Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive Analysis
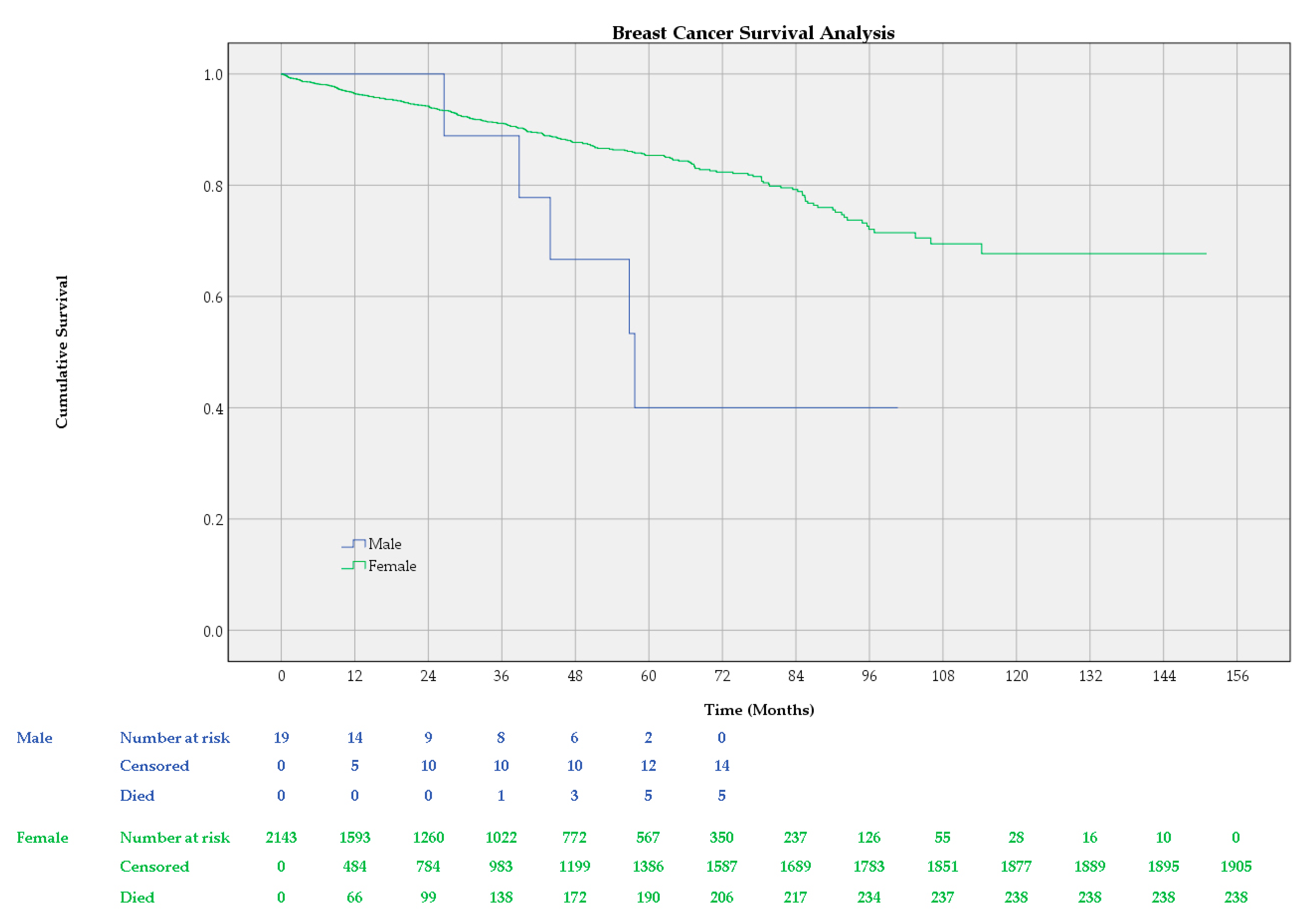
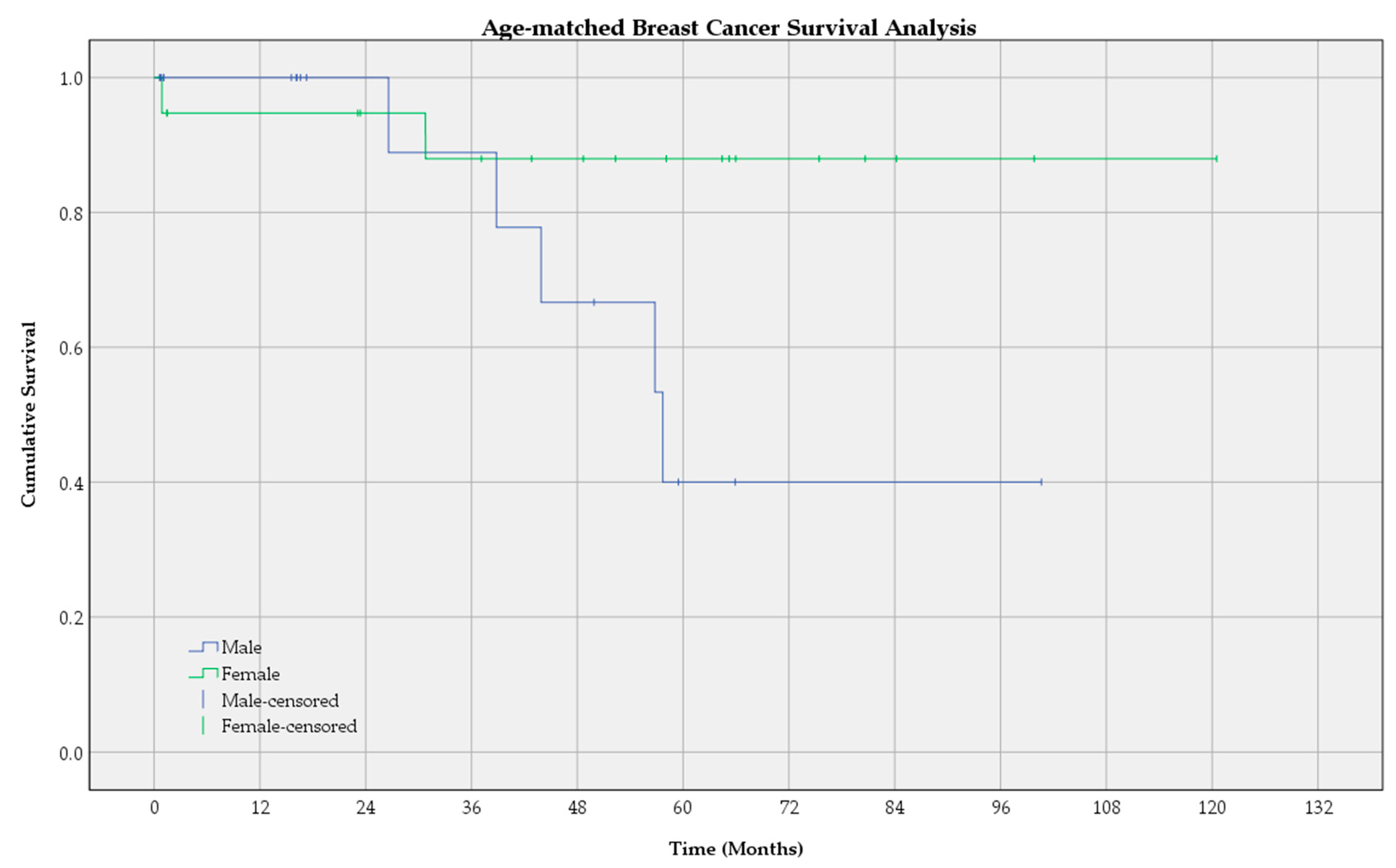
3.2. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Breast Cancer. Available online: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 13 May 2020).
