Simple Summary
Bladder cancer is an economically costly cancer, especially in Brazil, where treatment and diagnosis vary greatly across regions. This study revealed that, after the age of 50, mortality from this disease increases, and is more common among white people and residents of the southern region of Brazil. The lack of health professionals and the lower investment in health actions and services contributed to this increase in mortality. In addition, the performance of basic procedures, such as punctures and cystoscopy examinations, was lower in regions with higher mortality rates; however, more complex surgeries failed to reduce mortality rates. These results underscore the need to improve public health policies and ensure better access to healthcare for cancer, especially in Brazilian regions with fewer resources.
Abstract
Bladder cancer is one of the most economically costly types of cancer, but few studies have evaluated its mortality considering the factors that impact this outcome. This study aimed to investigate the impact of sociodemographic factors, period, cohort, and health services on bladder cancer mortality. This ecological study analyzed bladder cancer mortality data in Brazil from 2000 to 2022 and evaluated sociodemographic variables (race, region of residence), socioeconomic variables (gross domestic product per capita, Gini index of household income per capita, number of health professionals per inhabitant, expenditure on public health services, and consultations per inhabitant), and bladder cancer diagnosis and treatment procedures. These data were subjected to statistical analysis, which revealed that after the age of 50, there was a progressive increase in the risk of bladder cancer. Indigenous people had the lowest mortality rate, while white people had a significantly greater mortality rate than black and brown people. The North Region and Northeast Region presented the lowest mortality rates, whereas the South Region presented the highest mortality rates. In the South and Southeast Regions, a higher GDP was related to lower mortality. In the South, higher mortality was associated with a lower number of consultations per inhabitant per region. Fewer bladder punctures/aspirations and bladder biopsies were associated with higher mortality rates. In oncology, more procedures, such as total cystectomy, cystoenteroplasty, and total cystectomy with a single shunt, do not reduce the mortality rate. These results can serve as guidelines for adjusting public health policies.
1. Introduction
Bladder cancer is one of the main types of cancer affecting the population globally [1]. The global incidence has been stable, but increases have been observed in certain regions of the world, impacting mortality, probably due to exposure to carcinogens [1]. In 2022, there were approximately 614,000 new cases and 220,000 deaths worldwide, representing approximately 3% of all new cases and more than 2% of cancer deaths [2].
Bladder cancer is one of the most economically costly types of cancer owing to the intensive treatment and monitoring needed, burdening patients and healthcare systems [3]. There are differences in survival and mortality rates between different regions of the world, which can be partly explained by differences in treatment protocols, healthcare systems, and access to diagnosis and treatment facilities [4]. In Brazil, there were an estimated 11,370 new cases and 5119 deaths in 2023. The estimated risk corresponds to 5.25 cases per 100,000 inhabitants, with men being more affected than women. These values represent an estimated risk of 7.45 new cases per 100,000 men [5]. However, few studies have evaluated mortality in the Brazilian population, and the studies found in this country refer to a specific region [6] and short periods without considering the country’s regional socioeconomic differences [7].
The most well-established risk factor for bladder cancer is smoking, which accounts for 30 to 50% of all cases. Other risk factors include age and sex, all of which have significant impacts on the development and progression of bladder cancer [8]. Thus, analyzing how these factors impact mortality in different administrative regions can contribute to the formulation of public policies.
In terms of race, the black population was associated with worse survival than other races. Although studies suggest a more aggressive manifestation of the disease in black patients, the disparity in mortality mostly reflects social barriers that disproportionately affect this population. These barriers include difficulties in accessing diagnosis and treatment, often resulting in the disease being diagnosed at an advanced stage [9]. In this sense, we believe that in Brazil, owing to the racial distribution in different regions, we can verify specific mortality rates.
Access to the public health system in Brazil may have improved from 2008 to 2017, as not only did the number of individuals increase significantly but also the proportion of cystectomies decreased, which may reflect an improvement in early-stage treatment for this type of cancer [7]. Nevertheless, the distribution of services is uneven across the country, and a more specific analysis is needed.
In this context, this study aimed to investigate the impact of sociodemographic factors (age, race, and region), period, cohort, and health services on bladder cancer mortality in Brazil from 2000 to 2022. Our hypothesis is that individuals over the age of 50 years in conditions of greater vulnerability (residents of the North and Northeast Regions; black and indigenous race) and regions with the lowest availability of health services have the highest mortality rates from bladder cancer.
2. Methods
2.1. Type of Study and Ethical Aspects
This is an observational study with an ecological design [10] that uses secondary data from public information systems on bladder cancer mortality in Brazil between 2000 and 2022. As the databases are in the public domain, without identifying the individuals, there was no need for approval by a Research Ethics Committee, as per the guidelines of the National Health Council of Brazil, CNS Resolution No. 510 (4 July 2016).
2.2. Population
Secondary data on all individuals who died from bladder cancer of both sexes were reviewed. The deaths analyzed were from the period between 2000 and 2022 and were registered in the Department of Informatics of the Unified Health System (DATASUS) of the Ministry of Health of Brazil (https://datasus.saude.gov.br/, accessed on 11 August 2024).
