Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Search and Analysis
2.2. Surgical Treatment
2.3. Statistical Assessment
3. Results
3.1. Patients and Treatment Prior to Cytoreductive Surgery with HIPEC
3.2. Surgical Treatment and Findings from Pathology
3.3. Short-Term Outcomes
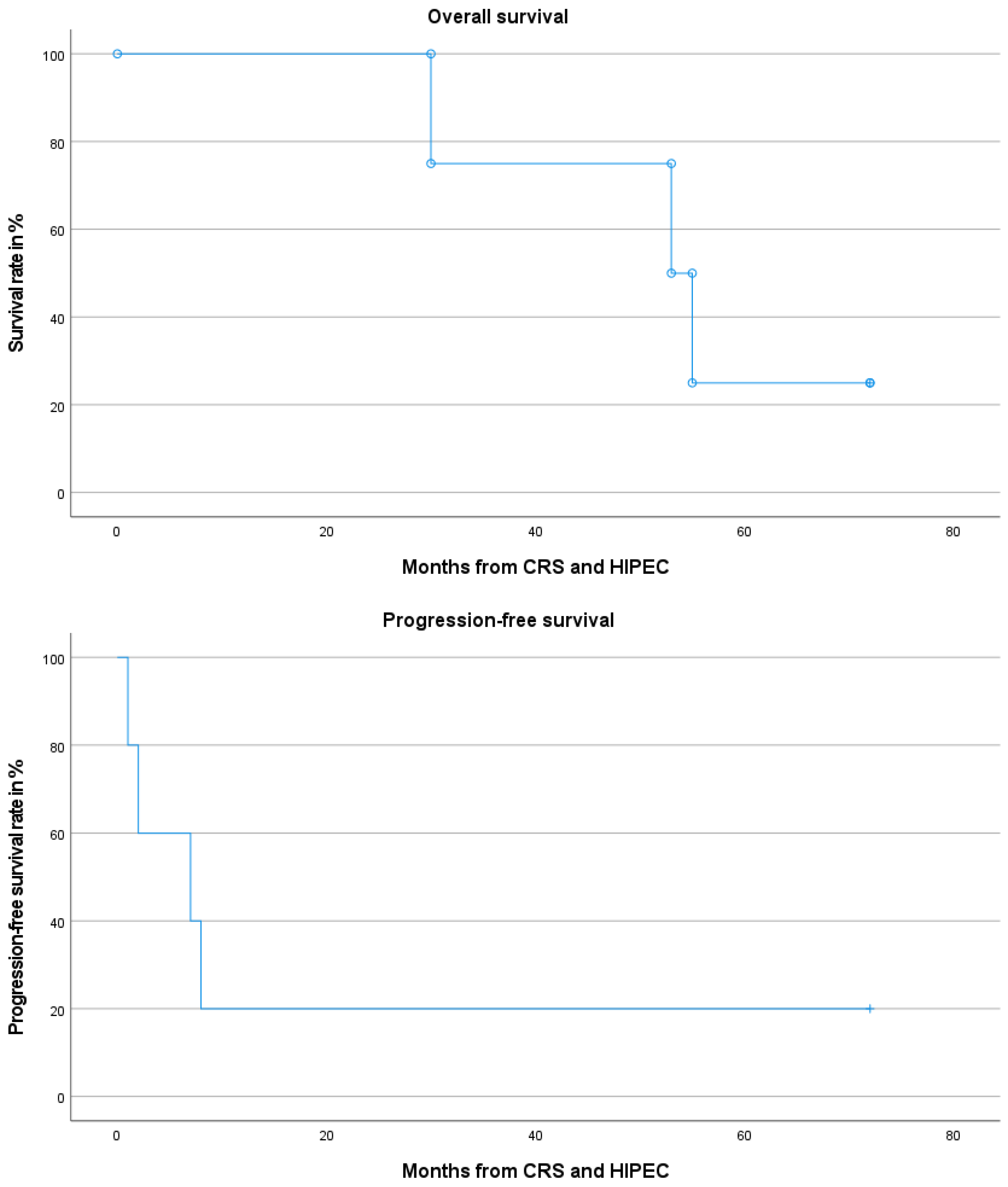
3.4. Long-Term Outcomes and Adjuvant Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilimoria, M.M.; Holtz, D.J.; Mirza, N.Q.; Feig, B.W.; Pisters, P.W.; Patel, S.; Pollock, R.E.; Benjamin, R.S.; Papadopoulos, N.E.; Plager, C.; et al. Tumor volume as a prognostic factor for sarcomatosis. Cancer 2002, 94, 2441–2446. [Google Scholar] [CrossRef]
- Anaya, D.A.; Lahat, G.; Liu, J.; Xing, Y.; Cormier, J.N.; Pisters, P.W.; Lev, D.C.; Pollock, R.E. Multifocality in retroperitoneal sarcoma: A prognostic factor critical to surgical decision-making. Ann. Surg. 2009, 249, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Karakousis, C.P.; Blumenson, L.E.; Canavese, G.; Rao, U. Surgery for disseminated abdominal sarcoma. Am. J. Surg. 1992, 163, 560–564. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Aronson, S.L.; Lopez-Yurda, M.; Koole, S.N.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van Gent, M.; Arts, H.J.G.; van Ham, M.; et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): Final survival analysis of a randomised, controlled, phase 3 trial. Lancet Oncol. 2023, 24, 1109–1118. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Chandrakumaran, K.; Carr, N.J.; Cecil, T.D.; Dayal, S.; Mohamed, F.; Thrower, A.; Moran, B.J. Single centre guidelines for radiological follow-up based on 775 patients treated by cytoreductive surgery and HIPEC for appendiceal pseudomyxoma peritonei. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Sleightholm, R.L.; Rusthoven, C.G.; Koshy, M.; Sher, D.J.; Grover, S.; Simone, C.B., 2nd. Malignant Peritoneal Mesothelioma: National Practice Patterns, Outcomes, and Predictors of Survival. Ann. Surg. Oncol. 2018, 25, 2018–2026. [Google Scholar] [CrossRef]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, 158, 683–691. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Goere, D.; Glehen, O.; Quenet, F.; Ducreux, M.; Guilloit, J.-M.; Texier, M.; Benhamou, E.; Elias, D.; BIG-RENAPE; PRODIGE. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-NTC01226394). J. Clin. Oncol. 2018, 36, 3531. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Cavalcanti, A.; Le Péchoux, C.; Terrier, P.; Vanel, D.; Blay, J.Y.; Le Cesne, A.; Elias, D. Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2005, 31, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaid, A.; Azzam, A.; Abuzaid, M.; Elhassan, T.; Albadawi, N.; Alkhatib, L.; AlOmar, O.; Alsuhaibani, A.; Amin, T.; Al-Badawi, I.A. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy for Management of Peritoneal Sarcomatosis: A Preliminary Single-Center Experience from Saudi Arabia. Gastroenterol. Res. Pract. 2016, 2016, 6567473. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Pennacchioli, E.; Kusamura, S.; Fiore, M.; Balestra, M.R.; Colombo, C.; Mingrone, E.; Gronchi, A.; Deraco, M. Peritoneal sarcomatosis: Is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann. Surg. Oncol. 2010, 17, 3220–3228. [Google Scholar] [CrossRef]
- Bryan, M.L.; Fitzgerald, N.C.; Levine, E.A.; Shen, P.; Stewart, J.H.; Votanopoulos, K.I. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in sarcomatosis from gastrointestinal stromal tumor. Am. Surg. 2014, 80, 890–895. [Google Scholar] [CrossRef]
- Díaz-Montes, T.P.; El-Sharkawy, F.; Lynam, S.; Harper, A.; Sittig, M.; MacDonald, R.; Gushchin, V.; Sardi, A. Efficacy of Hyperthermic Intraperitoneal Chemotherapy and Cytoreductive Surgery in the Treatment of Recurrent Uterine Sarcoma. Int. J. Gynecol. Cancer 2018, 28, 1130–1137. [Google Scholar] [CrossRef]
- Gusani, N.J.; Cho, S.W.; Colovos, C.; Seo, S.; Franko, J.; Richard, S.D.; Edwards, R.P.; Brown, C.K.; Holtzman, M.P.; Zeh, H.J.; et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann. Surg. Oncol. 2008, 15, 754–763. [Google Scholar] [CrossRef]
- Karamveri, C.; Pallas, N.; Kyziridis, D.; Hristakis, C.; Kyriakopoulos, V.; Kalakonas, A.; Vaikos, D.; Tentes, A.K. Cytoreductive Surgery in Combination with HIPEC in the Treatment of Peritoneal Sarcomatosis. Indian J Surg Oncol 2019, 10, 40–45. [Google Scholar] [CrossRef]
- Naffouje, S.A.; Tulla, K.A.; Salti, G.I. A Simplified Peritoneal Sarcomatosis Score for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2018, 9, 1138–1143. [Google Scholar] [CrossRef]
- Rossi, C.R.; Deraco, M.; De Simone, M.; Mocellin, S.; Pilati, P.; Foletto, M.; Cavaliere, F.; Kusamura, S.; Gronchi, A.; Lise, M. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: Clinical outcome and prognostic factors in 60 consecutive patients. Cancer 2004, 100, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Sardi, A.; Sipok, A.; Baratti, D.; Deraco, M.; Sugarbaker, P.; Salti, G.; Yonemura, Y.; Sammartino, P.; Glehen, O.; Bakrin, N.; et al. Multi-institutional study of peritoneal sarcomatosis from uterine sarcoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2017, 43, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Kopanakis, N.; Argyriou, E.O.; Vafias, E.; Efstathiou, E. Locoregional treatment of peritoneal sarcomatosis A single-centre experience. Ann. Ital. Chir. 2016, 87, 333–336. [Google Scholar] [PubMed]
