The Significance of Longitudinal Psoas Muscle Loss in Predicting the Maintenance Efficacy of Durvalumab Treatment Following Concurrent Chemoradiotherapy in Patients with Non-Small Cell Lung Cancer: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Data Collection
2.3. Evaluation of Muscle Quantity and Sarcopenia
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics and Treatment
3.2. Impact of Sarcopenia on Durvalumab’s Efficacy at Different Time Points in Patients with NSCLC
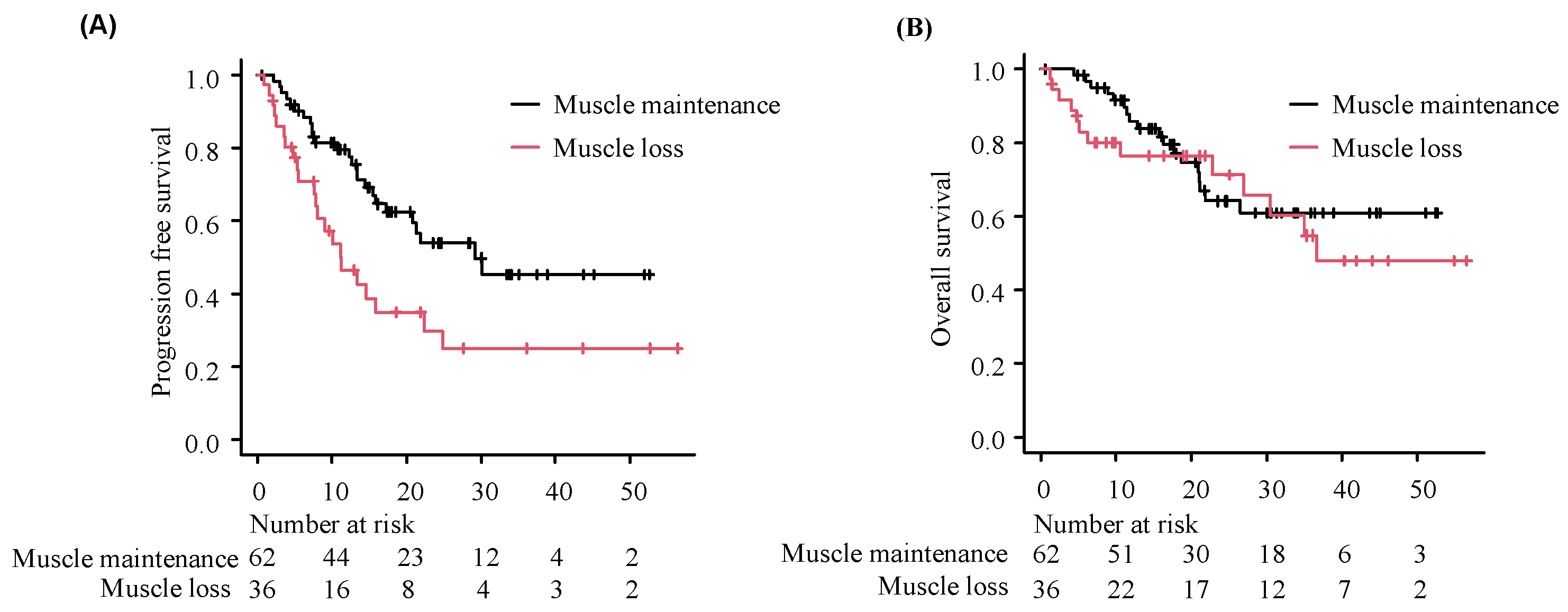
3.3. Impact of Longitudinal Muscle Loss on Clinical Outcomes of Durvalumab Treatment
3.4. Combined Analysis of Longitudinal Muscle Loss and Single Time Point Sarcopenia in Predicting Durvalumab’s Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Mansfield ASMurphy, S.J.; Peikert, T.; Yi, E.S.; Vasmatzis, G.; Wigle, D.A.; Aubry, M.C. Heterogeneity of Programmed Cell Death Ligand 1 Expression in Multifocal Lung Cancer. Clin. Cancer Res. 2016, 22, 2177–2182. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non–Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Lakhani, A.A.; Thompson, S.L.; Sheltzer, J.M. Aneuploidy in human cancer: New tools and perspectives. Trends Genet. 2023, 39, 968–980. [Google Scholar] [CrossRef]
- Salk, J.J.; Schmitt, M.W.; Loeb, L.A. Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat. Rev. Genet. 2018, 19, 269–285. [Google Scholar] [CrossRef]
- Spurr, L.F.; Weichselbaum, R.R.; Pitroda, S.P. Tumor aneuploidy predicts survival following immunotherapy across multiple cancers. Nat. Genet. 2022, 54, 1782–1785. [Google Scholar] [CrossRef]
- Antoun, S.; Morel, H.; Souquet, P.J.; Surmont, V.; Planchard, D.; Bonnetain, F.; Foucher, P.; Egenod, T.; Krakowski, I.; Gaudin, H.; et al. Staging of nutrition disorders in non-small-cell lung cancer patients: Utility of skeletal muscle mass assessment. J. Cachexia Sarcopenia Muscle 2019, 10, 782–793. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Baracos, V.E.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010, 91, 1133S–1137S. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Verna, L.; Porzio, G.; Bozzetti, F.; Palumbo, P.; Masciocchi, C.; Cannita, K.; Parisi, A.; Brocco, D.; Tinari, N.; et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac. Cancer 2019, 10, 347–351. [Google Scholar] [CrossRef]
- Tsukagoshi, M.; Yokobori, T.; Yajima, T.; Maeno, T.; Shimizu, K.; Mogi, A.; Araki, K.; Harimoto, N.; Shirabe, K.; Kaira, K. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine 2020, 99, e19059. [Google Scholar] [CrossRef]
- Roch, B.; Coffy, A.; Jean-Baptiste, S.; Palaysi, E.; Daures, J.P.; Pujol, J.L.; Bommart, S. Cachexia—Sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020, 143, 19–26. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Xu, S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis. Int. Immunopharmacol. 2020, 88, 106907. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Nishioka, N.; Uchino, J.; Hirai, S.; Katayama, Y.; Yoshimura, A.; Okura, N.; Tanimura, K.; Harita, S.; Imabayashi, T.; Chihara, Y.; et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 450. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Minami SIhara, S.; Tanaka, T.; Komuta, K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; Di Marino, P.; Tinari, N.; De Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef]