2.3. Inclusion and Exclusion Criteria
This study included patients who died between 2000 and 2022 and were classified under the ICD-10 code C67 (malignant neoplasm of the bladder) [11]. Individuals who died outside of the study period were excluded from the analysis, and individuals with unknown information were excluded from the analyses.
2.4. Database and Variables Analyzed
The variables investigated and their descriptions, the information systems consulted, and the collection period are listed in Table 1. The units of analysis were the five geographical regions of Brazil (North, Northeast, South, Southeast, and Midwest), according to the Brazilian Institute of Geography and Statistics.

Table 1.
Description of the variables analyzed, information systems consulted, and collection period.
The DATASUS of the Ministry of Health of Brazil offers a secondary open-access database. The mortality information used to calculate the age-standardized mortality rate (ASMR), as well as sociodemographic data, access factors, spending on public services, diagnosis, and treatment, were obtained from DATASUS through the records of the mortality information system (SIM), hospital information system (SIH), outpatient information system (SIA), and indicators and basic data (IDB).
The population data used to calculate the crude-specific mortality rate were found from a projection using population data from the Brazilian Institute of Geography and Statistics (IBGE) (https://ibge.gov.br/, accessed on 11 August 2024). The number of deaths used to calculate the age-adjusted standardized mortality rate was obtained according to place of residence. The world standard population according to the World Health Organization [12] was used to calculate the age-standardized mortality rate.
2.5. Statistical Analysis
To identify the influence of age, period, and birth cohort on deaths from bladder cancer, the APC Web tool (Biostatistics Branch, National Cancer Institute, Bethesda, MD, USA) was used [13]. The important functions of the APC offered by this tool are useful in oncological applications. As described in Nascimento et al. [14], the following parameters were studied in this model: net drift; all age, period, or cohort deviations; all period or cohort rate ratios (RRs); and all local drift. Additionally, Wald tests were applied followed by chi-square tests (x2) to identify statistically significant variables (p < 0.05) on the basis of age, period, and birth cohort. The age group of the study population and the number of deaths were grouped into 5-year intervals, resulting in 18 age groups (from 0–4 years to 85 years or more). In addition, 4 periods (2000–2004 to 2015–2019) and 21 birth cohorts were considered, each with an interval of 5 years (1915–2015).
The results of the descriptive analysis were expressed in measures of central tendency and dispersion. The normality of the data was evaluated with the Shapiro–Wilk test. The nonparametric Kruskal–Wallis test was used to verify whether there were statistically significant differences between the median crude mortality rates and those adjusted for race and geographic region.
A multivariate linear regression analysis was performed to assess the predictive value of the age-standardized mortality rate via the adjusted model.
The Durbin-Watson test was performed to verify the independence of the residuals, with an acceptable range of [1.5; 2.5]. A Cook’s distance below 1 meant that there were no outliers in the dataset that could impair the estimation of the coefficients. The variance inflation factor (VIF) (less than 10) and tolerance (greater than 0.2) values of the final model revealed the absence of multicollinearity.
In addition, we analyzed the Gaussian distribution and the P-P plot, in which a comparison between the “observed” and “expected” probabilities was used to test the normal distribution. We used the graph of “standardized residuals” versus “standardized predicted values” to verify the constancy of the variance of the residuals.
We conducted intergroup comparisons and multivariate linear regression analysis using the Statistical Package for Social Sciences (SPSS for Windows, version 21.0; IBM, Armonk, NY, USA), considering p-values less than 0.05 as statistically significant.
3. Results
We identified 78,015 bladder cancer deaths in Brazil from 2000 to 2022, with males accounting for 68.78% of these cases. Table 2 provides a descriptive analysis of the entire cohort.

Table 2.
Descriptive analysis of the entire cohort.