- Wong, L.C.K.; Li, Z.; Fan, Q.; Tan, J.W.; Tan, Q.X.; Wong, J.S.M.; Ong, C.J.; Chia, C.S. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal sarcomatosis-A systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 640–648. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Dubois, D.F. A formula to estimate the approximate surface area if height and body mass be known. Arch. Intern. Med. 1916, 17, 863–871. [Google Scholar] [CrossRef]
- Karakousis, C.P.; Kontzoglou, K.; Driscoll, D.L. Intraperitoneal chemotherapy in disseminated abdominal sarcoma. Ann. Surg. Oncol. 1997, 4, 496–498. [Google Scholar] [CrossRef]
- Klingler, F.; Ashmawy, H.; Häberle, L.; Esposito, I.; Schimmöller, L.; Knoefel, W.T.; Krieg, A. Treatment Pathways and Prognosis in Advanced Sarcoma with Peritoneal Sarcomatosis. Cancers 2023, 15, 1340. [Google Scholar] [CrossRef]
- Almasri, M.S.; Hakeam, H.A.; Alnajashi, N.S.; Alzamil, L.A.; Azzam, A.Z.; Amin, T.M. Cytoreductive Surgery with Bidirectional Intraoperative Chemotherapy (BDIC) Using Intravenous Ifosfamide Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Peritoneal Sarcomatosis: A Retrospective Cohort Study. Ann. Surg. Oncol. 2024, 31, 2368–2377. [Google Scholar] [CrossRef]
- Lopez-Ramirez, F.; Sardi, A.; Studeman, K.; King, M.C.; Falla-Zuniga, L.F.; Nikiforchin, A.; Baron, E.; Nieroda, C.; Gushchin, V.; Diaz-Montes, T. Outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal dissemination from ovarian carcinosarcoma. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2023, 49, 1495–1503. [Google Scholar] [CrossRef]
- Muñoz Casares, F.C.; Padillo Ruiz, F.J.; González de Pedro, C.; Gómez Barbadillo, J.; Martín Broto, J.; Almoguera González, F.; Díaz Gómez, D.; Fernández-Hernández, J.; González López, J.A.; Asencio Pascual, J.M. Radical cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal sarcomatosis: Results from a reference center and considerations based on current evidence. Cir. Esp. (Engl. Ed.), 2024; in press. [Google Scholar] [CrossRef]
- Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Konigsrainer, I.; Konigsrainer, A.; Beckert, S.; Loffler, M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018, 7, 567. [Google Scholar] [CrossRef] [PubMed]
| Sex—n | |
| Female | 9 |
| Male | 1 |
| Age—Median (min–max) | |
| Years | 46.1 (23–77) |
| Relevant comorbidities—n | |
| Asthma bronchiale | 2 |
| Arterial hypertension | 3 |
