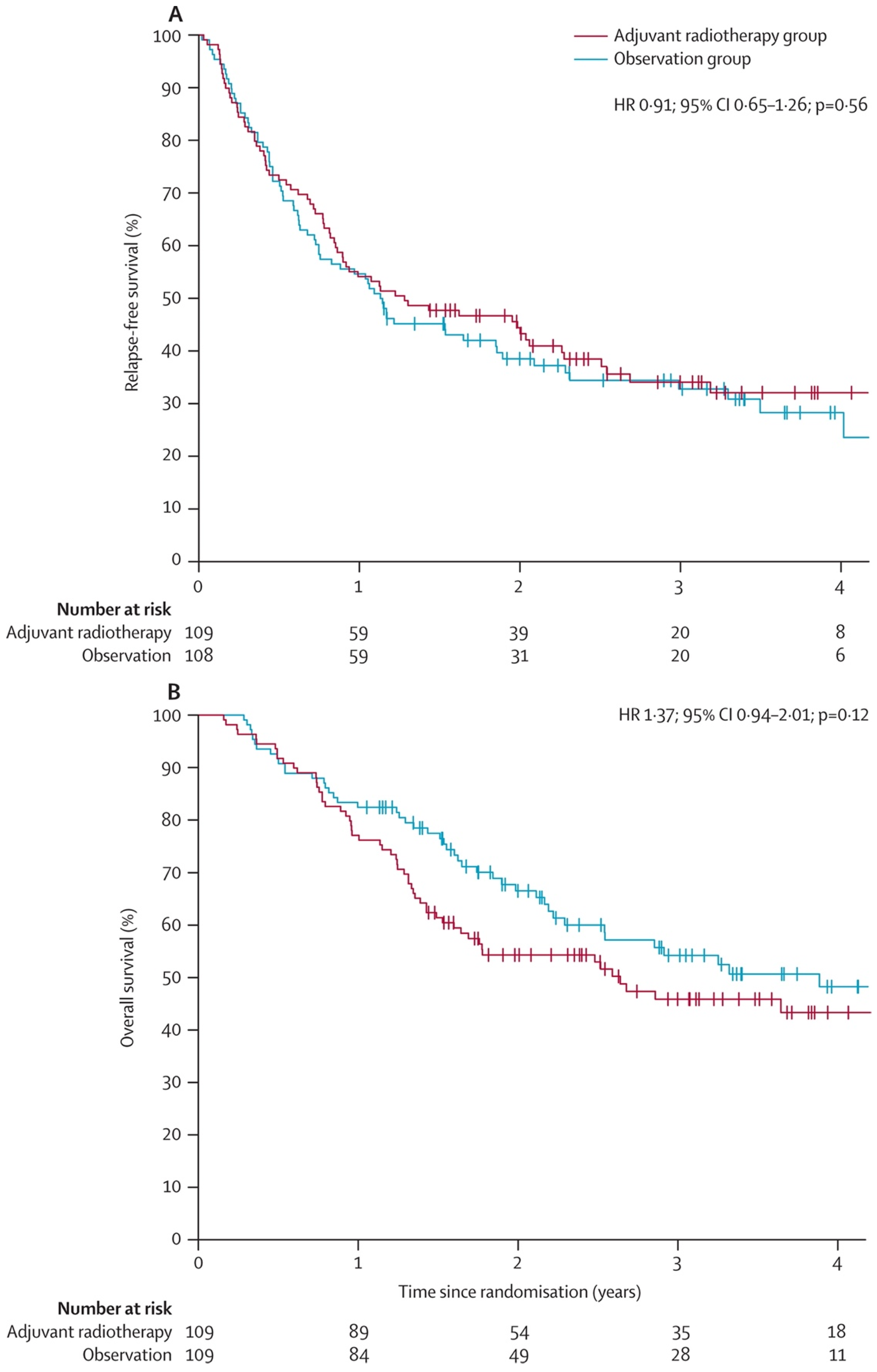
- Henderson, M.A.; Burmeister, B.H.; Ainslie, J.; Fisher, R.; Di Iulio, J.; Smithers, B.M.; Hong, A.; Shannon, K.; Scolyer, R.A.; Carruthers, S.; et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015, 16, 1049–1060. [Google Scholar] [CrossRef]
- Burmeister, B.H.; Henderson, M.A.; Ainslie, J.; Fisher, R.; Di Iulio, J.; Smithers, B.M.; Hong, A.; Shannon, K.; Scolyer, R.A.; Carruthers, S.; et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: A randomised trial. Lancet Oncol. 2012, 13, 589–597. [Google Scholar] [CrossRef]
- Swetter, S.M.; Johnson, D.; Albertini, M.R.; Barker, C.A.; Bateni, S.; Baumgartner, J.; Bhatia, S.; Bichakjian, C.; Boland, G.; Chandra, S.; et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Glitza, I.C.; Davies, M.A. Genotyping of cutaneous melanoma. Chin. Clin. Oncol. 2014, 3, 27. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, J.W.; Kim, Y.S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: A meta-analysis. Br. J. Dermatol. 2011, 164, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit With Adjuvant Dabrafenib Plus Trametinib in Patients With Resected BRAF V600–Mutant Stage III Melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef]
- Maio, M.; Lewis, K.; Demidov, L.; Mandalà, M.; Bondarenko, I.; Ascierto, P.A.; Herbert, C.; Mackiewicz, A.; Rutkowski, P.; Guminski, A.; et al. Adjuvant vemurafenib in resected, BRAFV600 mutation-positive melanoma (BRIM8): A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 510–520. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Lewis, K.D.; Di Giacomo, A.M.; Demidov, L.; Mandalà, M.; Bondarenko, I.; Herbert, C.; Mackiewicz, A.; Rutkowski, P.; Guminski, A.; et al. Prognostic impact of baseline tumour immune infiltrate on disease-free survival in patients with completely resected, BRAF(v600) mutation-positive melanoma receiving adjuvant vemurafenib. Ann. Oncol. 2020, 31, 153–159. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Systemic Immunostimulatory Effects of Radiation Therapy Improves the Outcomes of Patients With Advanced NSCLC Receiving Immunotherapy. Am. J. Clin. Oncol. 2020, 43, 218–228. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Demaria, S.; Golden, E.B.; Formenti, S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015, 1, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Straker, R.J.; Song, Y.; Sun, J.; Shannon, A.B.; Cohen, L.S.; Muradova, E.; Daou, H.; Krause, K.; Li, S.; Frederick, D.T.; et al. Adjuvant Radiation Therapy for Clinical Stage III Melanoma in the Modern Therapeutic Era. Ann. Surg. Oncol. 2021, 28, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.; Pan, L.; Sun, J. The positive feedback between lncRNA TNK2-AS1 and STAT3 enhances angiogenesis in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 507, 185–192. [Google Scholar] [CrossRef]
| NCT Study # | Phase | RT | ST | Primary Endpoint | Patient Population |
|---|---|---|---|---|---|
| NCT04594187 | 2 | 30 Gy in five fractions | Pembrolizumab, nivolumab | Time to regional nodal recurrence | Node-positive melanoma |
| NCT05229614 | 2 | Carbon ion RT | Pembrolizumab | Objective response rate (ORR) | Melanoma, non-small cell lung cancer, head/neck squamous cell carcinoma, and urothelial carcinoma |
| NCT05498805 | 2 | SBRT/hypofractionated | PD-1 inhibitor | Progression free survival | Metastatic melanoma |
| NCT04318717 | 2 | 30 Gy in five fractions | Pembrolizumab | Local tumor control rate | Mucosal melanoma |
| NCT02392871 | 1/2 | Palliative RT | Combi-AD | Toxicity profile for RT + Combi-AD | Metastatic/unresectable melanoma |
| NCT04902040 | 1b/2 | Hypofractionated | Plinabulin, pembrolizumab or nivolumab | Incidence of adverse events, ORR | Select advanced cancers |
| NCT04620603 | 1/2 | Brachytherapy | SOC immunotherapy | Number of participants with tumor response | Stage III and IV melanoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wuthrick, E. Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review. Cancers 2024, 16, 3027. https://doi.org/10.3390/cancers16173027
Zhou J, Wuthrick E. Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review. Cancers. 2024; 16(17):3027. https://doi.org/10.3390/cancers16173027
Chicago/Turabian StyleZhou, Jennifer, and Evan Wuthrick. 2024. "Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review" Cancers 16, no. 17: 3027. https://doi.org/10.3390/cancers16173027
APA StyleZhou, J., & Wuthrick, E. (2024). Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review. Cancers, 16(17), 3027. https://doi.org/10.3390/cancers16173027