3.1. Age–Period–Cohort Effect
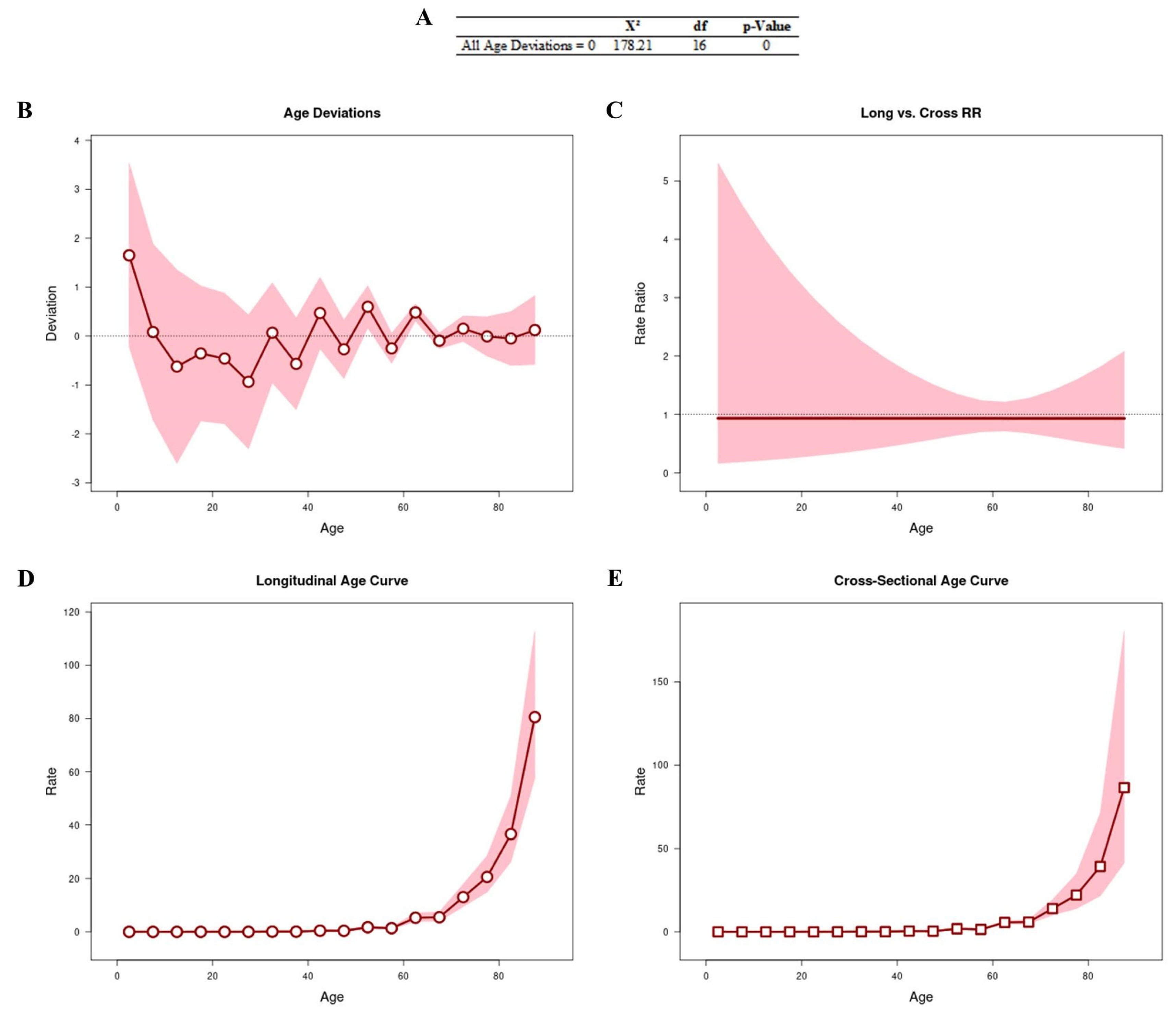
Figure 1 shows the results obtained from the APC analysis to assess whether age is a determining factor in the number of deaths. All age deviations (Figure 1A,B) showed that the adjusted longitudinal and transverse age curves were log-linear (Figure 1C). We observed that the age curves (longitudinal [RR: 1.74; 95% CI: 1.39–2.16] and cross-sectional [RR: 1.86; 95% CI: 1.32–2.61]) were linear, with an increased risk of progression after the age of 50 years (Figure 1D and Figure 1E, respectively).
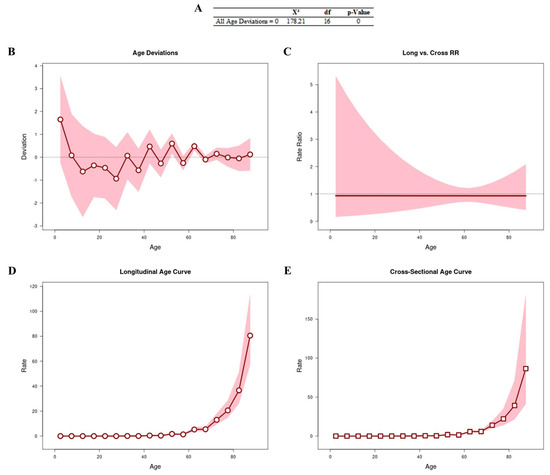
Figure 1.
APC analysis to assess whether age is a determining factor in the number of deaths. All age deviations are shown in (A,B). The age curves are log-linear (C). Longitudinal (D) and transverse (E) age curves.