- Katsui, K.; Ogata, T.; Sugiyama, S.; Yoshio, K.; Kuroda, M.; Hiraki, T.; Kiura, K.; Maeda, Y.; Toyooka, S.; Kanazawa, S. Sarcopenia is associated with poor prognosis after chemoradiotherapy in patients with stage III non-small-cell lung cancer: A retrospective analysis. Sci. Rep. 2021, 11, 11882. [Google Scholar] [CrossRef]
- Rounis, K.; Makrakis, D.; Tsigkas, A.P.; Georgiou, A.; Galanakis, N.; Papadaki, C.; Monastirioti, A.; Vamvakas, L.; Kalbakis, K.; Vardakis, N.; et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: Results from a prospective, observational study. Transl. Lung Cancer Res. 2021, 10, 3538–3549. [Google Scholar] [CrossRef]
- Loosen, S.H.; van den Bosch, V.; Gorgulho, J.; Schulze-Hagen, M.; Kandler, J.; Jördens, M.S.; Tacke, F.; Loberg, C.; Antoch, G.; Brümmendorf, T.; et al. Progressive Sarcopenia Correlates with Poor Response and Outcome to Immune Checkpoint Inhibitor Therapy. J. Clin. Med. 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Maccio, A.; Sanna, E.; Neri, M.; Oppi, S.; Madeddu, C. Cachexia as Evidence of the Mechanisms of Resistance and Tolerance during the Evolution of Cancer Disease. Int. J. Mol. Sci. 2021, 22, 2890. [Google Scholar] [CrossRef]
- Myers, J.S. Proinflammatory cytokines and sickness behavior: Implications for depression and cancer-related symptoms. Oncol. Nurs. Forum 2008, 35, 802–807. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Davis, J.M.; Muga, S.J.; Carson, J.A. Interleukin-6 and cachexia in ApcMin/+ mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R393–R401. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Almand, B.; Clark, J.I.; Nikitina, E.; van Beynen, J.; English, N.R.; Knight, S.C.; Carbone, D.P.; Gabrilovich, D.I. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J. Immunol. 2001, 166, 678–689. [Google Scholar] [CrossRef]
- Ballaro, R.; Beltrà, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [PubMed]
- Holmen Olofsson, G.; Jensen, A.W.P.; Idorn, M.; Thor Straten, P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. Int. J. Mol. Sci. 2020, 21, 3816. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–920,922. [Google Scholar]
- Steensberg, A. IL-6 enhances plasma IL-1ra, IL-10, and cortisol-in-humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, e433–e437. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor. Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, W.; Wang, H.; He, J.; Su, C.; Yu, Q. Impact of Smoking History on Response to Immunotherapy in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 703143. [Google Scholar] [CrossRef]