- Hassett, M.J.; Somerfield, M.R.; Baker, E.R.; Cardoso, F.; Kansal, K.J.; Kwait, D.C.; Plichta, J.K.; Ricker, C.; Roshal, A.; Ruddy, K.J.; et al. Management of male breast cancer: ASCO guideline. J. Clin. Oncol. 2020, 38, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Burga, A.M.; Fadare, O.; Lininger, R.A.; Tavassoli, F.A. Invasive carcinomas of the male breast: A morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006, 449, 507–512. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/ (accessed on 9 August 2024).
- Hong, J.H.; Ha, K.S.; Jung, Y.H.; Won, H.S.; An, H.J.; Lee, G.J.; Kang, D.; Park, J.C.; Park, S.; Byun, J.H.; et al. Clinical features of male breast cancer: Experiences from seven institutions over 20 years. Cancer Res. Treat. 2016, 48, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Bartlett, J.; Slaets, L.; van Deurzen, C.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 2018, 29, 405–417. [Google Scholar] [CrossRef]
- Giordano, S.H.; Buzdar, A.U.; Hortobagyi, G.N. Breast cancer in men. Ann. Intern. Med. 2002, 137, 678–687. [Google Scholar] [CrossRef]
- Hübner, J.; Katalinic, A.; Waldmann, A.; Kraywinkel, K. Long-term Incidence and Mortality Trends for Breast Cancer in Germany. Geburtshilfe Frauenheilkd. 2020, 80, 611–618. [Google Scholar] [CrossRef]
- Varzaru, V.B.; Eftenoiu, A.E.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Popescu, R.; Cobec, I.M. The Influence of Tumor-Specific Markers in Breast Cancer on Other Blood Parameters. Life 2024, 14, 458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angelico, G.; Broggi, G.; Tinnirello, G.; Puzzo, L.; Vecchio, G.M.; Salvatorelli, L.; Memeo, L.; Santoro, A.; Farina, J.; Mulé, A.; et al. Tumor Infiltrating Lymphocytes (TILS) and PD-L1 Expression in Breast Cancer: A Review of Current Evidence and Prognostic Implications from Pathologist’s Perspective. Cancers 2023, 15, 4479. [Google Scholar] [CrossRef]
- Pal, T.; Agnese, D.; Daly, M.; La Spada, A.; Litton, J.; Wick, M.; Klugman, S.; Esplin, E.D.; Jarvik, G.P.; Professional Practice and Guidelines Committee. Points to consider: Is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2020, 22, 681–685. [Google Scholar] [CrossRef]
- Campos, F.A.B.; Rouleau, E.; Torrezan, G.T.; Carraro, D.M.; da Rocha, J.C.C.; Mantovani, H.K.; da Silva, L.R.; Osório, C.A.B.d.T.; Sanches, S.M.; Caputo, S.M.; et al. Genetic Landscape of Male Breast Cancer. Cancers 2021, 13, 3535. [Google Scholar] [CrossRef]
- Valentini, V.; Bucalo, A.; Conti, G.; Celli, L.; Porzio, V.; Capalbo, C.; Silvestri, V.; Ottini, L. Gender-Specific Genetic Predisposition to Breast Cancer: BRCA Genes and Beyond. Cancers 2024, 16, 579. [Google Scholar] [CrossRef] [PubMed]
- Grigore, L.G.; Radoi, V.E.; Serban, A.; Mihai, A.D.; Stoica, I. The molecular detection of germline mutations in the BRCA1 and BRCA2 genes associated with breast and ovarian cancer in a Romanian cohort of 616 patients. Curr. Issues Mol. Biol. 2024, 46, 4630–4645. [Google Scholar] [CrossRef] [PubMed]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res. Treat. 2018, 172, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Huang, T.W.; Tam, K.W. Treatment of male breast cancer: Meta-analysis of real-world evidence. Br. J. Surg. 2021, 108, 1034–1042. [Google Scholar] [CrossRef]
- Garcia-Etienne, C.A.; Ferrari, A.; Della Valle, A.; Lucioni, M.; Ferraris, E.; Di Giulio, G.; Squillace, L.; Bonzano, E.; Lasagna, A.; Rizzo, G.; et al. Management of the axilla in patients with breast cancer and positive sentinel lymph node biopsy: An evidence-based update in a European breast center. Eur. J. Surg. Oncol. 2020, 46, 15–23. [Google Scholar] [CrossRef]
- Guidelinesprogramm Onkologie. Interdisciplinary S3-Guidelines for the Early Diagnostic, Diagnostic, Therapy and Follow up of the Breast Cancer, Longversion 4.4-June 2021 AWMF-Registernummer: 032-045OL. S3-Leitlinie Mammakarzinom. Available online: https://www.awmf.org/ (accessed on 13 May 2023).