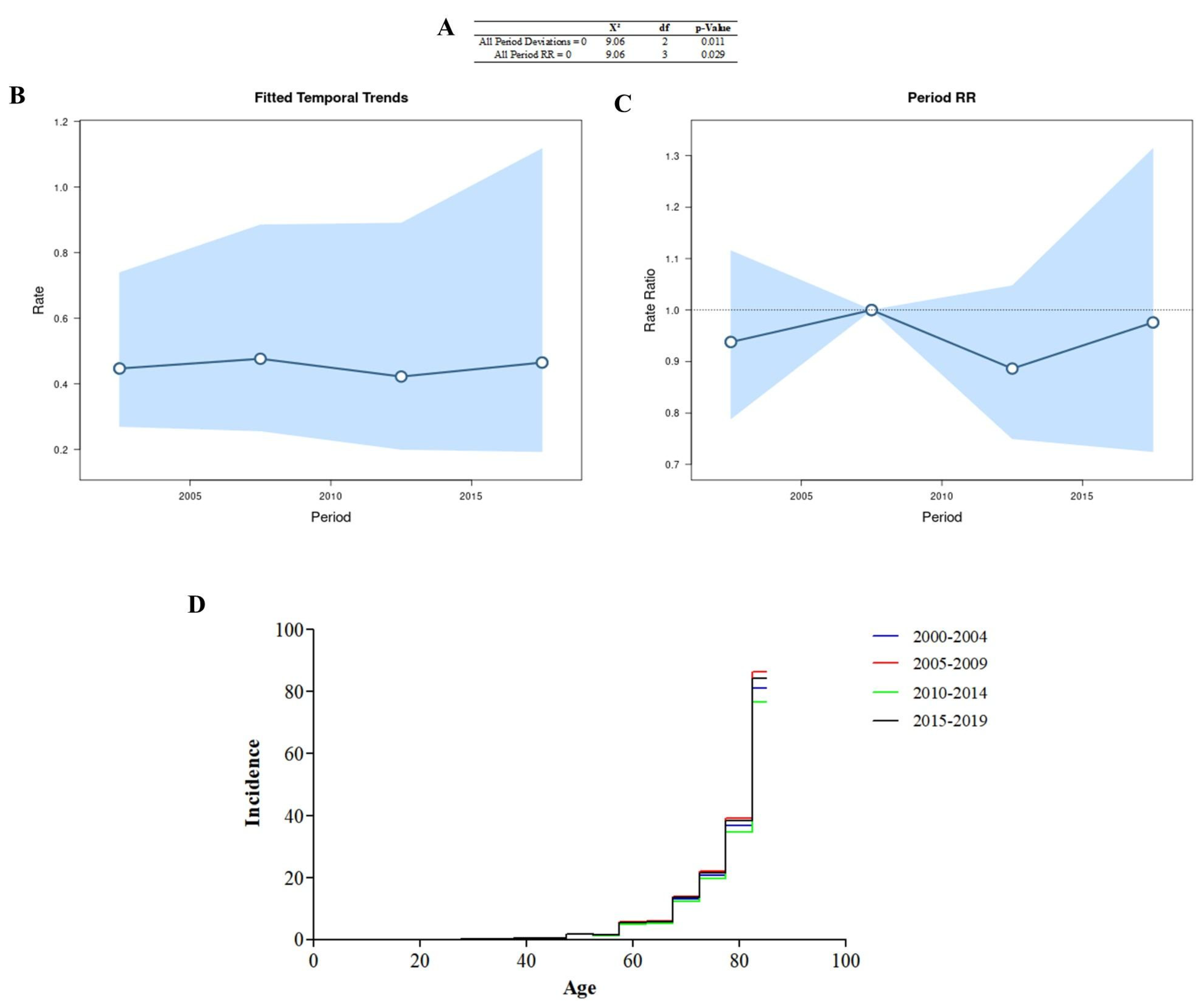
All period deviations were significant (Figure 2A), indicating that the adjusted time trends (Figure 2B) and period rates (Figure 2C) were log-linear. All period rate ratios were significant, and the age incidence pattern in each period (Figure 2D) was determined using the cross-sectional age curve (Figure 1E). The period 2005–2009 had the highest number of cases in the different years.
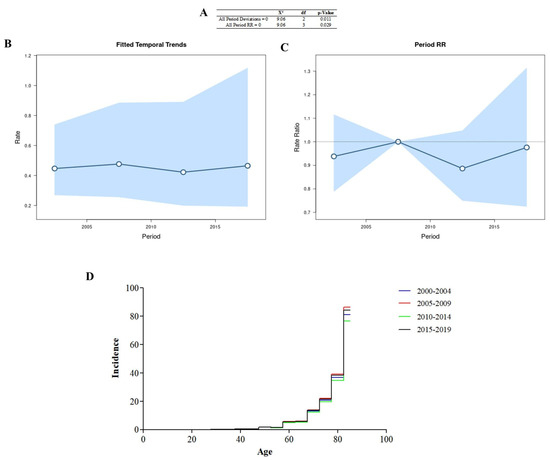
Figure 2.
Age–period–cohort analysis was performed via the Wald test (A), temporal trends (B), period rate ratios (C), and the age incidence pattern for every period (D).
The values for the rate (χ2 = 30.78; df = 20; p = 0.058) and deviation (χ2 =18.35; df = 19; p = 0.499) for the cohort were not significant.
3.2. Sociodemographic Factors
Table 3 presents the socioeconomic predictors of bladder cancer mortality in the different administrative regions of Brazil. We observed that in the South Region and Southeast Region, a higher GDP was related to lower mortality.

Table 3.
Socioeconomic predictors of bladder cancer mortality in the different administrative regions of Brazil.