| Adipositas (BMI ≥ 30 kg/m2) | 1 |
| Diabetes mellitus (any type) | 2 |
| Other malignancies | 1 |
| None | 4 |
| ASA score | |
| 1 | 0 |
| 2 | 1 |
| 3 | 9 |
| Primary tumor location—n | |
| Pelvis | 4 |
| Ovary | 2 |
| Tuba uterina | 1 |
| Uterus | 1 |
| Mesentery | 1 |
| Retroperitoneum | 1 |
| Primary tumor histological subtype—n | |
| Endometrial stromal sarcoma | 3 |
| Leiomyosarcoma | 3 |
| DSRCT | 2 |
| Retroperitoneal liposarcoma | 1 |
| Carcinosarcoma of the uterine tube | 1 |
| Grading—n | |
| G1 | 2 |
| G2 | 2 |
| G3 | 6 |
| Occurrence of peritoneal metastasis—n | |
| Synchronous | 4 |
| Metachronous | 6 |
| Treatment prior to CRS with HIPEC—n | |
| Induction chemotherapy | 6 |
| Surgery | 3 |
| Radiotherapy | 1 |
| None | 1 |
| PSI—n | |
| <10 | 4 |
| ≥10 | 6 |
| Surgical procedures—n | |
| Peritonectomy | 9 |
| Omental resection | 8 |
| Liver resection | 1 |
| Cholecystectomy | 1 |
| Small bowel resection | 3 |
| Large bowel resection | 5 |
| Rectal resection | 3 |
| Appendectomy | 4 |
| Nephrectomy | 1 |
| Bladder resection | 1 |
| Hysterectomy | 1 |
| Adnexectomy | 2 |
| Completeness of cytoreduction—n | |
| CC0 | 9 |
| CC1 | 1 |
| Features of HIPEC | |
| Duration | 60 min |
| Temperature | 42.0 °C |
| Technique | open–close |
| Drugs and dose | 75 mg cisplatin/m2 BSA |
| 15 mg doxorubicine/m2 BSA | |
| Perfusate | 3.000 mL of saline 0.9% |
| Regression following preoperative treatment | |
| No | 2 |
| ≤60% | 2 |
| >60% | 3 |
| Hospital stay—days (range) | |
| Total | 11.1 (6–17) |
| Intensive care unit | 1.24 (0.6–1.9) |
| Number of postoperative complications according to the Clavien–Dindo classification—n | |
| I | 4 |
| II | 3 |
| IIIa | 1 |
| ≥IIIb | 0 |
| CCI in number of patients—n | |
| 8.7 | 2 |
| 20.9 | 1 |
| 30.8 | 1 |
| 33.5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurttas, C.; Ladurner, R.; Mihaljević, A.L.; Strohäker, J. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis. Cancers 2024, 16, 3034. https://doi.org/10.3390/cancers16173034
Yurttas C, Ladurner R, Mihaljević AL, Strohäker J. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis. Cancers. 2024; 16(17):3034. https://doi.org/10.3390/cancers16173034
Chicago/Turabian StyleYurttas, Can, Ruth Ladurner, André L. Mihaljević, and Jens Strohäker. 2024. "Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis" Cancers 16, no. 17: 3034. https://doi.org/10.3390/cancers16173034
APA StyleYurttas, C., Ladurner, R., Mihaljević, A. L., & Strohäker, J. (2024). Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis. Cancers, 16(17), 3034. https://doi.org/10.3390/cancers16173034