| Characteristic n (%) | Total N = 98(%) | Muscle Maintenance Group N = 62(%) | Muscle Loss Group N = 36(%) | p-Value |
|---|---|---|---|---|
| Age (years) Median [range] <75 ≥75 | 71.5 [49–87] 60 (61.2) 38 (38.8) | 70.5 [49–87] 40 (64.5) 22 (35.5) | 72.0 [49–84] 20 (55.6) 16 (44.4) | 0.55 0.40 |
| Gender Male Female | 73 (74.5) 25 (25.5) | 46 (74.2) 16 (25.8) | 27 (75.0) 9 (25.0) | 1.00 |
| ECOG-PS 0 1 | 41 (41.8) 57 (58.2) | 25 (40.3) 37 (59.7) | 16 (44.4) 20 (55.6) | 0.83 |
| Stage IIB IIIA IIIB IIIC Postoperative recurrence | 4 (4.1) 35 (35.7) 39 (39.8) 14 (14.3) 6 (6.1) | 1 (1.6) 19 (30.6) 27 (43.5) 10 (16.1) 5 (8.1) | 3 (8.3) 16 (44.4) 12 (33.3) 4 (11.1) 1 (2.8) | 0.24 |
| Histology Adeno Others | 45 (45.9) 53 (54.1) | 25 (40.3) 37 (59.7) | 20 (55.6) 16 (44.4) | 0.21 |
| Driver oncogenes EGFR mutation ALK rearrangement Others Negative Unknown | 8 (8.2) 1 (1.0) 5 (5.1) 71 (72.4) 14 (13.3) | 4 (6.5) 1 (1.6) 4 (6.5) 45 (72.6) 9 (12.9) | 4 (11.1) 0 (0.0) 1 (2.8) 26 (72.2) 5 (13.9) | 0.86 |
| PD-L1 <50% ≥50% Unknown | 47 (46.9) 39 (40.8) 12 (12.2) | 28 (45.2) 27 (43.5) 7 (11.3) | 19 (52.8) 12 (33.3) 5 (13.9) | 0.62 |
| BMI-kg/m2 (pre-chemoradiotherapy) Median [range] <22 ≥22 | 21.9 [15.6–30.5] 52 (53.1) 46 (46.9) | 21.5 [16.5–28.3] 38 (61.3) 24 (38.7) | 23.0 [15.6–30.5] 14 (38.9) 22 (61.1) | 0.005 |
| BMI -kg/m2 (post-chemoradiotherapy) Median [range] <22 ≥22 | 21.2 [15.2–28.7] 63 (64.3) 35 (35.7) | 21.0 [15.4–28.7] 44 (71.0) 18 (29.0) | 21.8 [15.2–27.6] 19 (52.8) 17 (47.2) | 0.08 |
| PMI-cm2/m2 (pre-chemoradiotherapy) Median [range] Male Female | 5.03 [2.24–10.25] 3.52 [2.23–6.50] | 4.68 [2.24–10.25] 3.28 [2.23–6.14] | 5.25 [3.55–8.22] 4.32 [2.77–6.50] | 0.01 0.07 |
| PMI-cm2/m2 (post-chemoradiotherapy) Median [range] Male Female | 4.60 [2.31–9.58] 3.21 [2.09–6.28] | 4.66 [2.31–9.58] 3.16 [2.41–6.28] | 4.43 [2.97–6.47] 3.58 [2.09–4.38] | 0.61 0.89 |
| Sarcopenia (pre-chemoradiotherapy) yes no | 82 (83.7) 16 (16.3) | 54 (87.1) 8 (12.9) | 28 (77.8) 8 (22.2) | 0.26 |
| Sarcopenia (post-chemoradiotherapy) yes no | 87 (88.8) 11 (11.2) | 54 (87.1) 8 (12.9) | 33 (91.7) 3 (8.3) | 0.74 |
| Cachexia yes no | 53 (54.1) 45 (45.9) | 32 (51.6) 30 (48.4) | 21 (58.3) 17 (41.7) | 0.54 |
| Period between CCRT and ICIs (days) Median [range] | 15.0 [1.0–49.0] | 12.0 [1.0–45.0] | 21.5 [1.0–49.0] | 0.002 |
| Chemotherapy CDDP+DTX CBDCA+PTX Daily CBDCA CDDP+VNR Weekly CBDCA+PTX Others | 24 (24.5) 45 (45.9) 7 (7.1) 7 (7.1) 13 (13.3) 2 (2.0) | 17 (27.4) 26 (41.9) 5 (8.1) 4 (6.5) 9 (14.5) 1 (2.8) | 7 (19.4) 19 (52.8) 2 (5.6) 3 (8.3) 4 (11.1) 1 (1.6) | 0.87 |
| ICI completion rate | 49 (50.0) | 36 (58.1) | 13 (36.1) | 0.07 |
| Adverse Events during Concurrent Chemoradiotherapy | ||||
| Leukopenia Any grade Grade 3 or more | 49 (50) 40 (40.8) | 34 (54.8) 28 (45.2) | 15 (41.7) 12 (33.3) | 0.30 |
| Neutropenia Any grade Grade 3 or more | 43 (43.9) 35 (35.7) | 29 (46.8) 24 (38.7) | 14 (38.9) 11 (30.6) | 0.53 |
| Thrombocytopenia Any grade Grade 3 or more | 20 (20.4) 0 | 12 (21.0) 0 | 8 (22.2) 0 | 1.00 |