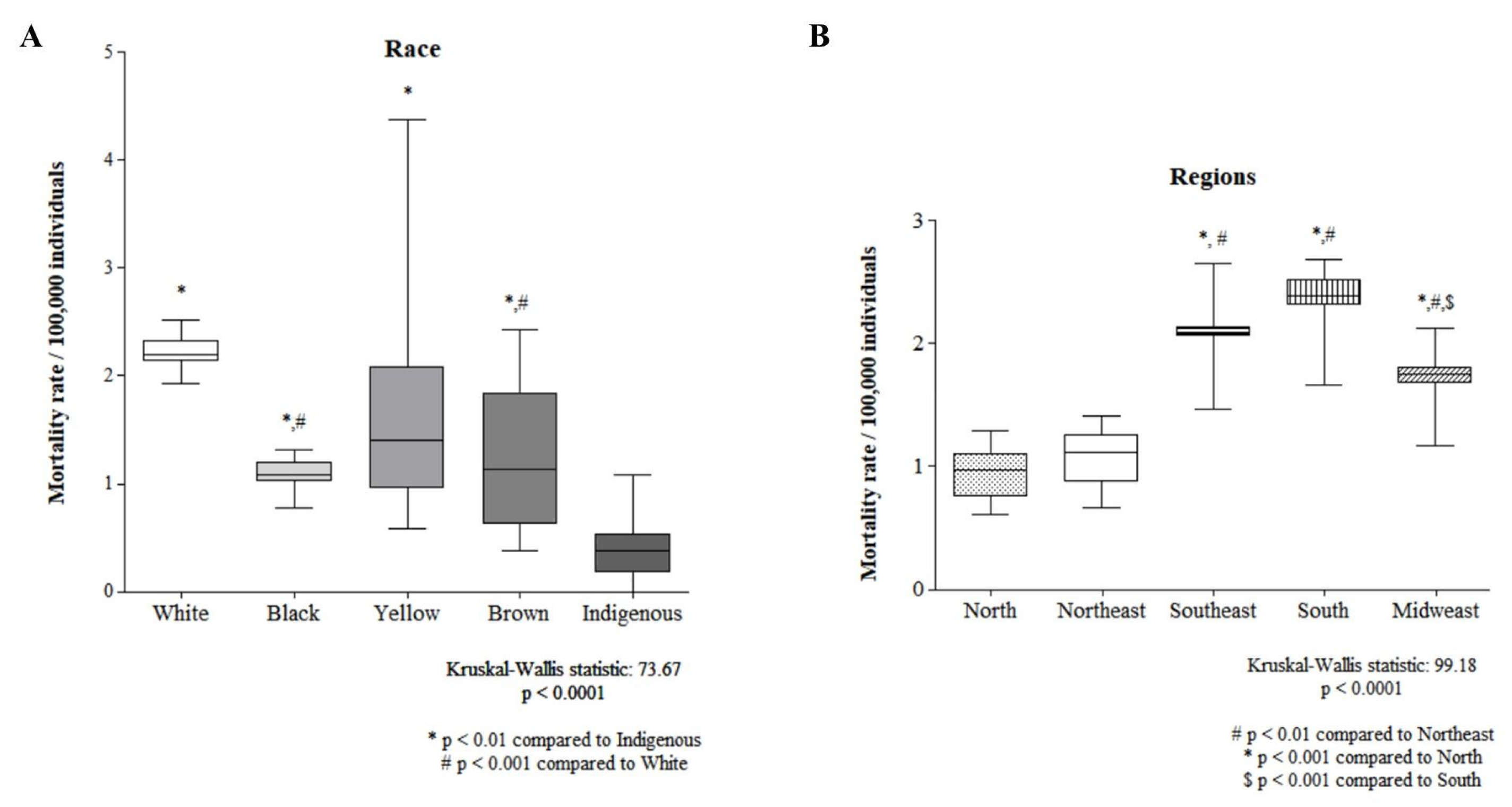
Figure 3 shows the comparison of mortality rates by race and regions of Brazil. The indigenous population had the lowest mortality rate, while that of the white population was significantly greater than that of the black and brown populations (Figure 3A). The North Region and Northeast Region presented the lowest mortality rates, whereas the South Region presented the highest mortality rate (Figure 3B).
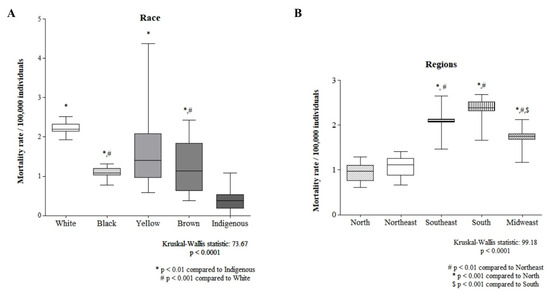
Figure 3.
A comparison between races (A) and regions (B) is shown.
3.3. Health Services, Diagnosis, and Treatment
Table 4 presents the access to and expenditures on public services and diagnoses that predict bladder cancer mortality in the different administrative regions of Brazil. In the Southeast Region, higher mortality was associated with a lower number of health professionals and lower expenses for public health actions and services. Nevertheless, in the South, higher mortality was associated with a lower number of consultations per inhabitant by region.

Table 4.
Access to and expenditure on public services and diagnoses that predict bladder cancer mortality in the different administrative regions of Brazil.