- Hennequin, C.; Belkacémi, Y.; Bourgier, C.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Lévi, J.M.; Pasquier, D.; Racadot, S.; Rivera, S. Radiotherapy of breast cancer. Cancer Radiother. 2022, 26, 221–230. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Ji, F.; Yang, C.Q.; Li, X.L.; Zhang, L.L.; Yang, M.; Li, J.Q.; Gao, H.F.; Zhu, T.; Cheng, M.Y.; Li, W.P.; et al. Risk of breast cancer-related death in women with a prior cancer. Aging 2020, 12, 5894–5906. [Google Scholar] [CrossRef]
- Cobec, I.M.; Moleriu, L.; Moatar, A.E.; Rempen, A. First clinical experience with CDK4/6 inhibitors in breast cancer therapy. Exp. Ther. Med. 2021, 21, 522. [Google Scholar] [CrossRef]
- Lin, R.-H.; Lin, C.-S.; Chuang, C.-L.; Kujabi, B.K.; Chen, Y.-C. Breast Cancer Survival Analysis Model. Appl. Sci. 2022, 12, 1971. [Google Scholar] [CrossRef]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Michowitz, M.; Noy, S.; Lazebnik, N.; Aladjem, D. Bilateral breast cancer. J. Surg. Oncol. 1985, 30, 109–112. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017.
- Wörmann, B. Breast cancer: Basics, screening, diagnostics and treatment. Med. Monatsschr Pharm. 2017, 40, 55–64. [Google Scholar] [PubMed]
- Fentiman, I.S.; Fourquet, A.; Hortobagyi, G.N. Male breast cancer. Lancet 2006, 367, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Stalsberg, H.; Thomas, D.B.; Rosenblatt, K.A.; Jimenez, L.M.; McTiernan, A.; Stemhagen, A.; Thompson, W.D.; Curnen, M.G.; Satariano, W.; Austin, D.F.; et al. Histologic types and hormone receptors in breast cancer in men: A population-based study in 282 United States men. Cancer Causes Control. 1993, 4, 143–151. [Google Scholar] [CrossRef]
- Ruddy, K.J.; Winer, E.P. Male breast cancer: Risk factors, biology, diagnosis, treatment, and survivorship. Ann. Oncol. 2013, 24, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, V.; Valentini, V.; Bucalo, A.; Conti, G.; Manzella, L.; Turchetti, D.; Russo, A.; Capalbo, C.; Ottini, L. HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy. Cancers 2024, 16, 548. [Google Scholar] [CrossRef]
- El-Tamer, M.B.; Komenaka, I.K.; Troxel, A.; Li, H.; Joseph, K.-A.; Ditkoff, B.-A.; Schnabel, F.R.; Kinne, D.W. Men with breast cancer have better disease-specific survival than women. Arch. Surg. 2004, 139, 1079–1082. [Google Scholar] [CrossRef]
- Marchal, F.; Salou, M.; Marchal, C.; Lesur, A.; Desandes, E. Men with breast cancer have same disease-specific and event-free survival as women. Ann. Surg. Oncol. 2009, 16, 972–978. [Google Scholar] [CrossRef]
- Fang, W.; Huang, Y.; Han, X.; Peng, J.; Zheng, M. Characteristics of metastasis and survival between male and female breast cancer with different molecular subtypes: A population-based observational study. Cancer Med. 2022, 11, 764–777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Shu, X.; Meszoely, I.; Pal, T.; Mayer, I.A.; Yu, Z.; Zheng, W.; Bailey, C.E.; Shu, X.O. Overall Mortality After Diagnosis of Breast Cancer in Men vs. Women. JAMA Oncol. 