| Febrile neutropenia Grade 3 or more | 6 (6.1) | 4 (6.5) | 2 (5.6) | 1.00 |
| Anemia Any grade Grade 3 or more | 25 (25.5) 3 (3.1) | 16 (25.8) 0 | 9 (25.0) 3 (8.3) | 1.00 |
| Radiation pneumonitis Any grade Grade 3 or more | 42 (42.9) 2 (2.0) | 27 (43.5) 1 (1.6) | 15 (41.7) 1 (2.8) | 1.00 |
| Anorexia Any grade Grade 3 or more | 34 (34.7) 11 (11.2) | 22 (35.5) 8 (12.9) | 12 (33.3) 3 (8.3) | 1.00 |
| Radiation esophagitis Any grade Grade 3 or more | 41 (41.8) 14 (14.3) | 26 (41.9) 8 (12.9) | 15 (41.7) 6 (16.7) | 1.00 |
| Hypoalbuminemia Any grade Grade 3 or more | 18 (18.4) 0 | 12 (19.4) 0 | 6 (16.7) 0 | 0.79 |
| Predictors of PFS | Crude HR | 95% CI | p-Value | Adjusted HR | 95 CI | p-Value |
|---|---|---|---|---|---|---|
| Muscle loss rate-% (<10 vs. ≥10) | 0.47 | 0.26–0.83 | p < 0.05 | 0.478 | 0.26–0.86 | p < 0.05 |
| Age (years) (<75 vs. ≥75) | 0.54 | 0.31–0.97 | p < 0.05 | 0.59 | 0.33–1.07 | 0.08 |
| ECOG-PS (0 vs. 1) | 1.11 | 0.62–1.97 | 0.73 | 1.28 | 0.60–1.97 | 0.78 |
| Histology (Adeno vs. others) | 0.69 | 0.38–1.23 | 0.21 | NI | NI | NI |
| Mutation (+ vs. −) | 0.83 | 0.35–1.95 | 0.67 | NI | NI | NI |
| PD-L1 (≥50% vs <50%) | 1.06 | 0.70–1.63 | 0.78 | 1.18 | 0.64–2.18 | 0.59 |
| Pre-chemotherapy BMI (kg/m2) (≥22 vs. <22) | 1.18 | 0.67–2.10 | 0.57 | NI | NI | NI |
| Post-chemotherapy BMI (kg/m2) (≥22 vs. <22) | 0.77 | 0.41–1.43 | 0.40 | NI | NI | NI |
| Cachexia (No vs. Yes) | 0.68 | 0.38–1.22 | 0.19 | NI | NI | NI |
| Sarcopenia (pre-chemotherapy) (No vs. Yes) | 0.44 | 0.16–1.22 | 0.11 | NI | NI | NI |
| Sarcopenia (post-chemotherapy) (No vs. Yes) | 0.32 | 0.08–1.33 | 0.12 | NI | NI | NI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuno, H.; Nishioka, N.; Yamada, T.; Kunimatsu, Y.; Yoshimura, A.; Hirai, S.; Futamura, S.; Masui, T.; Egami, M.; Chihara, Y.; et al. The Significance of Longitudinal Psoas Muscle Loss in Predicting the Maintenance Efficacy of Durvalumab Treatment Following Concurrent Chemoradiotherapy in Patients with Non-Small Cell Lung Cancer: A Retrospective Study. Cancers 2024, 16, 3037. https://doi.org/10.3390/cancers16173037
Kuno H, Nishioka N, Yamada T, Kunimatsu Y, Yoshimura A, Hirai S, Futamura S, Masui T, Egami M, Chihara Y, et al. The Significance of Longitudinal Psoas Muscle Loss in Predicting the Maintenance Efficacy of Durvalumab Treatment Following Concurrent Chemoradiotherapy in Patients with Non-Small Cell Lung Cancer: A Retrospective Study. Cancers. 2024; 16(17):3037. https://doi.org/10.3390/cancers16173037
Chicago/Turabian StyleKuno, Haruka, Naoya Nishioka, Tadaaki Yamada, Yusuke Kunimatsu, Akihiro Yoshimura, Soichi Hirai, Shun Futamura, Taiki Masui, Masashi Egami, Yusuke Chihara, and et al. 2024. "The Significance of Longitudinal Psoas Muscle Loss in Predicting the Maintenance Efficacy of Durvalumab Treatment Following Concurrent Chemoradiotherapy in Patients with Non-Small Cell Lung Cancer: A Retrospective Study" Cancers 16, no. 17: 3037. https://doi.org/10.3390/cancers16173037