A lower rate of bladder puncture/aspiration was associated with higher mortality rates in Brazil, especially in the Northeast Region and South Region. Fewer cystoscopies and/or ureteroscopies and/or urethroscopies were associated with higher mortality in the North and Midwest Regions. In addition, fewer bladder biopsies were associated with higher death rates in Brazil, especially in the Midwest region.
Table 5 presents the treatments that predict bladder cancer mortality in the different administrative regions of Brazil. In the northern region, we found that less “cystostomy” was associated with a higher mortality rate. However, in oncology, more procedures, such as total cystectomy, cystoenteroplasty, and total cystectomy with a single shunt, do not reduce the mortality rate. A lower percentage of patients who underwent total cystectomy was related to a higher mortality rate in the Southeast Region.

Table 5.
Treatment that predicts bladder cancer mortality in the different administrative regions of Brazil.
4. Discussion
Bladder cancer is one of the most common neoplasms and is associated with high morbidity and mortality for patients [6], in addition to having a high cost of diagnosis and treatment [3]. In Brazil, regional disparities contribute to different patterns of mortality distribution [15]. In this study, we observed that age, period, birth cohort, sociodemographic factors, and those related to health services, diagnosis, and treatment in the different administrative regions of Brazil impacted the determinants of bladder cancer mortality between 2000 and 2022.
Men were the most affected during the evaluated period, representing approximately 70% of the deaths. Another associated factor was age, with a progressive increase in risk from the age of 50 in all regions of Brazil, corroborating the findings of other studies [7] that have also identified these factors. In addition, the number of hospital admissions after the age of 60 has been shown to be high in Brazil [15]. However, the age range found in our study differed from that reported in other studies, which indicated a predominance from the age of 60 years [6,16].
In 2008, the Ministry of Health established the National Policy for Comprehensive Care for Men’s Health, developed in partnership with managers of the Unified Health System and Scientific Society, with the aim of promoting health actions that increase life expectancy and reduce morbidity and mortality from preventable and preventable causes in this population, such as bladder cancer [17]. This may explain why the period from 2005 to 2009 had the highest number of cases, followed by a positive effect of this policy in subsequent years.
In the comparison between races and regions, indigenous people had the lowest mortality rate, whereas whites had significantly higher rates than blacks and browns. The lower mortality rate of indigenous people may be associated with lower exposure to risk factors for occupational exposure, such as aluminum production, rubber production, painting with artificial dyes, such as magenta, or environmental exposure to radiation, medications (cyclophosphamide), opium consumption, and Schistosoma infection [1], which are common in urban centers and are part of the daily life of Caucasian individuals; in another study, most of the men were white [7]. Another point to be highlighted is the barriers encountered by indigenous people in health services and high-cost diagnostic tests, which may have contributed to a lower detection of cases in this population [18].
In our study, the North and Northeast Regions had the lowest mortality rates, whereas the South Region presented the highest mortality rate. This finding converges with other studies that reported a 76% higher mortality rate and a 146% higher hospitalization rate in the South Region than the national average [6]. This phenomenon may be influenced by the cultural habit of consuming chimarrão, a drink based on Yerba mate, since it is associated with regular tobacco consumption [6], which is the greatest predictor of incidence and mortality from bladder cancer [8].
We observed that in the South Region and Southeast Region, a higher GDP was related to lower mortality. Studies have shown that countries with decreasing trends in bladder cancer mortality have a greater average GDP per capita than those with increasing trends [8]. A higher GDP may be associated with better eating habits, greater awareness of the disease, and better access to health services.
The results regarding health services, diagnosis, and treatment were alarming, showing that the lack of access to health services is associated with higher mortality in all the Federative regions of Brazil in different dimensions. In the Southeast Region, higher mortality was associated with a lower number of health professionals and lower expenditures on actions and public services. In the South, higher mortality is associated with a lower number of consultations per inhabitant, indicating that insufficient access to regular medical care can lead to late diagnoses and less effective treatments [19].
In addition, fewer procedures, such as bladder puncture/aspiration (Northeast and South), cystoscopy (North and Midwest), bladder biopsy (Midwest), and cystostomy (North), were associated with higher mortality rates. Although these procedures are often used only for diagnosis or prior treatment, their unavailability or delay in being performed can delay the start of definitive treatment, leading to the progression of the disease at the cost of a worsening prognosis [6].
Despite the fact that more procedures, such as total cystectomy (with or without a single shunt) and cystoenteroplasty, have been performed in the northern region than in the southern region, these procedures have not reduced the mortality rate, which reinforces the slowness of diagnosis and treatment since these procedures are performed in more serious cases and emergencies, possibly with a late diagnosis. Although mortality rates are higher for procedures such as these than for endoscopic procedures [7], it is important to take into account that total cystectomy offers an acceptable rate of perioperative complications and can be offered as a viable treatment option, even in elderly patients [20]. For the cystoenteroplasty procedure, since it involves reconstruction of the bladder via laparoscopy [21], these reconstruction procedures may be indicated in elderly patients if the patient’s condition is favorable [22].