2019, 5, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baojiang, L.; Tingting, L.; Gang, L.; Li, Z. Male breast cancer: A retrospective study comparing survival with female breast cancer. Oncol. Lett. 2012, 4, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Shi, W.; Liu, T.; Siyin, S.T.; Wang, W.; Duan, N.; Xu, G.; Qu, J. Clinicopathologic characteristics and prognosis for male breast cancer compared to female breast cancer. Sci. Rep. 2022, 12, 220. [Google Scholar] [CrossRef]
- Scomersi, S.; Giudici, F.; Cacciatore, G.; Losurdo, P.; Fracon, S.; Cortinovis, S.; Ceccherini, R.; Zanconati, F.; Tonutti, M.; Bortul, M. Comparison between male and female breast cancer survival using propensity score matching analysis. Sci. Rep. 2021, 11, 11639. [Google Scholar] [CrossRef] [PubMed]
- Sanli, A.N.; Tekcan Sanli, D.E.; Altundag, M.K.; Aydogan, F. Is There a Survival Difference Between Male and Female Breast Cancer Subtypes According to the Prognostic Staging System? A Population-Based Cohort Study. Am. Surg. 2024, 90, 788–799. [Google Scholar] [CrossRef]
- Greif, J.M.; Pezzi, C.M.; Klimberg, V.S.; Bailey, L.; Zuraek, M. Gender differences in breast cancer: Analysis of 13,000 breast cancers in men from the national cancer data base. Ann. Surg. Oncol. 2012, 19, 3199–3204. [Google Scholar] [CrossRef]
- Restrepo, D.J.; Boczar, D.; Huayllani, M.T.; Sisti, A.; McLaughlin, S.A.; Spaulding, A.; Parker, A.S.; Carter, R.E.; Leppin, A.L.; Forte, A.J. Survival Disparities in Male Patients with Breast Cancer. Anticancer. Res. 2019, 39, 5669–5674. [Google Scholar] [CrossRef]
- Potter, A.M.; Bentz, B.; Crue, L.; Leiby, S.; Bashi, S.; Maguire, K.; Meyers, J.; Mieczkowski, K. Men’s Lived Experiences of Breast Cancer and Changes in Occupation. Occup. Ther. Int. 2023, 2023, 9641922. [Google Scholar] [CrossRef]
- Anderson, W.F.; Jatoi, I.; Tse, J.; Rosenberg, P.S. Male breast cancer: A population-based comparison with female breast cancer. J. Clin. Oncol. 2010, 28, 232–239. [Google Scholar] [CrossRef]
- Caetano Dos Santos, F.L.; Michalek, I.M.; Wojciechowska, U.; Didkowska, J. Changes in the survival of patients with breast cancer: Poland, 2000-2019. Breast Cancer Res Treat. 2023, 197, 623–631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meisel, J.L.; Venur, V.A.; Gnant, M.; Carey, L. Evolution of targeted therapy in breast cancer: Where precision medicine began. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 78–86. [Google Scholar] [CrossRef]
- DiPippo, A.J.; Patel, N.K.; Barnett, C.M. Cyclin-dependent kinase inhibitors for the treatment of breast cancer: Past, present, and future. Pharmacotherapy 2016, 36, 652–667. [Google Scholar] [CrossRef]