The limitations of our study are that the use of secondary databases introduces an ecological bias since the analyses were carried out at the population level and not at the individual level. In addition, the quality of the data from these databases can vary significantly, impacting mortality estimates. The absence of outpatient and hospitalization data from health services not covered by the Brazilian Unified Health System also limits the scope of the results. There is the possibility of delays or errors when entering data into the Ministry of Health’s system, mainly due to disparities in connectivity infrastructure in the different regions of the country. Another important limitation is racial classification, which is determined by the medical team responsible for completing the death certificate, increasing the possibility of divergence in self-reported data. In addition, the nature of ecological studies prevents direct inferences of causality, and it is essential to carry out case–control or cohort studies.
5. Conclusions
This study revealed that there was an increase in the mortality rate from this condition after the age of 50 years in the period 2005–2009, which was more prevalent among white people and those living in the southern region of Brazil. A lower number of health professionals per inhabitant per region and lower spending on health actions and services had an impact on increasing mortality. A lower performance of “bladder puncture/aspiration”, “cystoscopy and/or ureteroscopy, and/or urethroscopy” and “cystostomy” was associated with higher mortality rates. However, the use of “total cystectomy”, “cystoenteroplasty”, or “total cystectomy with a single shunt in oncology” did not reduce the mortality rate.
Author Contributions
Conceptualization, J.S.d.M.N.; data curation, J.S.d.M.N., A.L.R.R., A.M.d.S., D.S.S., D.B.S.d.N., I.N.R. and R.L.M.G.; formal analysis, J.S.d.M.N., A.L.R.R., A.M.d.S., D.S.S., D.B.S.d.N., I.N.R. and R.L.M.G.; investigation, J.S.d.M.N., A.L.R.R., A.M.d.S., D.S.S., D.B.S.d.N., I.N.R. and R.L.M.G.; methodology, J.S.d.M.N., A.L.R.R., A.M.d.S., D.S.S., D.B.S.d.N., I.N.R. and R.L.M.G.; supervision, J.S.d.M.N., S.F.M., D.d.R.G. and A.L.M.d.N.; writing—original draft, J.S.d.M.N., S.F.M., A.L.R.R., A.M.d.S., D.S.S., D.B.S.d.N., I.N.R., R.L.M.G., D.d.R.G. and A.L.M.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable to this study since the database used in this research contains only consolidated information, without identifying individuals.
Informed Consent Statement
Not applicable. This study analyzes secondary data from Open Access.
Data Availability Statement
All data were made available at https://opendatasus.saude.gov.br/dataset, accessed on 11 August 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0302283823027070 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Van Hoogstraten, L.M.C.; Vrieling, A.; Van Der Heijden, A.G.; Kogevinas, M.; Richters, A.; Kiemeney, L.A. Global trends in the epidemiology of bladder cancer: Challenges for public health and clinical practice. Nat. Rev. Clin. Oncol. 2023, 20, 287–304. Available online: https://www.nature.com/articles/s41571-023-00744-3 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Wéber, A.; Vignat, J.; Shah, R.; Morgan, E.; Laversanne, M.; Nagy, P.; Kenessey, I.; Znaor, A. Global burden of bladder cancer mortality in 2020 and 2040 according to GLOBOCAN estimates. World J. Urol. 2024, 42, 237. Available online: https://link.springer.com/10.1007/s00345-024-04949-8 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Instituto Nacional de Câncer. Estimativa 2023: Incidência de câncer no Brasil; Instituto Nacional De Câncer: Rio de Janeiro, Brasil, 2023. [Google Scholar]
- Chielle, E.O.; Kuiava, V.; Perin, A.T. Epidemiologia da neoplasia maligna de bexiga: Um estudo das taxas de mortalidade e de internação hospitalar. Rev. Atenção Saúde 2020, 17, 62. Available online: https://seer.uscs.edu.br/index.php/revista_ciencias_saude/article/view/5633 (accessed on 26 June 2024).
- Timoteo, F.; Korkes, F.; Baccaglini, W.; Glina, S. Bladder cancer trends and mortality in the brazilian public health system. Int. Braz. J. Urol. 2020, 46, 224–233. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1677-55382020000200224&tlng=en (accessed on 26 June 2024). [CrossRef] [PubMed]
- Teoh, J.Y.; Huang, J.; Ko, W.Y.; Lok, V.; Choi, P.; Ng, C.F.; Sengupta, S.; Mostafid, H.; Kamat, A.M.; Black, P.C.; et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur. Urology 2020, 78, 893–906. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0302283820306977 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Paster, I.C.; Zeng, J.; Recio-Boiles, A.; Chipollini, J. Gender, Racial and Ethnic Differences in Pathologic Response Following Neoadjuvant Chemotherapy for Bladder Cancer Patients. Urology 2023, 178, 105–113. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0090429523004466 (accessed on 26 June 2024). [CrossRef] [PubMed]
- Thiese, M.S. Observational and interventional study design types; an overview. Biochem. Med. 2014, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.H.C.; Bay-Nielsen, H.; Braun, R.; Israel, R.A.; Laurenti, R.; Maguin, P.; Taylor, E. CID-10: Classificação Estatística Internacional de Doenças e Problemas Relacionados à Saúde; EDUSP: São Paulo, Brazil, 2011. [Google Scholar]
- Ahmad, O.B.; Boschi Pinto, C.; Lopez, A.D. Age Standardization of Rates: A New WHO Standard. GPE Discussion Paper Series: No 31. 2001; pp.10–12. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/gpe_discussion_paper_series_paper31_2001_age_standardization_rates.pdf (accessed on 26 June 2024).
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A Web Tool for Age–Period–Cohort Analysis of Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2296–2302. Available online: https://aacrjournals.org/cebp/article/23/11/2296/14669/A-Web-Tool-for-Age-Period-Cohort-Analysis-of (accessed on 4 July 2024). [CrossRef] [PubMed]
- Nascimento, A.Q.; Dantas, D.B.; Melo, G.S.; Gomes, F.D.C.; De Melo Neto, J.S. Impact of sociodemographic factors and screening, diagnosis, and treatment strategies on colorectal cancer mortality in Brazil: A 20-year ecological study. Patel GK, organizador. PLoS ONE 2022, 17, e0274572. Available online: https://dx.plos.org/10.1371/journal.pone.0274572 (accessed on 4 July 2024). [CrossRef] [PubMed]
- Palmeira, I.P.; Guimarães, L.D.S.; Santos, A.K.T.D.; Andrade, R.L.B.D.; Figueiredo, M.B.G.D.A.; Nunes, M.A.P.; Jesus, C.V.F.; Lima, S.O. Evolução comparativa e temporal das tendências de mortalidade por Câncer Colorretal em Sergipe e Nordeste no período de 2008 a 2018. BJHR 2020, 3, 9058–9074. Available online: https://www.brazilianjournals.com/index.php/BJHR/article/view/13712/11485 (accessed on 5 July 2024). [CrossRef]
- Morais Neto, J.F.D.; Mendes, L.M.C.; Ferreira Filho, M.A.G.; Mendes, L.C.; Lino, L.A.; Silva, A.P.D.; Matos, L.F.F.; Garcia, J.P.; Madeira, J.P.B.; Delgado, R.L.O. Análise da internação por neoplasia maligna da bexiga no Brasil entre o período de 2011 a agosto de 2022. RSD 2023, 12, e27112240205. Available online: https://rsdjournal.org/index.php/rsd/article/view/40205 (accessed on 4 July 2024). [CrossRef]
- Chakora, E.S. Política Nacional de Atenção Integral à Saúde do Homem. Esc. Anna Nery 2014, 18. Available online: https://www.scielo.br/j/ean/a/YT4pgHZWTmrzVRdmCn8bTLw/?lang=pt&format=pdf (accessed on 4 July 2024).
- Gomes, S.C.; Esperidião, M.A. Acesso dos usuários indígenas aos serviços de saúde de Cuiabá, Mato Grosso, Brasil. Cad. Saúde Pública 2017, 33, 5. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2017000505010&lng=pt&tlng=pt (accessed on 4 July 2024). [CrossRef] [PubMed]
- Mourão, T.C.; Curado, M.P.; De Oliveira, R.A.R.; Santana, T.B.M.; Favaretto, R.D.L.; Guimarães, G.C. Epidemiology of Urological Cancers in Brazil: Trends in Mortality Rates Over More Than Two Decades. J. Epidemiol. Glob. Health 2022, 12, 239–247. Available online: https://link.springer.com/10.1007/s44197-022-00042-8 (accessed on 4 July 2024). [CrossRef] [PubMed]
- Tamalunas, A.; Volz, Y.; Schlenker, B.A.; Buchner, A.; Kretschmer, A.; Jokisch, F.; Rodler, S.; Schulz, G.; Eismann, L.; Pfitzinger, P.; et al. Is It Safe to Offer Radical Cystectomy to Patients above 85 Years of Age? A Long-Term Follow-Up in a Single-Center Institution. Urol Int. 2020, 104, 975–981. Available online: https://pubmed.ncbi.nlm.nih.gov/32871580/ (accessed on 14 August 2024). [CrossRef] [PubMed]
- Hsueh, T.Y.; Huang, Y.H.; Chiu, A.W.; Huan, S.K.; Lee, Y.H. Survival analysis in patients with upper urinary tract transitional cell carcinoma: A comparison between open and hand-assisted laparoscopic nephroureterectomy. BJU Int. 2007, 99, 632–636. Available online: https://bjui-journals.onlinelibrary.wiley.com/doi/10.1111/j.1464-410X.2006.06665.x (accessed on 20 August 2024). [CrossRef] [PubMed]
- Saika, T.; Suyama, B.; Murata, T.; Manabe, D.; Kurashige, T.; Nasu, Y.; Tsushima, T.; Kumon, H. Orthotopic neobladder reconstruction in elderly bladder cancer patients. Int. J. Urol. 2008, 8, 533–538. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1442-2042.2001.00367.x?sid=nlm%3Apubmed (accessed on 20 August 2024). [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).