- Grinda, T.; Antoine, A.; Jacot, W.; Blaye, C.; Cottu, P.-H.; Diéras, V.; Dalenc, F.; Gonçalves, A.; Debled, M.; Patsouris, A.; et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open 2021, 6, 100114. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Burciu, O.M.; Sas, I.; Popoiu, T.A.; Merce, A.G.; Moleriu, L.; Cobec, I.M. Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer. Int. J. Mol. Sci. 2024, 25, 8506. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, V.B.; Vlad, T.; Popescu, R.; Vlad, C.S.; Moatar, A.E.; Cobec, I.M. Triple-Negative Breast Cancer: Molecular Particularities Still a Challenge. Diagnostics 2024, 14, 1875. [Google Scholar] [CrossRef]
| Histopathological Type of Breast Cancer | Total (n = 2162) | Female (n = 2143) | Male (n = 19) |
|---|---|---|---|
| Invasive ductal carcinoma | 1727 (79.9%) | 1709 (79.7%) | 18 (94.7%) |
| Invasive lobular carcinoma | 314 (14.5%) | 314 (14.7%) | - |
| Mucinous carcinoma | 45 (2.1%) | 45 (2.1%) | - |
| Tubular carcinoma | 23 (1.1%) | 23 (1.1%) | - |
| Papillary carcinoma | 13 (0.6%) | 13 (0.6%) | - |
| Breast carcinoma with neuroendocrine differentiation | 10 (0.5%) | 10 (0.5%) | - |
| Medullary carcinoma | 8 (0.4%) | 8 (0.4%) | - |
| Metaplastic breast cancer | 7 (0.3%) | 7 (0.3%) | - |
| Intracystic breast carcinoma | 6 (0.3%) | 5 (0.2%) | 1 (5.3%) |
| Malign Phyllodes tumor | 4 (0.2%) | 4 (0.2%) | - |
| Cribriform breast cancer | 2 (0.1%) | 2 (0.1%) | - |
| Mammary Paget disease | 2 (0.1%) | 2 (0.1%) | - |
| Breast angiosarcoma | 1 (<0.1%) | 1 (<0.1%) | - |
| ER | PR | Her2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (N = 2162) | Male (N = 19) | Female (N = 2143) | Total (N = 2162) | Male (N = 19) | Female (N = 2143) | Total (N = 2162) | Male (N = 19) | Female (N = 2143) | |
| Negative | 353 (16.3%) | 1 (2.3%) | 352 (16.4%) | 552 (25.5%) | 4 (21.1%) | 548 (25.6%) | 1667 (77.1%) | 13 (68.4%) | 1654 (77.2%) |
| Positive | 1779 (82.3%) | 18 (94.7%) | 1761 (82.2%) | 1579 (73.0%) | 15 (78.9%) | 1564 (73.0%) | 323 (14.9%) | 3 (15.8%) | 320 (14.9%) |
| Unknown | 30 (1.4%) | - | 30 (1.4%) | 31 (1.4%) | - | 31 (1.4%) | 172 (8.0%) | 3 (15.8%) | 169 (7.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varzaru, V.B.; Anastasiu-Popov, D.-M.; Eftenoiu, A.-E.; Popescu, R.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Puscasiu, D.; Cobec, I.M. Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival. Cancers 2024, 16, 3049. https://doi.org/10.3390/cancers16173049
Varzaru VB, Anastasiu-Popov D-M, Eftenoiu A-E, Popescu R, Vlad DC, Vlad CS, Moatar AE, Puscasiu D, Cobec IM. Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival. Cancers. 2024; 16(17):3049. https://doi.org/10.3390/cancers16173049
Chicago/Turabian StyleVarzaru, Vlad Bogdan, Diana-Maria Anastasiu-Popov, Anca-Elena Eftenoiu, Roxana Popescu, Daliborca Cristina Vlad, Cristian Sebastian Vlad, Aurica Elisabeta Moatar, Daniela Puscasiu, and Ionut Marcel Cobec. 2024. "Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival" Cancers 16, no. 17: 3049. https://doi.org/10.3390/cancers16173049
APA StyleVarzaru, V. B., Anastasiu-Popov, D.-M., Eftenoiu, A.-E., Popescu, R., Vlad, D. C., Vlad, C. S., Moatar, A. E., Puscasiu, D., & Cobec, I. M. (2024). Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival. Cancers, 16(17), 3049. https://doi.org/10.3390/cancers16173049







